Long-Term Outcomes of a Randomized Study of Neoadjuvant Induction Dual HER2 Blockade with Trastuzumab and Lapatinib Followed by Weekly Paclitaxel Plus Dual HER2 Blockade for HER2-Positive Primary Breast Cancer (Neo-Lath Study)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Patients
2.3. Observation and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patients
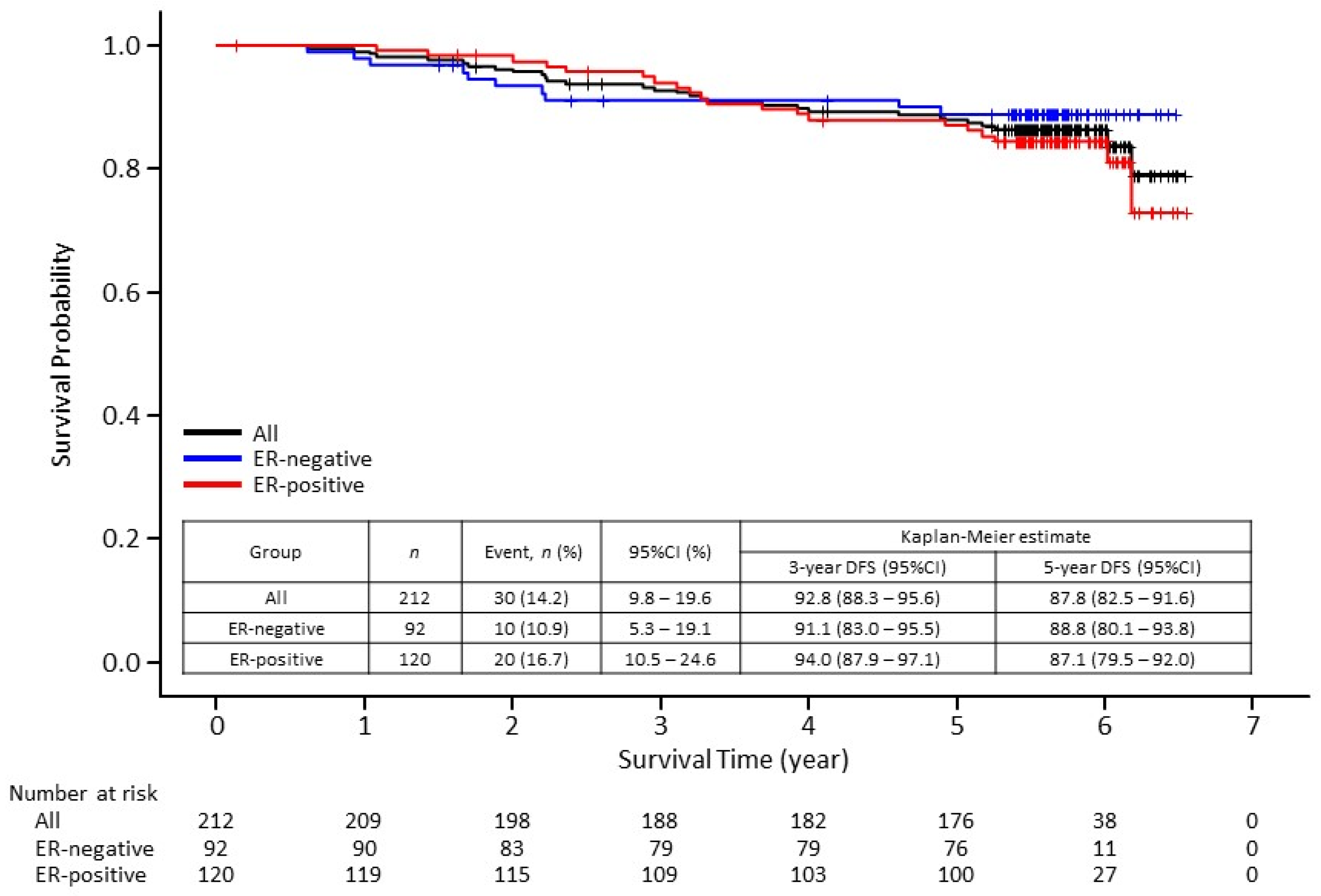
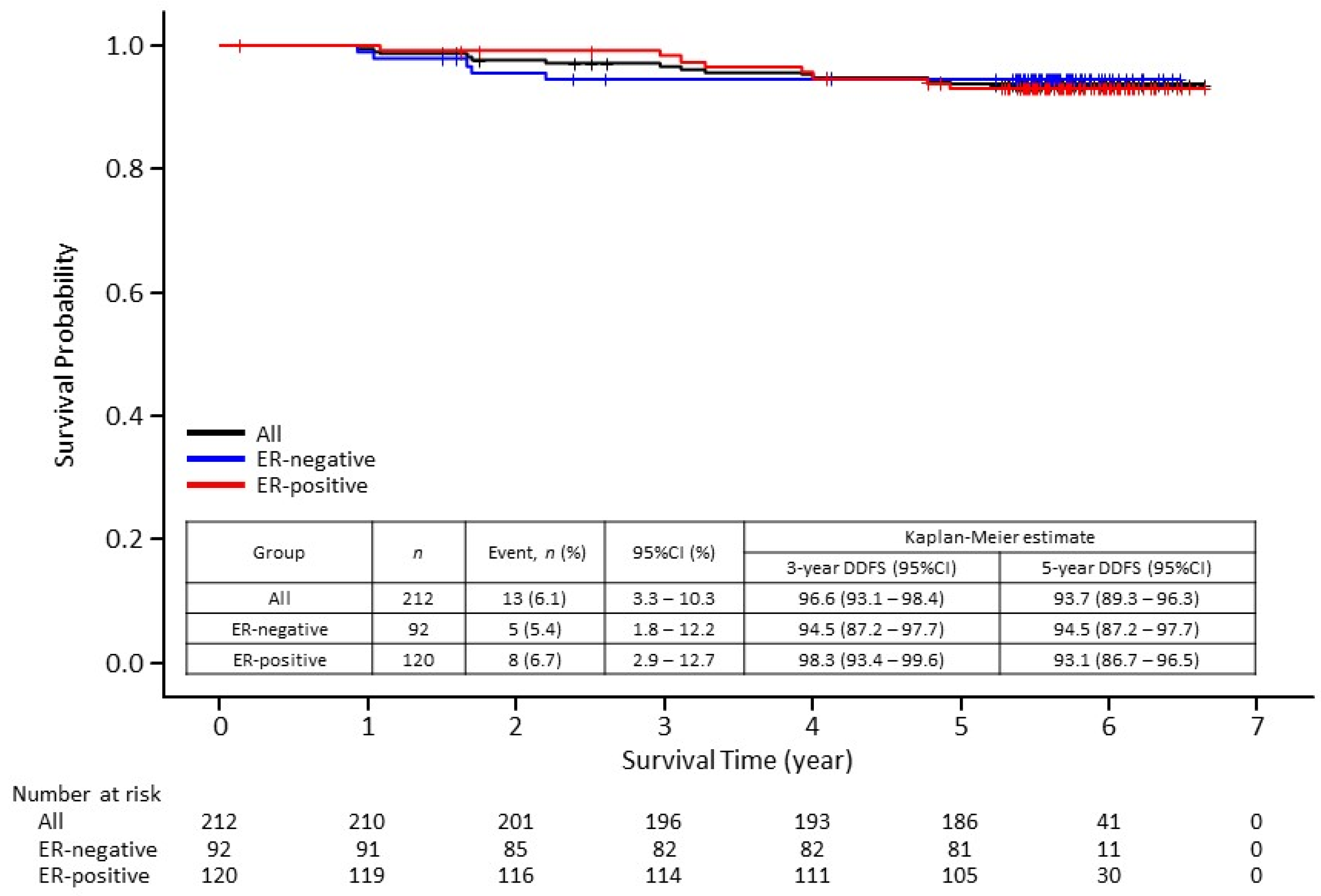
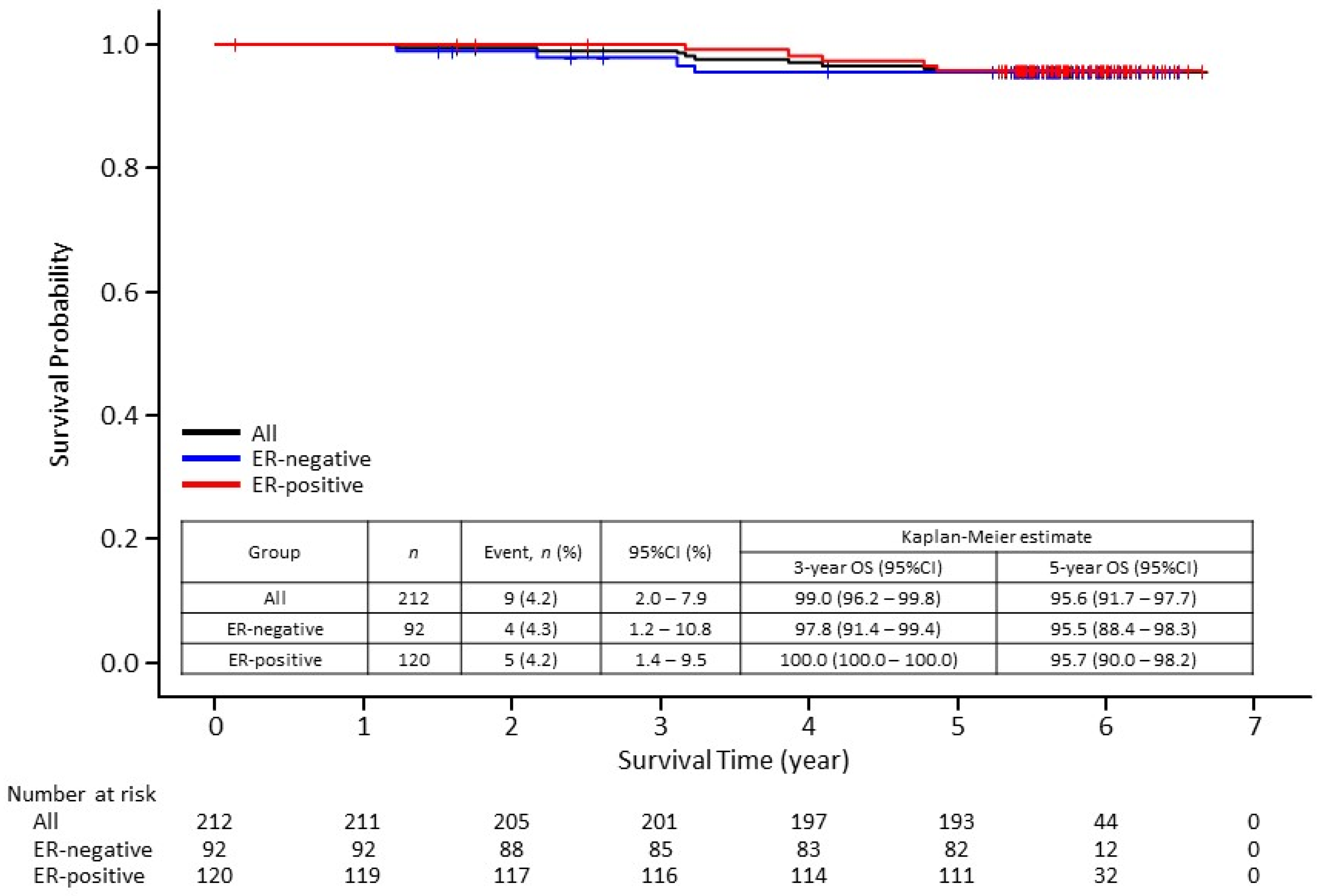
3.2. Survival Outcomes
3.3. Subgroup Analyses
3.3.1. Stratified by Response to Neoadjuvant Treatment
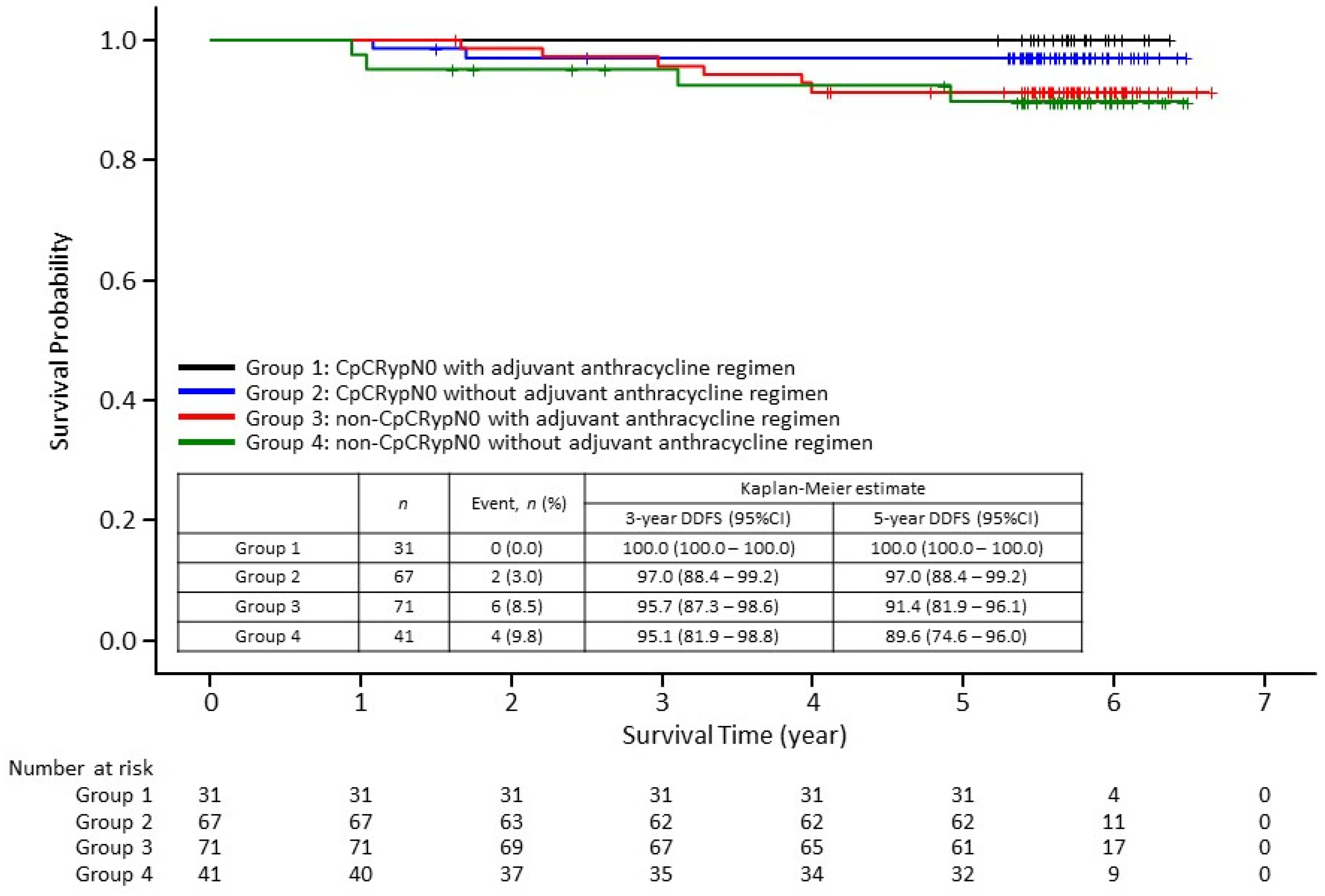
3.3.2. Stratified by Response to Neoadjuvant Treatment and by Use of Adjuvant Anthracycline Therapy
3.4. Exploratory Analysis
4. Discussion
4.1. Survival Outcomes
4.2. Omission of Adjuvant Anthracycline Therapy
4.3. Outcomes in Patients with Small Node-Negative Tumor
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, N.E.; Stern, D.F. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta 1994, 1198, 165–184. [Google Scholar]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, G.; Fabi, A.; Felici, A.; Papaldo, P. Improved prognosis by trastuzumab of women with HER2-positive breast cancer compared with those with HER2-negative disease. J. Clin. Oncol. 2010, 28, e337. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M.; et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010, 375, 377–384. [Google Scholar] [PubMed]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2012, CD006243. [Google Scholar] [CrossRef]
- Balduzzi, S.; Mantarro, S.; Guarneri, V.; Tagliabue, L.; Pistotti, V.; Moja, L.; D’Amico, R. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst. Rev. 2014, 2014, CD006242. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Fu, F.; Li, J.; Huang, M.; Zeng, B.; Lin, Y.; Mei, Q.; Lv, J.; Wang, C. Dual HER2 Blockade versus a single agent in trastuzumab-containing regimens for HER2-positive early breast cancer: A systematic review and meta-analysis of randomized controlled trials. J. Oncol. 2020, 2020, 5169278. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Advani, P.; Cornell, L.; Chumsri, S.; Moreno-Aspitia, A. Dual HER2 blockade in the neoadjuvant and adjuvant treatment of HER2-positive breast cancer. Breast Cancer 2015, 7, 321–335. [Google Scholar] [CrossRef] [Green Version]
- Pop, L.; Suciu, I.D.; Ionescu, O.; Ionescu, P.; Toader, O.D. The dual blockade in the neoadjuvant setting of HER-2 positive early-stage breast cancer. J. Med. Life 2019, 12, 329–331. [Google Scholar] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Toi, M.; Yamamoto, N.; Iwata, H.; Kuroi, K.; Bando, H.; Ohtani, S.; Takano, T.; Inoue, K.; Yanagita, Y.; et al. Efficacy and safety of trastuzumab, lapatinib, and paclitaxel neoadjuvant treatment with or without prolonged exposure to anti-HER2 therapy, and with or without hormone therapy for HER2-positive primary breast cancer: A randomised, five-arm, multicentre, open-label phase II trial. Breast Cancer 2018, 25, 407–415. [Google Scholar]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- de Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Huober, J.; Holmes, E.; Baselga, J.; de Azambuja, E.; Untch, M.; Fumagalli, D.; Sarp, S.; Lang, I.; Smith, I.; Boyle, F.; et al. Survival outcomes of the NeoALTTO study (BIG 1-06): Updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. Eur. J. Cancer 2019, 118, 169–177. [Google Scholar] [CrossRef]
- Broglio, K.R.; Quintana, M.; Foster, M.; Olinger, M.; McGlothlin, A.; Berry, S.M.; Boileau, J.F.; Brezden-Masley, C.; Chia, S.; Dent, S.; et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: A meta-analysis. JAMA Oncol. 2016, 2, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.; Holmes, E.; Baselga, J.; de Azambuja, E.; Dueck, A.C.; Viale, G.; Zujewski, J.A.; Goldhirsch, A.; Armour, A.; Pritchard, K.I.; et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: Results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 2016, 34, 1034–1042. [Google Scholar] [CrossRef] [Green Version]
- Lambertini, M.; Campbell, C.; Gelber, R.D.; Viale, G.; McCullough, A.; Hilbers, F.; Korde, L.A.; Werner, O.; Chumsri, S.; Jackisch, C.; et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: Exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res. Treat. 2019, 177, 103–114. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Giuliano, M.; Hu, H.; Wang, Y.C.; Fu, X.; Nardone, A.; Herrera, S.; Mao, S.; Contreras, A.; Gutierrez, C.; Wang, T.; et al. Upregulation of ER signaling as an adaptive mechanism of cell survival in HER2-positive breast tumors treated with anti-HER2 therapy. Clin. Cancer Res. 2015, 21, 3995–4003. [Google Scholar] [CrossRef] [Green Version]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef] [Green Version]
- Rimawi, M.F.; Cecchini, R.S.; Rastogi, P.; Geyer, C.E., Jr.; Fehrenbacher, L.; Stella, P.J.; Dayao, Z.; Rabinovitch, R.; Dyar, S.H.; Flynn, P.J.; et al. A phase III trial evaluating pCR in patients with HR+, HER2-positive breast cancer treated with neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) +/− estrogen deprivation: NRG Oncology/NSABP B-52. In Proceedings of the 2016 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2016. [Google Scholar]
- Brenton, J.D.; Carey, L.A.; Ahmed, A.A.; Caldas, C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol. 2005, 23, 7350–7360. [Google Scholar] [CrossRef] [Green Version]
- von Minckwitz, G. Neoadjuvant chemotherapy in breast cancer-insights from the German experience. Breast Cancer 2012, 19, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Ueno, T.; Masuda, N.; Sato, N.; Ohtani, S.; Yamamura, J.; Matsunami, N.; Kashiwaba, M.; Takano, T.; Takahashi, M.; Kaneko, K.; et al. Multicenter study of primary systemic therapy with docetaxel, cyclophosphamide and trastuzumab for HER2-positive operable breast cancer: The JBCRG-10 study. Jpn. J. Clin. Oncol. 2020, 50, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Tamura, K.; Tsurutani, J.; Takahashi, S.; Iwata, H.; Krop, I.E.; Redfern, C.; Sagara, Y.; Doi, T.; Park, H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: A dose-expansion, phase 1 study. Lancet Oncol. 2019, 20, 816–826. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Tolaney, S.M.; Guo, H.; Pernas, S.; Barry, W.T.; Dillon, D.A.; Ritterhouse, L.; Schneider, B.P.; Shen, F.; Fuhrman, K.; Baltay, M.; et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2019, 37, 1868–1875. [Google Scholar] [CrossRef]
| Characteristics | Group A n = 44 | Group B n = 48 | Group C n = 41 | Group D n = 39 | Group E n = 40 | All n = 212 | |
|---|---|---|---|---|---|---|---|
| Age, years | Median | 56 | 56 | 52 | 53 | 49 | 53 |
| Range | 33–69 | 36–69 | 32–70 | 26–66 | 28–68 | 26–70 | |
| TNM staging before treatment start | |||||||
| T: primary lesion | cT1 | 4 (9.1) | 6 (12.5) | 11 (26.8) | 8 (20.5) | 11 (27.5) | 40 (18.9) |
| T2 | 31 (70.5) | 29 (60.4) | 26 (63.4) | 26 (66.7) | 26 (65.0) | 138 (65.1) | |
| T3 | 9 (20.5) | 13 (27.1) | 4 (9.8) | 5 (12.8) | 3 (7.5) | 34 (16.0) | |
| N: regional lymph node | N0 | 22 (50.0) | 26 (54.2) | 23 (56.1) | 22 (56.4) | 24 (60.0) | 117 (55.2) |
| N1 | 22 (50.0) | 22 (45.8) | 18 (43.9) | 17 (43.6) | 16 (40.0) | 95 (44.8) | |
| Histological grade (B&R) | 1 | 0 | 2 (4.2) | 1 (2.4) | 0 | 0 | 3 (1.4) |
| 2 | 6 (13.6) | 7 (14.6) | 13 (31.7) | 10 (25.6) | 13 (32.5) | 49 (23.1) | |
| 3 | 11 (25.0) | 11 (22.9) | 9 (22.0) | 12 (30.8) | 8 (20.0) | 51 (24.1) | |
| Unknown | 27 (61.4) | 28 (58.3) | 18 (43.9) | 17 (43.6) | 19 (47.5) | 109 (51.4) | |
| Lymph node metastasis after surgery | pN0 | 40 (90.9) | 44 (91.7) | 33 (80.5) | 31 (79.5) | 32 (80.0) | 180 (84.9) |
| pN (+) | 4 (9.1) | 2 (4.2) | 7 (17.1) | 6 (15.4) | 5 (12.5) | 24 (11.3) | |
| Unknown | 0 | 2 (4.2) | 1 (2.4) | 2 (5.1) | 3 (7.5) | 8 (3.8) | |
| Response to neoadjuvant chemotherapy (CpCRypN0) | Yes | 29 (65.9) | 28 (58.3) | 13 (31.7) | 13 (33.3) | 15 (37.5) | 98 (46.2) |
| No | 15 (34.1) | 20 (41.7) | 28 (68.3) | 26 (66.7) | 24 (60.0) | 113 (53.3) | |
| Unknown | 0 | 0 | 0 | 0 | 1 (2.5) | 1 (0.5) | |
| Surgical procedure | Breast-conserving surgery | 28 (63.6) | 26 (54.2) | 29 (70.7) | 21 (53.8) | 26 (65.0) | 130 (61.3) |
| Total mastectomy | 16 (36.4) | 21 (43.8) | 12 (29.3) | 18 (46.2) | 12 (30.0) | 79 (37.3) | |
| Did not undergo surgery | 0 | 1 (2.1) | 0 | 0 | 2 (5.0) | 3 (1.4) | |
| Axillary dissection procedure | Axillary dissection | 21 (47.7) | 19 (39.6) | 16 (39.0) | 12 (30.8) | 13 (32.5) | 81 (38.2) |
| Axillary sampling dissection | 2 (4.5) | 5 (10.4) | 4 (9.8) | 1 (2.6) | 3 (7.5) | 15 (7.1) | |
| SLN biopsy | 21 (47.7) | 23 (47.9) | 21 (51.2) | 26 (66.7) | 22 (55.0) | 113 (53.3) | |
| Did not undergo surgery | 0 | 1 (2.1) | 0 | 0 | 2 (5.0) | 3 (1.4) | |
| Postoperative radiation a | |||||||
| Patients undergoing breast conservation (N = 130) | Yes (with regional lymph node irradiation) | 3 (6.8) | 1 (2.1) | 2 (4.9) | 0 | 1 (2.5) | 7 (3.3) |
| Yes (without regional lymph node irradiation) | 23 (52.3) | 24 (50.0) | 26 (63.4) | 21 (53.8) | 24 (60.0) | 118 (55.7) | |
| No | 2 (4.5) | 1 (2.1) | 1 (2.4) | 0 | 1 (2.5) | 5 (2.4) | |
| Surgical procedure: Total mastectomy (N = 79) | Yes (with regional lymph node irradiation) | 4 (9.1) | 6 (12.5) | 2 (4.9) | 3 (7.7) | 0 | 15 (7.1) |
| Yes (without regional lymph node irradiation) | 0 | 0 | 1 (2.4) | 1 (2.6) | 0 | 2 (0.9) | |
| No | 12 (27.3) | 15 (31.3) | 9 (22.0) | 14 (35.9) | 12 (30.0) | 62 (29.2) | |
| Adjuvant chemotherapyAnthracycline b | Yes | 18 (40.9) | 15 (31.3) | 24 (58.5) | 23 (59.0) | 22 (55.0) | 102 (48.1) |
| No | 26 (59.1) | 33 (68.8) | 17 (41.5) | 16 (41.0) | 17 (42.5) | 109 (51.4) | |
| Endocrine therapy b | Yes | 11 (25.0) | 6 (12.5) | 38 (92.7) | 37 (94.9) | 35 (87.5) | 127 (59.9) |
| No | 33 (75.0) | 42 (87.5) | 3 (7.3) | 2 (5.1) | 4 (10.0) | 84 (39.6) | |
| ER status at registration | Negative | 44 (100.0) | 48 (100.0) | 0 | 0 | 0 | 92 (43.4) |
| (central assessment) | Positive | 0 | 0 | 41 (100.0) | 39 (100.0) | 40 (100.0) | 120 (56.6) |
| Positive (1–9%) | 0 | 0 | 4 (9.8) | 5 (12.8) | 10 (25.0) | 19 (9.0) | |
| Positive (≥10%) | 0 | 0 | 37 (90.2) | 34 (87.2) | 30 (75.0) | 101 (47.6) | |
| Hormonal status of postoperative residual tumor cells in the breast c | Not performed | 0 | 6 (40.0) | 3 (13.0) | 2 (8.3) | 2 (11.8) | 13 (14.8) |
| HR− | 7 (77.8) | 3 (20.0) | 2 (8.7) | 0 | 1 (5.9) | 13 (14.8) | |
| HR+ | 2 (22.2) | 6 (40.0) | 18 (78.3) | 22 (91.7) | 14 (82.4) | 62 (70.5) | |
| HER2 status before treatment start | IHC3+ | 43 (97.7) | 45 (93.8) | 37 (90.2) | 33 (84.6) | 37 (92.5) | 195 (92.0) |
| IHC2 + DISH+ | 1 (2.3) | 3 (6.3) | 4 (9.8) | 6 (15.4) | 3 (7.5) | 17 (8.0) | |
| HER2 status of postoperative residual tumor cells in the breast c | Not performed | 0 | 6 (40.0) | 6 (26.1) | 5 (20.8) | 3 (17.6) | 20 (22.7) |
| IHC3+ | 6 (66.7) | 6 (40.0) | 8 (34.8) | 12 (50.0) | 8 (47.1) | 40 (45.5) | |
| IHC2 + FISH+ | 2 (22.2) | 0 | 3 (13.0) | 2 (8.3) | 2 (11.8) | 9 (10.2) | |
| IHC2 + FISH unknown | 0 | 1 (6.7) | 1 (4.3) | 3 (12.5) | 1 (5.9) | 6 (6.8) | |
| IHC1+/0 | 1 (11.1) | 2 (13.3) | 5 (21.7) | 2 (8.3) | 3 (17.6) | 13 (14.8) | |
| ER Status | Achieved CpCRypN0 | Residual Invasive Disease | Total |
|---|---|---|---|
| ER-negative | 29.8% (17/57) | 45.7% (16/35) | 35.9% (33/92) |
| ER-positive | 34.1% (14/41) | 71.4% (55/77) | 58.5% (69/118) |
| Total | 31.6% (31/98) | 63.4% (71/112) | 48.6% (102/210 a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokunaga, E.; Masuda, N.; Yamamoto, N.; Iwata, H.; Bando, H.; Aruga, T.; Ohtani, S.; Fujisawa, T.; Takano, T.; Inoue, K.; et al. Long-Term Outcomes of a Randomized Study of Neoadjuvant Induction Dual HER2 Blockade with Trastuzumab and Lapatinib Followed by Weekly Paclitaxel Plus Dual HER2 Blockade for HER2-Positive Primary Breast Cancer (Neo-Lath Study). Cancers 2021, 13, 4008. https://doi.org/10.3390/cancers13164008
Tokunaga E, Masuda N, Yamamoto N, Iwata H, Bando H, Aruga T, Ohtani S, Fujisawa T, Takano T, Inoue K, et al. Long-Term Outcomes of a Randomized Study of Neoadjuvant Induction Dual HER2 Blockade with Trastuzumab and Lapatinib Followed by Weekly Paclitaxel Plus Dual HER2 Blockade for HER2-Positive Primary Breast Cancer (Neo-Lath Study). Cancers. 2021; 13(16):4008. https://doi.org/10.3390/cancers13164008
Chicago/Turabian StyleTokunaga, Eriko, Norikazu Masuda, Naohito Yamamoto, Hiroji Iwata, Hiroko Bando, Tomoyuki Aruga, Shoichiro Ohtani, Tomomi Fujisawa, Toshimi Takano, Kenichi Inoue, and et al. 2021. "Long-Term Outcomes of a Randomized Study of Neoadjuvant Induction Dual HER2 Blockade with Trastuzumab and Lapatinib Followed by Weekly Paclitaxel Plus Dual HER2 Blockade for HER2-Positive Primary Breast Cancer (Neo-Lath Study)" Cancers 13, no. 16: 4008. https://doi.org/10.3390/cancers13164008
APA StyleTokunaga, E., Masuda, N., Yamamoto, N., Iwata, H., Bando, H., Aruga, T., Ohtani, S., Fujisawa, T., Takano, T., Inoue, K., Suganuma, N., Takada, M., Aogi, K., Sakurai, K., Shigematsu, H., Kuroi, K., Haga, H., Ohno, S., Morita, S., & Toi, M. (2021). Long-Term Outcomes of a Randomized Study of Neoadjuvant Induction Dual HER2 Blockade with Trastuzumab and Lapatinib Followed by Weekly Paclitaxel Plus Dual HER2 Blockade for HER2-Positive Primary Breast Cancer (Neo-Lath Study). Cancers, 13(16), 4008. https://doi.org/10.3390/cancers13164008








