IL-27 Mediates PD-L1 Expression and Release by Human Mesothelioma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
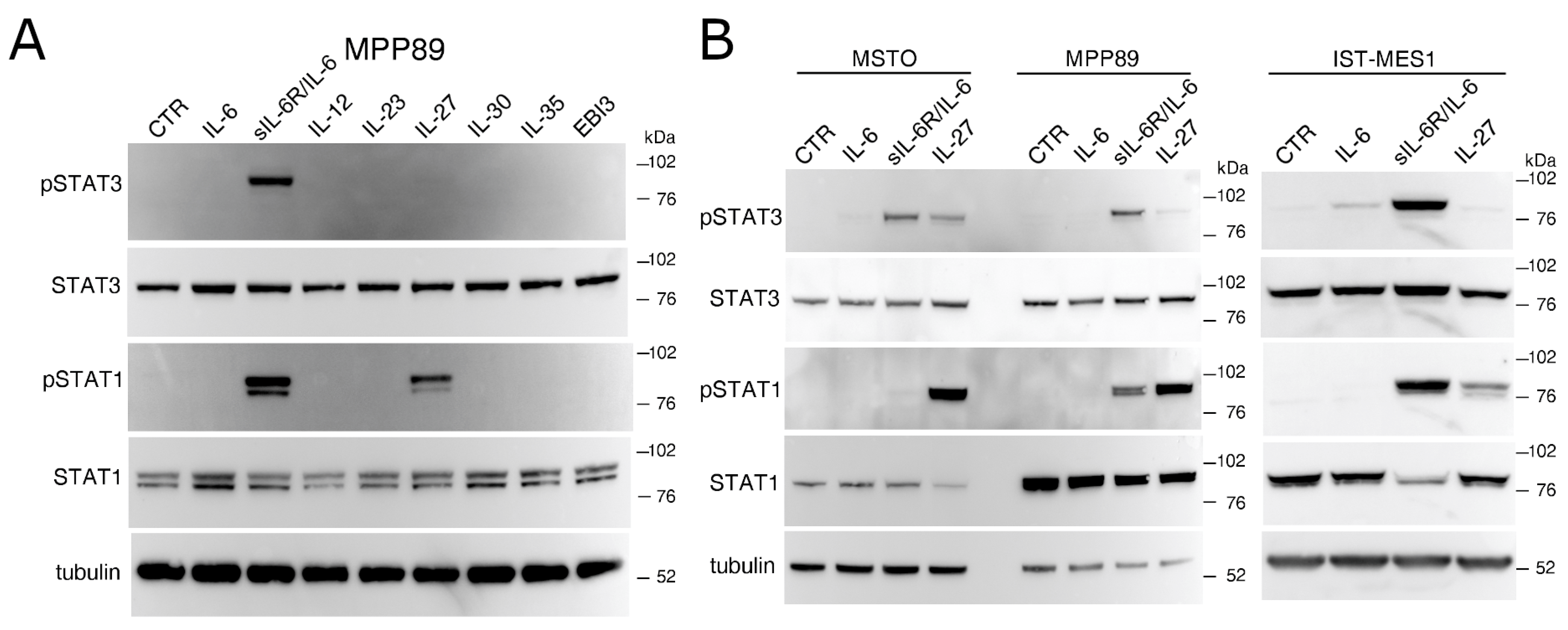
2.1. IL-27 and a sIL-6R/IL-6 Chimeric Protein Mediated Signal Transduction in Human MM Cells
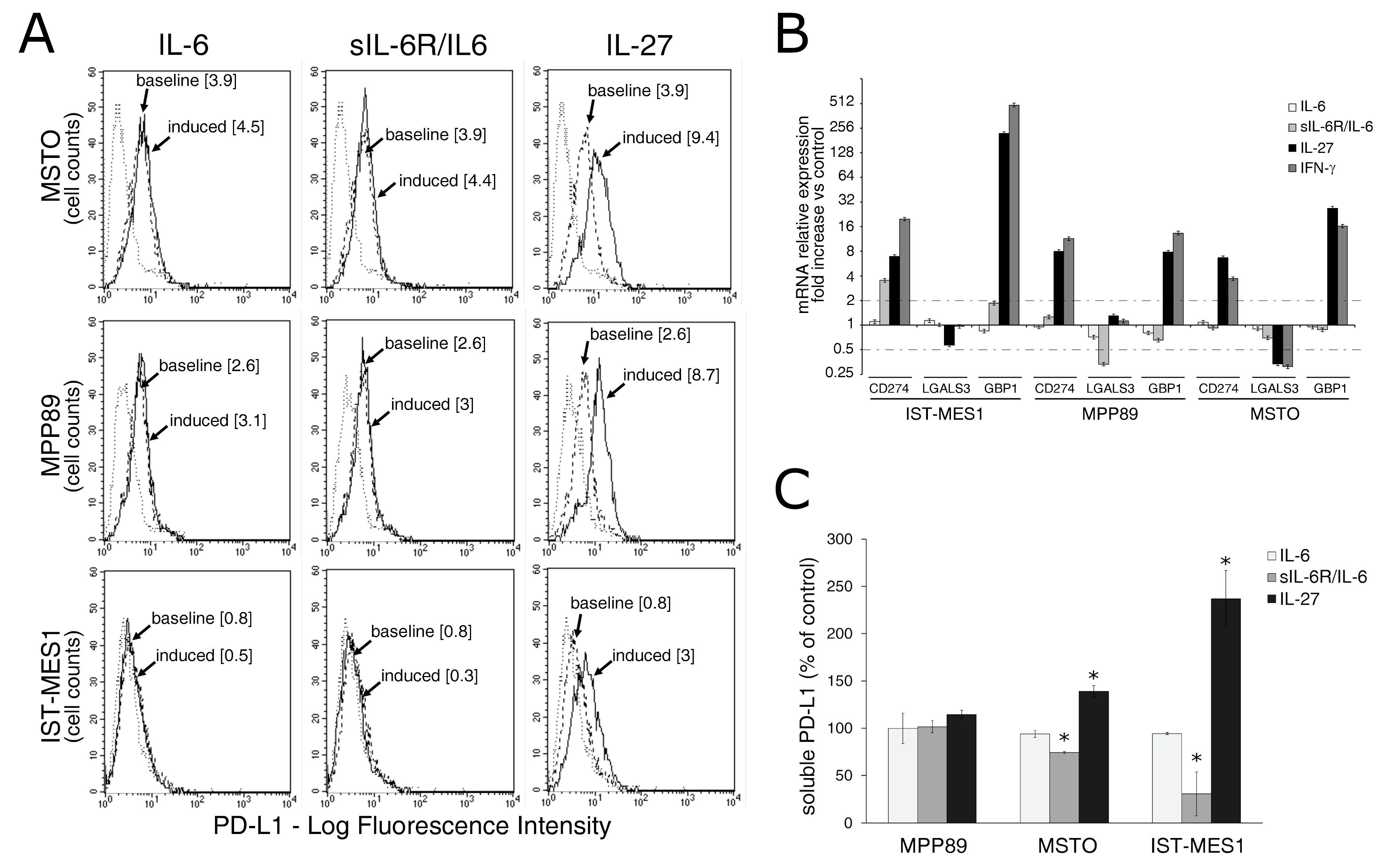
2.2. IL-27, but Not sIL-6R/IL-6 Chimeric Protein, Mediates Expression of Surface PD-L1 Molecule and Release of Soluble PD-L1 by MM Cells
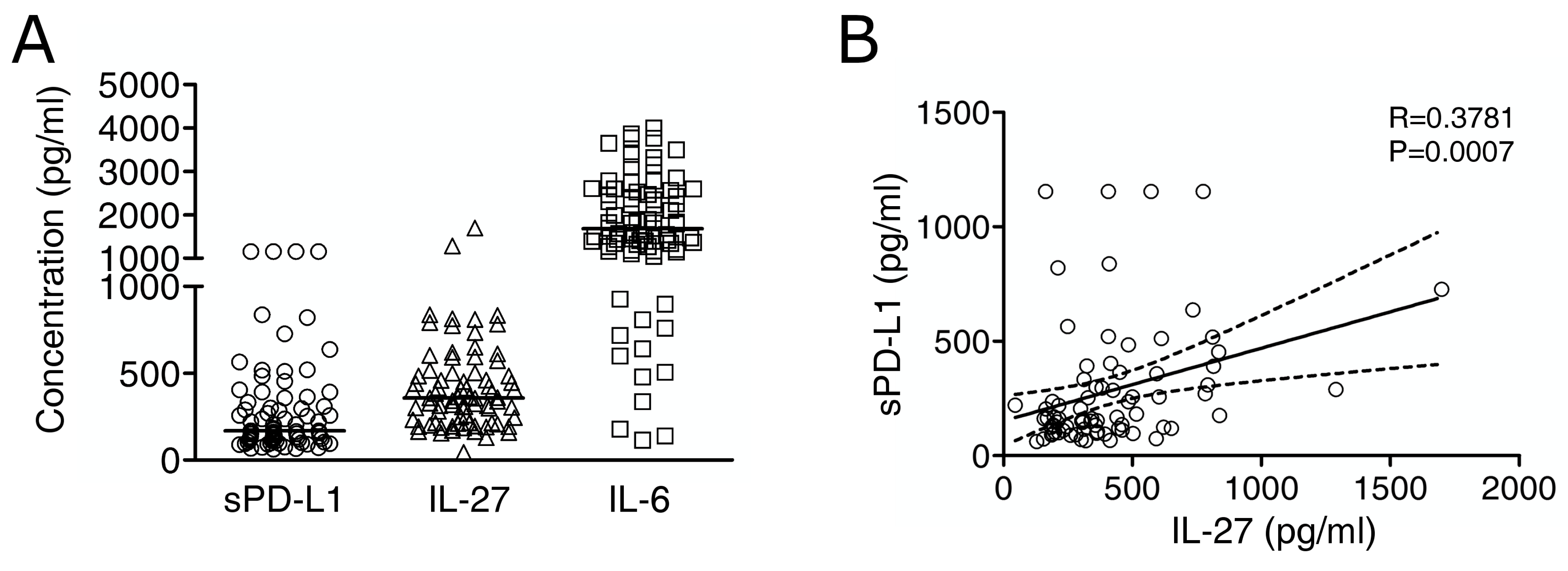
2.3. Immuno-Reactive IL-27 Is Detectable in MM Pleural Exudates and Correlates with Soluble PD-L1 Expression
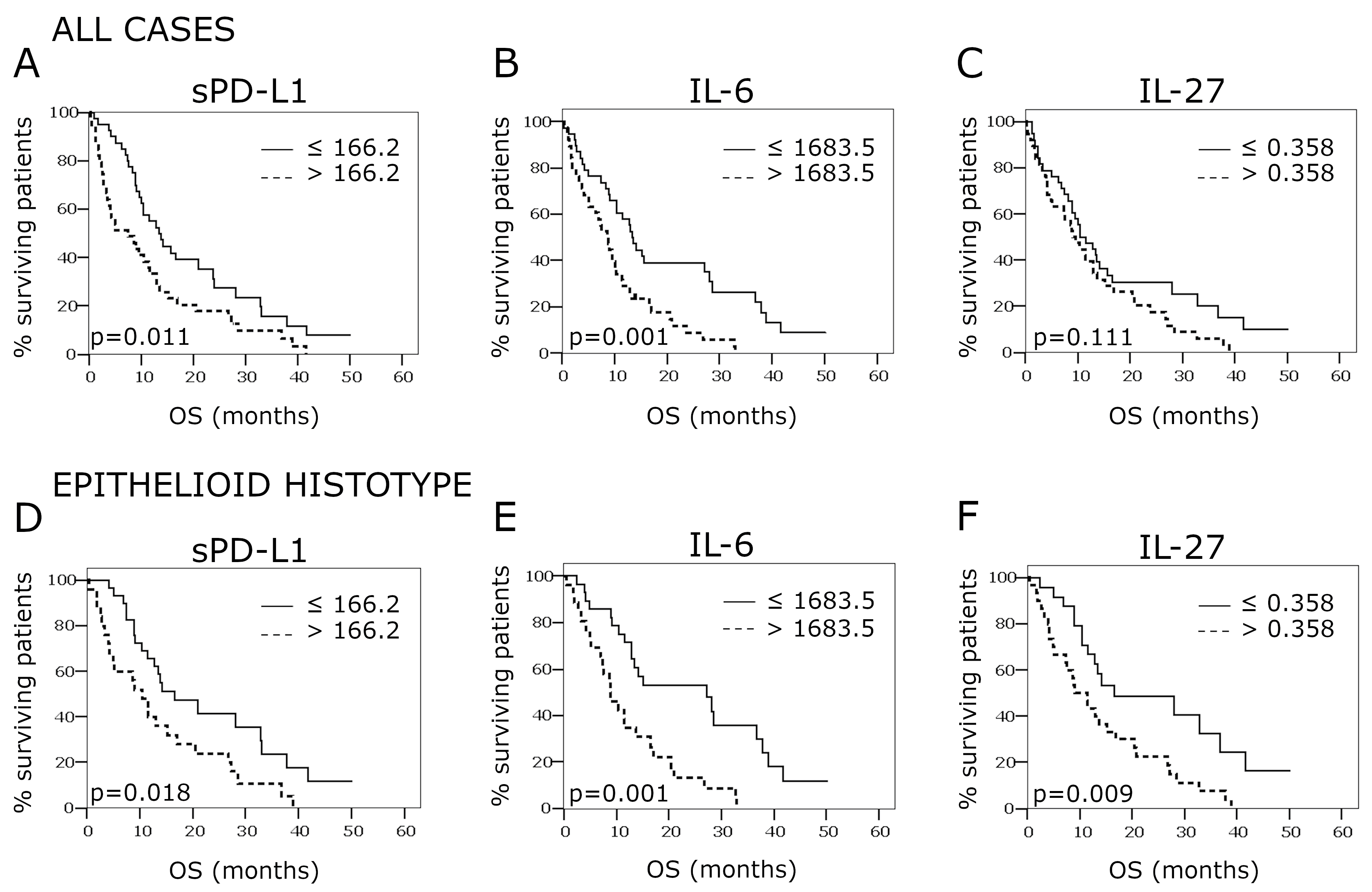
2.4. Overall Survival According to PD-L1, IL-27, and IL-6 Concentrations in the Pleural Effusion
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. Cells and Treatments
4.3. Western Blot
4.4. Immunofluorescence and Flow Cytometry
4.5. RT-PCR Analysis
4.6. Cytokine Dosage and ELISA Tests
4.7. Patients
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, W.; Linkov, F.; Landsittel, D.P.; Silverstein, J.C.; Bashara, W.; Gaudioso, C.; Feldman, M.D.; Pass, H.I.; Melamed, J.; Friedberg, J.S.; et al. Factors Influencing Malignant Mesothelioma Survival: A Retrospective Review of the National Mesothelioma Virtual Bank Cohort. F1000Research 2018, 7, 1184. [Google Scholar] [CrossRef]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as Second-Line or Third-Line Treatment in Relapsed Malignant Mesothelioma (DETERMINE): A Multicentre, International, Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef]
- Calabrò, L.; Morra, A.; Giannarelli, D.; Amato, G.; D’Incecco, A.; Covre, A.; Lewis, A.; Rebelatto, M.C.; Danielli, R.; Altomonte, M.; et al. Tremelimumab Combined with Durvalumab in Patients with Mesothelioma (NIBIT-MESO-1): An Open-Label, Non-Randomised, Phase 2 Study. Lancet Respir. Med. 2018, 6, 451–460. [Google Scholar] [CrossRef]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Dô, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or Nivolumab plus Ipilimumab in Patients with Relapsed Malignant Pleural Mesothelioma (IFCT-1501 MAPS2): A Multicentre, Open-Label, Randomised, Non-Comparative, Phase 2 Trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Hassan, R.; Thomas, A.; Nemunaitis, J.J.; Patel, M.R.; Bennouna, J.; Chen, F.L.; Delord, J.-P.; Dowlati, A.; Kochuparambil, S.T.; Taylor, M.H.; et al. Efficacy and Safety of Avelumab Treatment in Patients with Advanced Unresectable Mesothelioma: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 351–357. [Google Scholar] [CrossRef]
- Metaxas, Y.; Rivalland, G.; Mauti, L.A.; Klingbiel, D.; Kao, S.; Schmid, S.; Nowak, A.K.; Gautschi, O.; Bartnick, T.; Hughes, B.G.; et al. Pembrolizumab as Palliative Immunotherapy in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 1784–1791. [Google Scholar] [CrossRef] [Green Version]
- Fennell, D.A.; Kirkpatrick, E.; Cozens, K.; Nye, M.; Lester, J.; Hanna, G.; Steele, N.; Szlosarek, P.; Danson, S.; Lord, J.; et al. CONFIRM: A Double-Blind, Placebo-Controlled Phase III Clinical Trial Investigating the Effect of Nivolumab in Patients with Relapsed Mesothelioma: Study Protocol for a Randomised Controlled Trial. Trials 2018, 19, 233. [Google Scholar] [CrossRef] [Green Version]
- Alley, E.W.; Lopez, J.; Santoro, A.; Morosky, A.; Saraf, S.; Piperdi, B.; van Brummelen, E. Clinical Safety and Activity of Pembrolizumab in Patients with Malignant Pleural Mesothelioma (KEYNOTE-028): Preliminary Results from a Non-Randomised, Open-Label, Phase 1b Trial. Lancet Oncol. 2017, 18, 623–630. [Google Scholar] [CrossRef]
- Forde, P.M.; Scherpereel, A.; Tsao, A.S. Use of Immune Checkpoint Inhibitors in Mesothelioma. Curr. Treat. Options Oncol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-Line Nivolumab plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Brcic, L.; Klikovits, T.; Megyesfalvi, Z.; Mosleh, B.; Sinn, K.; Hritcu, R.; Laszlo, V.; Cufer, T.; Rozman, A.; Kern, I.; et al. Prognostic Impact of PD-1 and PD-L1 Expression in Malignant Pleural Mesothelioma: An International Multicenter Study. Transl. Lung Cancer Res. 2021, 10, 1594–1607. [Google Scholar] [CrossRef]
- Jin, L.; Gu, W.; Li, X.; Xie, L.; Wang, L.; Chen, Z. PD-L1 and Prognosis in Patients with Malignant Pleural Mesothelioma: A Meta-Analysis and Bioinformatics Study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920962362. [Google Scholar] [CrossRef]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed Necrosis Induced by Asbestos in Human Mesothelial Cells Causes High-Mobility Group Box 1 Protein Release and Resultant Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.; Yang, H. Molecular Pathways: Targeting Mechanisms of Asbestos and Erionite Carcinogenesis in Mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillegass, J.M.; Miller, J.M.; MacPherson, M.B.; Westbom, C.M.; Sayan, M.; Thompson, J.K.; Macura, S.L.; Perkins, T.N.; Beuschel, S.L.; Alexeeva, V.; et al. Asbestos and Erionite Prime and Activate the NLRP3 Inflammasome That Stimulates Autocrine Cytokine Release in Human Mesothelial Cells. Part. Fibre Toxicol. 2013, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Comar, M.; Zanotta, N.; Zanconati, F.; Cortale, M.; Bonotti, A.; Cristaudo, A.; Bovenzi, M. Chemokines Involved in the Early Inflammatory Response and in Pro-Tumoral Activity in Asbestos-Exposed Workers from an Italian Coastal Area with Territorial Clusters of Pleural Malignant Mesothelioma. Lung Cancer 2016, 94, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.; Thomas, P.; Govindarajan, V.; Sharma, P.; Loggie, B. Malignant Peritoneal Mesothelioma: Characterization of the Inflammatory Response in the Tumor Microenvironment. Ann. Surg. Oncol. 2016, 23, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.N.A.; Ho, G.Y.; Coward, J.I.G. The Role of Interleukin-6 in Malignant Mesothelioma. Transl. Lung Cancer Res. 2015, 4, 55–66. [Google Scholar] [CrossRef]
- Nakano, T.; Chahinian, A.P.; Shinjo, M.; Tonomura, A.; Miyake, M.; Togawa, N.; Ninomiya, K.; Higashino, K. Interleukin 6 and Its Relationship to Clinical Parameters in Patients with Malignant Pleural Mesothelioma. Br. J. Cancer 1998, 77, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Monti, G.; Jaurand, M.C.; Monnet, I.; Chretien, P.; Saint-Etienne, L.; Zeng, L.; Portier, A.; Devillier, P.; Galanaud, P.; Bignon, J. Intrapleural Production of Interleukin 6 during Mesothelioma and Its Modulation by Gamma-Interferon Treatment. Cancer Res. 1994, 54, 4419–4423. [Google Scholar]
- Rose-John, S.; Neurath, M.F. IL-6 Trans-Signaling: The Heat Is On. Immunity 2004, 20, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Adachi, Y.; Aoki, C.; Yoshio-Hoshino, N.; Takayama, K.; Curiel, D.T.; Nishimoto, N. Interleukin-6 Induces Both Cell Growth and VEGF Production in Malignant Mesotheliomas. Int. J. Cancer 2006, 119, 1303–1311. [Google Scholar] [CrossRef]
- Lamano, J.B.; Lamano, J.B.; Li, Y.D.; DiDomenico, J.D.; Choy, W.; Veliceasa, D.; Oyon, D.E.; Fakurnejad, S.; Ampie, L.; Kesavabhotla, K.; et al. Glioblastoma-Derived IL6 Induces Immunosuppressive Peripheral Myeloid Cell PD-L1 and Promotes Tumor Growth. Clin. Cancer Res. 2019, 25, 3643–3657. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Ishibashi, M.; Yamashita, T.; Tanosaki, S.; Okuyama, N.; Kondo, A.; Hyodo, H.; Shinya, E.; Takahashi, H.; Dong, H.; et al. Marrow Stromal Cells Induce B7-H1 Expression on Myeloma Cells, Generating Aggressive Characteristics in Multiple Myeloma. Leukemia 2013, 27, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Dual Roles of IL-27 in Cancer Biology and Immunotherapy. Mediat. Inflamm. 2017, 2017, 3958069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a Heterodimeric Cytokine Composed of EBI3 and P28 Protein, Induces Proliferation of Naive CD4+ T Cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Petretto, A.; Carbotti, G.; Inglese, E.; Lavarello, C.; Pistillo, M.P.; Rigo, V.; Croce, M.; Longo, L.; Martini, S.; Vacca, P.; et al. Proteomic Analysis Uncovers Common Effects of IFN-γ and IL-27 on the HLA Class I Antigen Presentation Machinery in Human Cancer Cells. Oncotarget 2016, 7, 72518–72536. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, I.; Ribatti, D. Regulation of Angiostatic Chemokines Driven by IL-12 and IL-27 in Human Tumors. J. Leukoc. Biol. 2011, 90, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.; Wiesinger, M.Y.; Nordhoff, C.; Schoenherr, C.; Haan, C.; Ludwig, S.; Weiskirchen, R.; Kato, N.; Heinrich, P.C.; Haan, S. Interleukin-27 Displays Interferon-Gamma-like Functions in Human Hepatoma Cells and Hepatocytes. Hepatology 2009, 50, 585–591. [Google Scholar] [CrossRef]
- Feng, X.M.; Liu, N.; Yang, S.G.; Hu, L.Y.; Chen, X.L.; Fang, Z.H.; Ren, Q.; Lu, S.H.; Liu, B.; Han, Z.C. Regulation of the Class II and Class I MHC Pathways in Human THP-1 Monocytic Cells by Interleukin-27. Biochem. Biophys. Res. Commun. 2008, 367, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Carbotti, G.; Nikpoor, A.R.; Vacca, P.; Gangemi, R.; Giordano, C.; Campelli, F.; Ferrini, S.; Fabbi, M. IL-27 Mediates HLA Class I up-Regulation, Which Can Be Inhibited by the IL-6 Pathway, in HLA-Deficient Small Cell Lung Cancer Cells. J. Exp. Clin. Cancer Res. 2017, 36, 140. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, E.; Sorrentino, C.; Zorzoli, A.; Di Meo, S.; Tupone, M.G.; Ognio, E.; Mincione, G.; Airoldi, I. The Antitumor Potential of Interleukin-27 in Prostate Cancer. Oncotarget 2014, 5, 10332–10341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, T.; Morishima, N.; Mizoguchi, I.; Shimizu, M.; Nagai, H.; Oniki, S.; Oka, M.; Nishigori, C.; Mizuguchi, J. Antiproliferative Activity of IL-27 on Melanoma. J. Immunol. 2008, 180, 6527–6535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and T(Regs) in the Melanoma Tumor Microenvironment Is Driven by CD8(+) T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-J.; Jang, B.-C.; Lee, S.-W.; Yang, Y.-I.; Suh, S.-I.; Park, Y.-M.; Oh, S.; Shin, J.-G.; Yao, S.; Chen, L.; et al. Interferon Regulatory Factor-1 Is Prerequisite to the Constitutive Expression and IFN-Gamma-Induced Upregulation of B7-H1 (CD274). FEBS Lett. 2006, 580, 755–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbotti, G.; Barisione, G.; Airoldi, I.; Mezzanzanica, D.; Bagnoli, M.; Ferrero, S.; Petretto, A.; Fabbi, M.; Ferrini, S. IL-27 Induces the Expression of IDO and PD-L1 in Human Cancer Cells. Oncotarget 2015, 6, 43267–43280. [Google Scholar] [CrossRef] [Green Version]
- Dozin, B.; Carbotti, G.; Roncella, S.; Ferro, P.; Dessanti, P.; Canessa, P.A.; Ferrini, S.; Fabbi, M. Assessment of Interferon-γ in Pleural Fluid as a Prognostic Factor of Survival in Malignant Pleural Mesothelioma. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef]
- Kondo, A.; Yamashita, T.; Tamura, H.; Zhao, W.; Tsuji, T.; Shimizu, M.; Shinya, E.; Takahashi, H.; Tamada, K.; Chen, L.; et al. Interferon-Gamma and Tumor Necrosis Factor-Alpha Induce an Immunoinhibitory Molecule, B7-H1, via Nuclear Factor-KappaB Activation in Blasts in Myelodysplastic Syndromes. Blood 2010, 116, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Pistillo, M.P.; Carosio, R.; Banelli, B.; Morabito, A.; Mastracci, L.; Ferro, P.; Varesano, S.; Venè, R.; Poggi, A.; Roncella, S. IFN-γ Upregulates Membranous and Soluble PD-L1 in Mesothelioma Cells: Potential Implications for the Clinical Response to PD-1/PD-L1 Blockade. Cell. Mol. Immunol. 2020, 17, 410–411. [Google Scholar] [CrossRef]
- Carbotti, G.; Petretto, A.; Naschberger, E.; Stürzl, M.; Martini, S.; Mingari, M.C.; Filaci, G.; Ferrini, S.; Fabbi, M. Cytokine-Induced Guanylate Binding Protein 1 (GBP1) Release from Human Ovarian Cancer Cells. Cancers 2020, 12, 488. [Google Scholar] [CrossRef] [Green Version]
- Carosio, R.; Fontana, V.; Mastracci, L.; Ferro, P.; Grillo, F.; Banelli, B.; Canessa, P.A.; Dessanti, P.; Vigani, A.; Morabito, A.; et al. Characterization of Soluble PD-L1 in Pleural Effusions of Mesothelioma Patients: Potential Implications in the Immune Response and Prognosis. J. Cancer Res. Clin. Oncol. 2021, 147, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Christmas, T.I.; Manning, L.S.; Davis, M.R.; Robinson, B.W.; Garlepp, M.J. HLA Antigen Expression and Malignant Mesothelioma. Am. J. Respir. Cell. Mol. Biol. 1991, 5, 213–220. [Google Scholar] [CrossRef]
- Mutti, L.; Valle, M.T.; Balbi, B.; Orengo, A.M.; Lazzaro, A.; Alciato, P.; Gatti, E.; Betta, P.G.; Pozzi, E. Primary Human Mesothelioma Cells Express Class II MHC, ICAM-1 and B7-2 and Can Present Recall Antigens to Autologous Blood Lymphocytes. Int. J. Cancer 1998, 78, 740–749. [Google Scholar] [CrossRef]
- Orengo, A.M.; Spoletini, L.; Procopio, A.; Favoni, R.E.; De Cupis, A.; Ardizzoni, A.; Castagneto, B.; Ribotta, M.; Betta, P.G.; Ferrini, S.; et al. Establishment of Four New Mesothelioma Cell Lines: Characterization by Ultrastructural and Immunophenotypic Analysis. Eur. Respir. J. 1999, 13, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 Blockade Induces Responses by Inhibiting Adaptive Immune Resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Ribas, A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov. 2015, 5, 915–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, A.; Carrier, Y.; Peron, J.P.S.; Bettelli, E.; Kamanaka, M.; Flavell, R.A.; Kuchroo, V.K.; Oukka, M.; Weiner, H.L. A Dominant Function for Interleukin 27 in Generating Interleukin 10-Producing Anti-Inflammatory T Cells. Nat. Immunol. 2007, 8, 1380–1389. [Google Scholar] [CrossRef]
- Yi, J.; Chen, Z.; Xu, F.; Wang, Z.; Zhang, A.; Liu, T.; Zhao, N.; Xiong, Y.; Jiang, G.; Ma, J.; et al. IL-27 Promotes Human Placenta-Derived Mesenchymal Stromal Cell Ability To Induce the Generation of CD4+IL-10+IFN-Γ+ T Cells via the JAK/STAT Pathway in the Treatment of Experimental Graft-versus-Host Disease. J. Immunol. 2019, 202, 1124–1136. [Google Scholar] [CrossRef]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-Induced Modulation of Autoimmunity and Its Therapeutic Potential. Autoimmun. Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pot, C.; Apetoh, L.; Awasthi, A.; Kuchroo, V.K. Induction of Regulatory Tr1 Cells and Inhibition of TH17 Cells by IL-27. Semin. Immunol. 2011, 23, 438–445. [Google Scholar] [CrossRef] [Green Version]
- DeLong, J.H.; Hall, A.; Rausch, M.; Moodley, D.; Perry, J.; Park, J.; Phan, A.T.; Beiting, D.P.; Kedl, R.M.; Hill, J.A.; et al. IL-27 and TCR Stimulation Promote T Cell Expression of Multiple Inhibitory Receptors. Immunohorizons 2019, 3, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orme, J.J.; Jazieh, K.A.; Xie, T.; Harrington, S.; Liu, X.; Ball, M.; Madden, B.; Charlesworth, M.C.; Azam, T.U.; Lucien, F.; et al. ADAM10 and ADAM17 Cleave PD-L1 to Mediate PD-(L)1 Inhibitor Resistance. OncoImmunology 2020, 9, 1744980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, Y.; Wise, R.; Zolkiewska, A. Proteolytic Processing of PD-L1 by ADAM Proteases in Breast Cancer Cells. Cancer Immunol. Immunother. 2020, 69, 43–55. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Shukla, S.A.; Patsoukis, N.; Chaudhri, A.; Browne, E.P.; Arazi, A.; Eisenhaure, T.M.; Pendergraft, W.F.; Hua, P.; Pham, H.C.; et al. A Secreted PD-L1 Splice Variant That Covalently Dimerizes and Mediates Immunosuppression. Cancer Immunol. Immunother. 2019, 68, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Hassounah, N.B.; Malladi, V.S.; Huang, Y.; Freeman, S.S.; Beauchamp, E.M.; Koyama, S.; Souders, N.; Martin, S.; Dranoff, G.; Wong, K.-K.; et al. Identification and Characterization of an Alternative Cancer-Derived PD-L1 Splice Variant. Cancer Immunol. Immunother. 2019, 68, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a Soluble Form of B7-H1 That Retains Immunosuppressive Activity and Is Associated with Aggressive Renal Cell Carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Kiyotani, K.; Sakata, S.; Nagano, S.; Kumehara, S.; Baba, S.; Besse, B.; Yanagitani, N.; Friboulet, L.; Nishio, M.; et al. Secreted PD-L1 Variants Mediate Resistance to PD-L1 Blockade Therapy in Non-Small Cell Lung Cancer. J. Exp. Med. 2019, 216, 982–1000. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy—Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Thomas, A.; Abate-Daga, D.; Zhang, J.; Morrow, B.; Steinberg, S.M.; Orlandi, A.; Ferroni, P.; Schlom, J.; Guadagni, F.; et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3+ T Cells Highly Expressing PD-L1 and the PD-L1+ Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti-PD-L1 Antibody Avelumab. J. Thorac. Oncol. 2016, 11, 1993–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti–PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Dilger, P.; Bird, C.; Wadhwa, M. IL-27 Promotes Proliferation of Human Leukemic Cell Lines Through the MAPK/ERK Signaling Pathway and Suppresses Sensitivity to Chemotherapeutic Drugs. J. Interferon Cytokine Res. 2016, 36, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Molle, C.; Nguyen, M.; Flamand, V.; Renneson, J.; Trottein, F.; De Wit, D.; Willems, F.; Goldman, M.; Goriely, S. IL-27 Synthesis Induced by TLR Ligation Critically Depends on IFN Regulatory Factor 3. J. Immunol. 2007, 178, 7607–7615. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Kamimura, D.; Hirano, T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 2019, 50, 812–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Total | sPD-L1 ≥ 166.2 pg/mL | |||||
|---|---|---|---|---|---|---|
| N | % | OR * | 95% CI ¶ | p-Value | ||
| IL-27 < 0.358 ng/mL | 38 | 12 | 30.8 | 1 (ref) | - | 0.001 |
| IL-27 ≥ 0.358 ng/mL | 39 | 27 | 69.2 | 4.86 | 1.86–12.79 | |
| IL-6 < 1683.5 pg/mL | 39 | 20 | 51.3 | 1 (ref) | - | 0.910 |
| IL-6 ≥ 1683.5 pg/mL | 38 | 19 | 48.7 | 0.950 | 0.39–2.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbotti, G.; Dozin, B.; Martini, S.; Giordano, C.; Scordamaglia, F.; Croce, M.; Filaci, G.; Ferrini, S.; Fabbi, M. IL-27 Mediates PD-L1 Expression and Release by Human Mesothelioma Cells. Cancers 2021, 13, 4011. https://doi.org/10.3390/cancers13164011
Carbotti G, Dozin B, Martini S, Giordano C, Scordamaglia F, Croce M, Filaci G, Ferrini S, Fabbi M. IL-27 Mediates PD-L1 Expression and Release by Human Mesothelioma Cells. Cancers. 2021; 13(16):4011. https://doi.org/10.3390/cancers13164011
Chicago/Turabian StyleCarbotti, Grazia, Beatrice Dozin, Stefania Martini, Chiara Giordano, Francesca Scordamaglia, Michela Croce, Gilberto Filaci, Silvano Ferrini, and Marina Fabbi. 2021. "IL-27 Mediates PD-L1 Expression and Release by Human Mesothelioma Cells" Cancers 13, no. 16: 4011. https://doi.org/10.3390/cancers13164011
APA StyleCarbotti, G., Dozin, B., Martini, S., Giordano, C., Scordamaglia, F., Croce, M., Filaci, G., Ferrini, S., & Fabbi, M. (2021). IL-27 Mediates PD-L1 Expression and Release by Human Mesothelioma Cells. Cancers, 13(16), 4011. https://doi.org/10.3390/cancers13164011






