Modelling Radiation-Induced Salivary Dysfunction during IMRT and Chemotherapy for Nasopharyngeal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment: Delineation of Organs at Risk, Constraint Planning and Selection of Toxicity-Related Organs at Risk
2.3. Endpoint Toxicity Definition
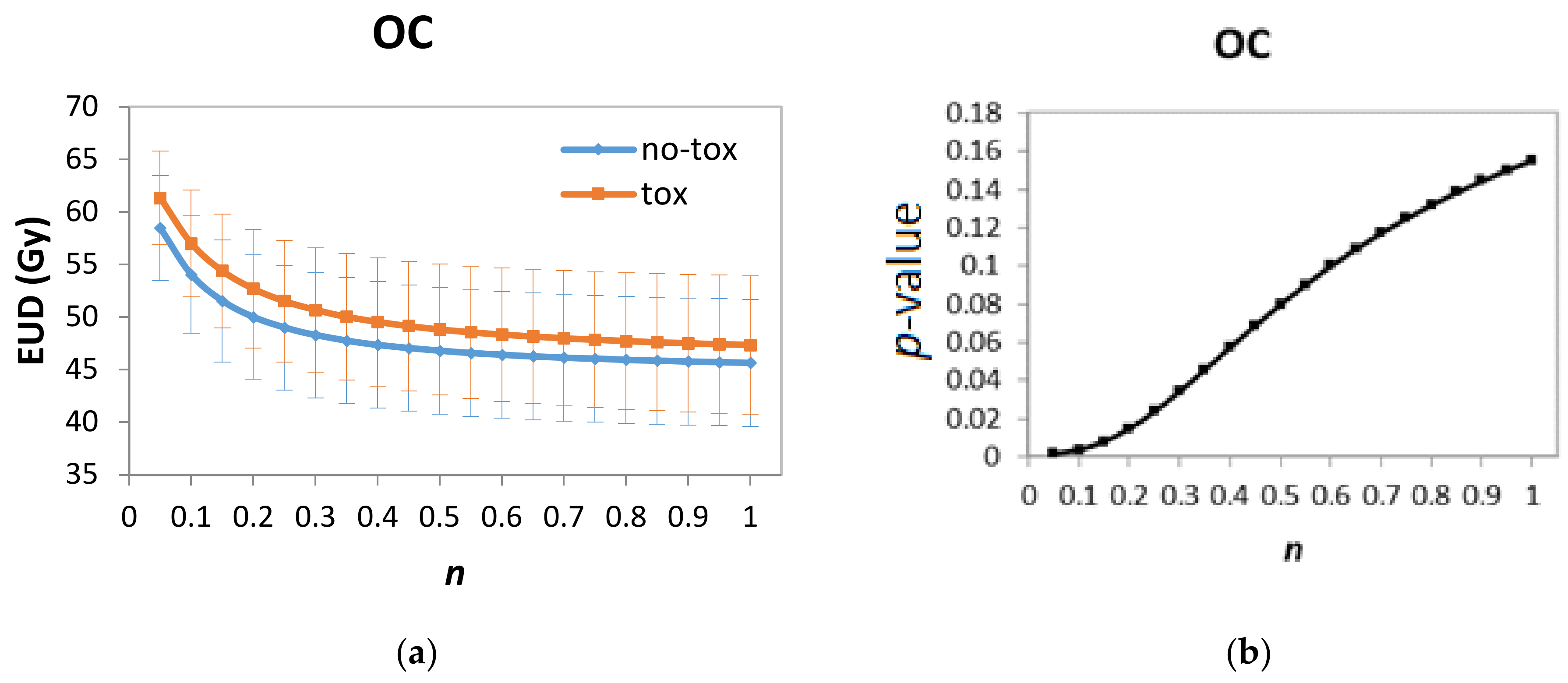
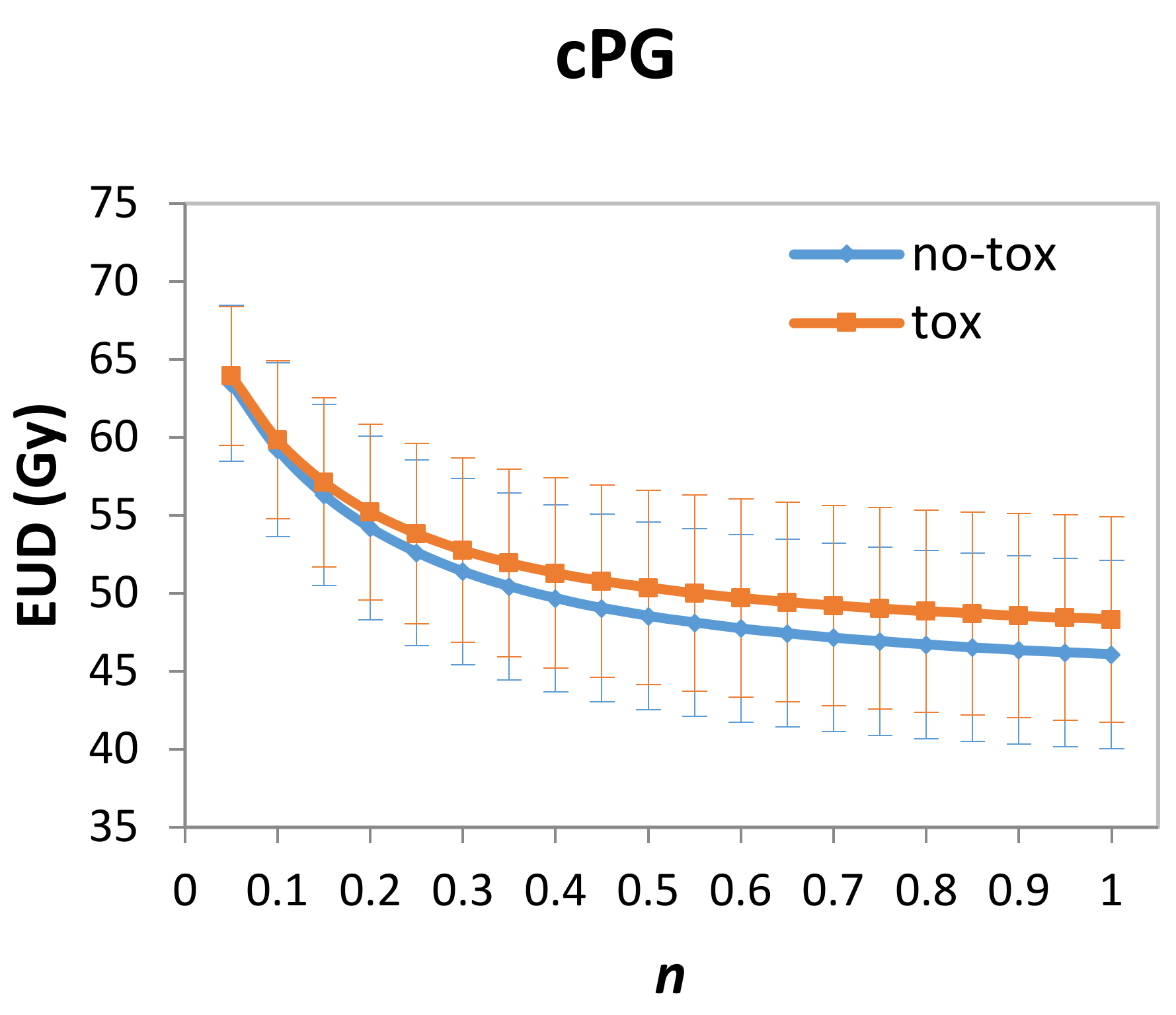
2.4. Modelling and Statistical Analysis
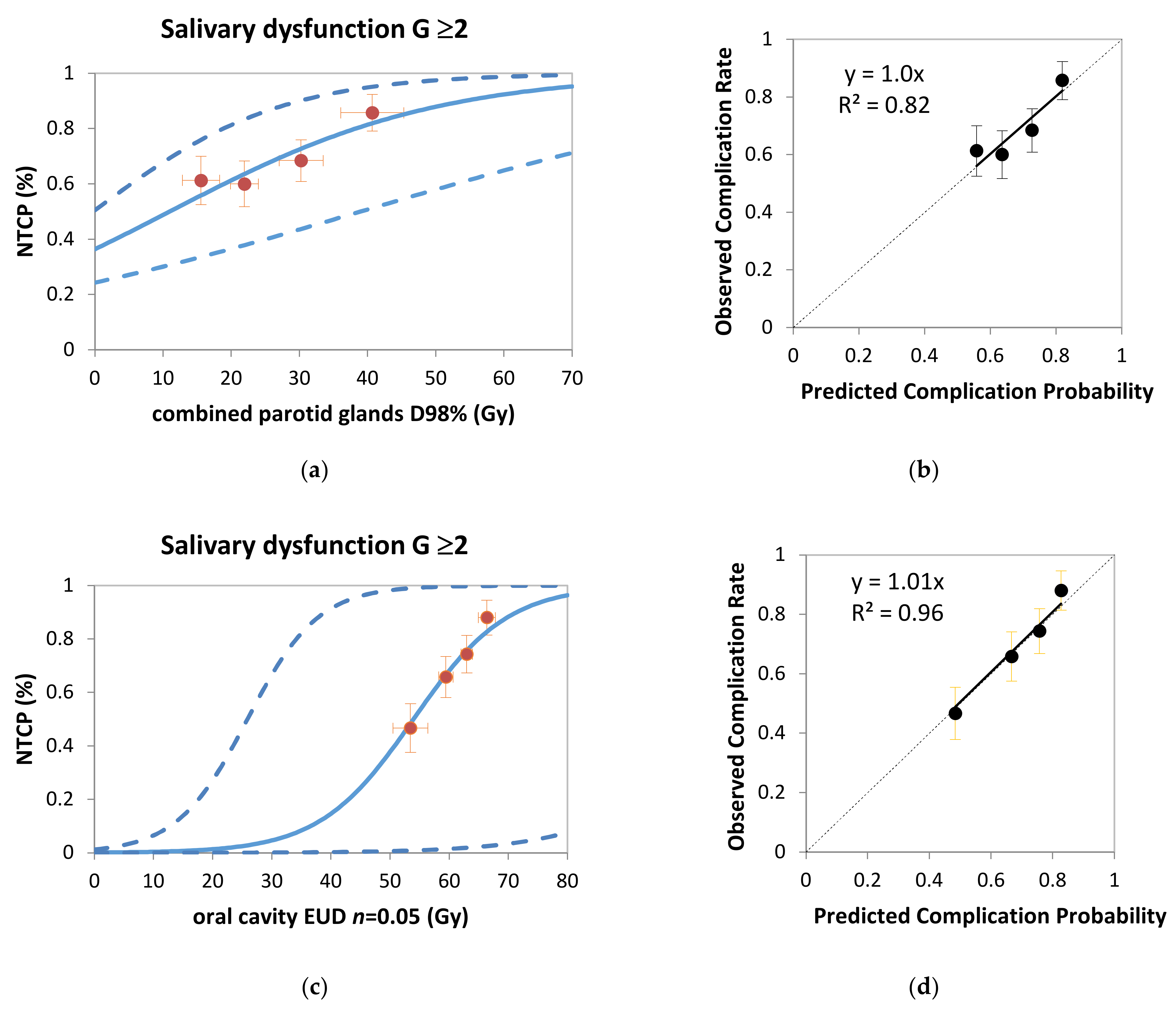
3. Results
3.1. Population for Model Development
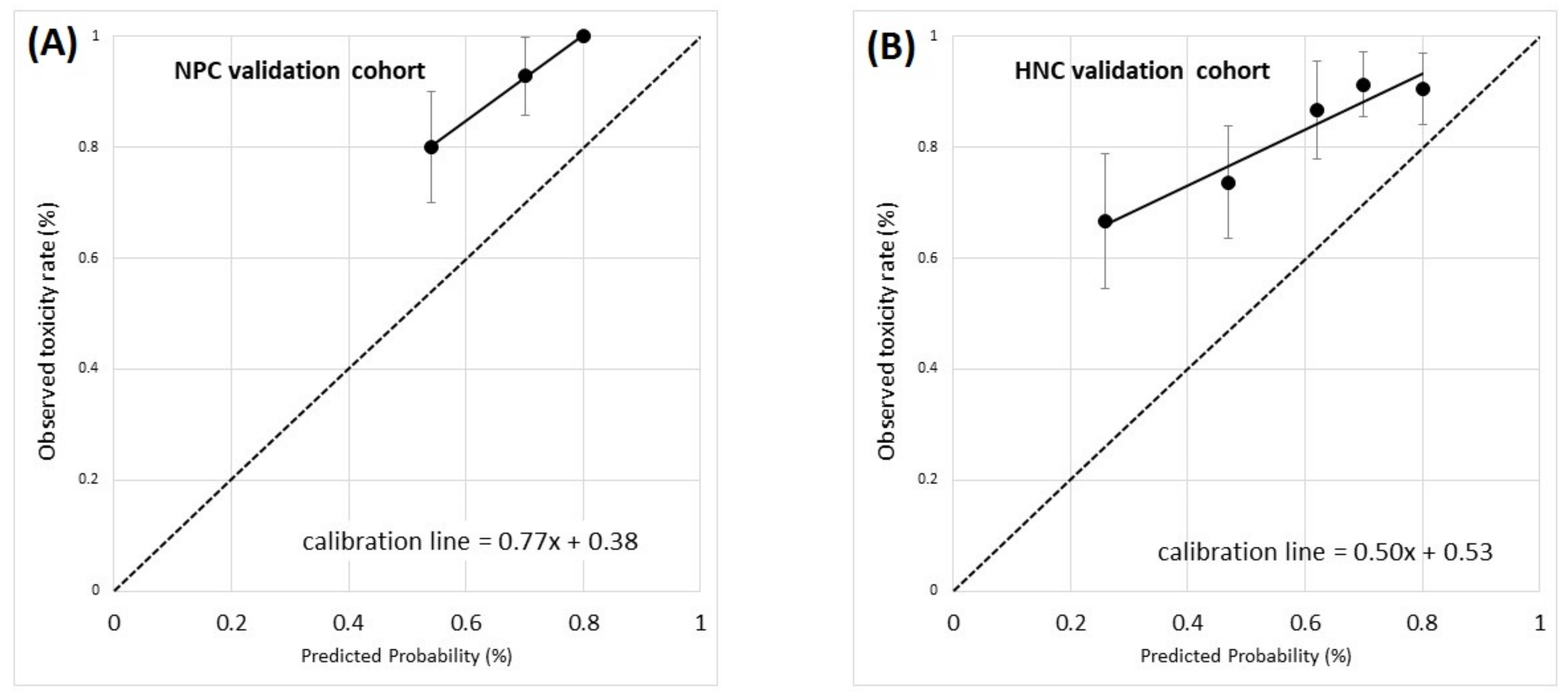
3.2. Model Validation Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Coefficient | SE | p-Value | OR | 95% CI |
|---|---|---|---|---|---|
| Gender = Male vs. Female | −0.470 | 0.426 | 0.27 | 0.63 | 0.27–1.44 |
| Age (5-year interval) | 0.064 | 0.070 | 0.36 | 1.07 | 0.93–1.22 |
| BMI (continuous variable) | 0.023 | 0.041 | 0.57 | 1.02 | 0.94–1.11 |
| BMI ≥ 25 kg/m2 = 1 | 0.066 | 0.382 | 0.86 | 1.07 | 0.51–2.26 |
| BMI ≥ 30 kg/m2 = 1 | 0.223 | 0.491 | 0.65 | 1.25 | 0.48–3.27 |
| Smoke = Yes | 0.405 | 0.514 | 0.43 | 1.50 | 0.55–4.11 |
| Comorbidities | |||||
| Hypertension = yes | 0.013 | 0.533 | 0.98 | 1.01 | 0.36–2.88 |
| Diabetes mellitus = yes | 5.483 | 6.752 | 0.42 | 240.57 | 0.0–1.3 × 108 |
| Cardiological = yes | 0.880 | 1.112 | 0.43 | 2.41 | 0.27–21.32 |
| Haematological = yes | 1.075 | 1.097 | 0.33 | 2.93 | 0.34–25.13 |
| Histology = SCC (reference) vs. undifferentiated | −0.054 | 0.632 | 0.93 | 0.95 | 0.27–3.27 |
| Stage low-intermediate (reference) vs. high | 0.065 | 0.377 | 0.86 | 1.07 | 0.51–2.23 |
| T low-intermediate (reference) vs. high | −0.606 | 0.438 | 0.17 | 0.55 | 0.23–1.29 |
| N 0 (reference) vs. 1 | 0.019 | 0.456 | 0.97 | 1.02 | 0.42–2.49 |
| GTVN Volume (cc) | 0.024 | 0.026 | 0.36 | 1.02 | 0.97–1.08 |
| Chemotherapy RT-CHT (reference) vs. iCHT + RT-CHT | 0.090 | 0.442 | 0.84 | 1.09 | 0.46–2.60 |
| RT Mode IMRT (reference) vs. VMAT | 0.243 | 0.376 | 0.52 | 1.28 | 0.61–2.67 |
| Fractionation 2.0 Gy/fraction (reference) vs 2.12 Gy/fraction | −0.217 | 0.433 | 0.62 | 0.81 | 0.34–1.88 |
| DOSIMETRIC PARAMETERS | |||||
| cPG D98% (continuous) | 0.051 | 0.022 | 0.02 | 1.05 | 1.01–1.10 |
| Constant | −0.557 | 0.577 | |||
| OC EUD (n = 0.05, continuous) | 0.126 | 0.041 | 0.002 | 1.13 | 1.05–1.23 |
| Constant | −6.809 | 2.474 |
References
- Buglione, M.; Cavagnini, R.; Di Rosario, F.; Maddalo, M.; Vassalli, L.; Grisanti, S.; Salgarello, S.; Orlandi, E.; Bossi, P.; Majorana, A.; et al. Oral Toxicity Management in Head and Neck Cancer Patients Treated with Chemotherapy and Radiation: Xerostomia and Trismus (Part 2). Literature Review and Consensus Statement. Crit. Rev. Oncol. Hematol. 2016, 102, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tian, Y.M.; Sun, X.M.; Chen, C.Y.; Han, F.; Xiao, W.W.; Deng, X.W.; Lu, T.X. Late Toxicities after Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: Patient and Treatment-Related Risk Factors. Br. J. Cancer 2014, 110, 49–54. [Google Scholar] [CrossRef]
- Ou, X.; Zhou, X.; Shi, Q.; Xing, X.; Yang, Y.; Xu, T.; Shen, C.; Wang, X.; He, X.; Kong, L.; et al. Treatment Outcomes and Late Toxicities of 869 Patients with Nasopharyngeal Carcinoma Treated with Definitive Intensity Modulated Radiation Therapy: New Insight into the Value of Total Dose of Cisplatin and Radiation Boost. Oncotarget 2015, 6, 38381. [Google Scholar] [CrossRef] [PubMed]
- Poon, D.M.C.; Kam, M.K.M.; Johnson, D.; Mo, F.; Tong, M.; Chan, A.T.C. Durability of the parotid-sparing effect of intensity-modulated radiotherapy (IMRT) in early stage nasopharyngeal carcinoma: A 15-year follow-up of a randomised prospective study of IMRT versus two-dimensional radiotherapy. Head Neck 2021, 43, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Sinha, S.; Ghosh-Laskar, S.; Budrukkar, A.; Mummudi, N.; Swain, M.; Phurailatpam, R.; Prabhash, K.; Agarwal, J.P. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: A randomised controlled trial. Radiother. Oncol. 2012, 104, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Miah, A.B.; Gulliford, S.L.; Morden, J.; Newbold, K.L.; Bhide, S.A.; Zaidi, S.H.; Hall, E.; Harrington, K.J.; Nutting, C.M. Recovery of salivary function: Contralateral parotid-sparing Intensity-modulated Radiotherapy versus bilateral superficial lobe parotid-sparing Intensity-modulated Radiotherapy. Clin. Oncol. 2016, 28, e69–e76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Jiang, W.; Lakshminarayanan, P.; Han, P.; Cheng, Z.; Bowers, M.; Hui, X.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; et al. Spatial radiation dose influence on xerostomia recovery and its comparison to acute incidence in patients with Head and Neck Cancer. Adv. Radiat. Oncol. 2020, 5, 221–230. [Google Scholar] [CrossRef]
- McDowell, L.J.; Rock, K.; Xu, W.; Chan, B.; Waldron, J.; Lu, L.; Ezzat, S.; Pothier, D.; Bernstein, L.J.; So, N.; et al. Long-Term Late Toxicity, Quality of Life, and Emotional Distress in Patients With Nasopharyngeal Carcinoma Treated With Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 340–352. [Google Scholar] [CrossRef]
- Guchelaar, H.J.; Vermes, A.; Meerwaldt, J.H. Radiation-Induced Xerostomia: Pathophysiology, Clinical Course and Supportive Treatment. Support. Care Cancer 1997, 5, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Seikaly, H.; Jha, N.; Mcgaw, T.; Coulter, L.; Liu, R. Submandibular Gland Transfer: A New Method of Preventing. Laryngoscope 2001, 111, 347–352. [Google Scholar] [CrossRef]
- Grundmann, O.; Mitchell, G.C.; Limesand, K.H. Sensitivity of Salivary Glands to Radiation: From Animal Models to Therapies. J. Dent. Res. 2009, 88, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Radfar, L.; Sirois, D.A. Structural and Functional Injury in Minipig Salivary Glands Following Fractionated Exposure to 70 Gy of Ionizing Radiation: An Animal Model for Human Radiation-Induced Salivary Gland Injury. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2003, 96, 267–274. [Google Scholar] [CrossRef]
- Jasmer, K.J.; Gilman, K.E.; Forti, K.M.; Weisman, G.A.; Limesand, K.H. Radiation-Induced Salivary Gland Dysfunction: Mechanisms, Therapeutics and Future Directions. J. Clin. Med. 2020, 9, 4095. [Google Scholar] [CrossRef]
- Ferraiolo, D.M.; Veitz-Keenan, A. Insufficient Evidence for Interventions to Prevent Dry Mouth and Salivary Gland Dysfunction Post Head and Neck Radiotherapy. Evid. Based Dent. 2018, 19, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Deasy, J.O.; Moiseenko, V.; Marks, L.; Clifford Chao, K.S.; Nam, J.; Eisbruch, A. Radiotherapy Dose-Volume Effects on Salivary Gland Function. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. 3), 58–63. [Google Scholar] [CrossRef] [Green Version]
- Moiseenko, V.; Wu, J.; Hovan, A.; Saleh, Z.; Apte, A.; Deasy, J.O.; Harrow, S.; Rabuka, C.; Muggli, A.; Thompson, A. Treatment Planning Constraints to Avoid Xerostomia in Head-and-Neck Radiotherapy: An Independent Test of QUANTEC Criteria Using a Prospectively Collected Dataset. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, P.G.; Lee, J.Y.; Mao, Y.; Li, P.; Green, M.; Worden, F.P.; Swiecicki, P.L.; Mierzwa, M.L.; Spector, M.E.; Schipper, M.J.; et al. Sparing All Salivary Glands with IMRT for Head and Neck Cancer: Longitudinal Study of Patient-Reported Xerostomia and Head-and-Neck Quality of Life. Radiother. Oncol. 2018, 126, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Beetz, I.; Schilstra, C.; Visink, A.; van der Schaaf, A.; Bijl, H.P.; van der Laan, B.F.; Steenbakkers, R.J.; Langendijk, J.A. Role of Minor Salivary Glands in Developing Patient-Rated Xerostomia and Sticky Saliva during Day and Night. Radiother. Oncol. 2013, 109, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, L.V.; Noordzij, W.; Brouwer, C.L.; Boellaard, R.; Burgerhof, J.G.M.; Langendijk, J.A.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. 18F-FDG PET Image Biomarkers Improve Prediction of Late Radiation-Induced Xerostomia. Radiother. Oncol. 2018, 126, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, L.V.; Thor, M.; Steenbakkers, R.J.H.M.; Apte, A.; Zhai, T.T.; Borra, R.; Noordzij, W.; Estilo, C.; Lee, N.; Langendijk, J.A.; et al. Parotid Gland Fat Related Magnetic Resonance Image Biomarkers Improve Prediction of Late Radiation-Induced Xerostomia. Radiother. Oncol. 2018, 128, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Van der Laan, H.P.; Bijl, H.P.; Steenbakkers, R.J.; van der Schaaf, A.; Chouvalova, O.; Vemer-van den Hoek, J.G.; Gawryszuk, A.; van der Laan, B.F.; Oosting, S.F.; Roodenburg, J.L.; et al. Acute Symptoms during the Course of Head and Neck Radiotherapy or Chemoradiation Are Strong Predictors of Late Dysphagia. Radiother. Oncol. 2015, 115, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, E.; Tomatis, S.; Potepan, P.; Bossi, P.; Mongioj, V.; Carrara, M.; Palazzi, M.; Franceschini, M.; Bergamini, C.; Locati, L.; et al. Critical Analysis of Locoregional Failures Following Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. Future Oncol. 2013, 9, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, N.A.; Cicchetti, A.; Cavallo, A.; Alfieri, S.; Locati, L.; Ivaldi, E.; Ingargiola, R.; Romanello, D.A.; Bossi, P.; Cavalieri, S.; et al. Role of IMRT/VMAT-Based Dose and Volume Parameters in Predicting 5-Year Local Control and Survival in Nasopharyngeal Cancer Patients. Front. Oncol. 2020, 10, 518110. [Google Scholar] [CrossRef] [PubMed]
- Van de Water, T.A.; Bijl, H.P.; Westerlaan, H.E.; Langendijk, J.A. Delineation Guidelines for Organs at Risk Involved in Radiation-Induced Salivary Dysfunction and Xerostomia. Radiother. Oncol. 2009, 93, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and Its Predictors Following Parotid-Sparing Irradiation of Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; v4.03: 14 June 2010; U.S. Department of Health and Human Services National Institutes of Health: Bethesda, MD, USA, 2009.
- Alfieri, S.; Ripamonti, C.I.; Marceglia, S.; Orlandi, E.; Iacovelli, N.A.; Granata, R.; Cavallo, A.; Pozzi, P.; Boffi, R.; Bergamini, C.; et al. Temporal Course and Predictive Factors of Analgesic Opioid Requirement for Chemoradiation-induced Oral Mucositis in Oropharyngeal Cancer. Head Neck 2016, 38 (Suppl. 1), E1521–E1527. [Google Scholar] [CrossRef] [PubMed]
- Kutcher, G.J.; Burman, C.; Brewster, L.; Goitein, M.; Mohan, R. Histogram Reduction Method for Calculating Complication Probabilities for Three-Dimensional Treatment Planning Evaluations. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 137–146. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularisation Paths for Generalised Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazzi, M.; Orlandi, E.; Bossi, P.; Pignoli, E.; Potepan, P.; Guzzo, M.; Franceschini, M.; Scaramellini, G.; Cantù, G.; Licitra, L.; et al. Further Improvement in Outcomes of Nasopharyngeal Carcinoma With Optimised Radiotherapy and Induction Plus Concomitant Chemotherapy: An Update of the Milan Experience. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, M.; Orlandi, E.; Pignoli, E.; Lualdi, M.; Zonca, G.; Sangalli, C.; Stucchi, C.; Olmi, P. Target Coverage in Head and Neck Cancer Treated with Intensity-Modulated Radiotherapy: A Comparison between Conventional and Conformal Techniques. Tumori 2006, 92, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Moroney, L.B.; Helios, J.; Ward, E.C.; Crombie, J.; Wockner, L.F.; Burns, C.L.; Spurgin, A.L.; Blake, C.; Kenny, L.; Hughes, B.G. Patterns of Dysphagia and Acute Toxicities in Patients with Head and Neck Cancer Undergoing Helical IMRT ± Concurrent Chemotherapy. Oral Oncol. 2017, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beetz, I.; Schilstra, C.; Burlage, F.R.; Koken, P.W.; Doornaert, P.; Bijl, H.P.; Chouvalova, O.; Leemans, C.R.; de Bock, G.H.; Christianen, M.E.; et al. Development of NTCP Models for Head and Neck Cancer Patients Treated with Three-Dimensional Conformal Radiotherapy for Xerostomia and Sticky Saliva: The Role of Dosimetric and Clinical Factors. Radiother. Oncol. 2012, 105, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beetz, I.; Schilstra, C.; van der Schaaf, A.; van den Heuvel, E.R.; Doornaert, P.; van Luijk, P.; Vissink, A.; van der Laan, B.F.; Leemans, C.R.; Bijl, H.P.; et al. NTCP Models for Patient-Rated Xerostomia and Sticky Saliva after Treatment with Intensity Modulated Radiotherapy for Head and Neck Cancer: The Role of Dosimetric and Clinical Factors. Radiother. Oncol. 2012, 105, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.F.; Liou, M.H.; Ting, H.M.; Chang, L.; Lee, H.Y.; Wan Leung, S.; Huang, C.J.; Chao, P.J. Patient- and Therapy-Related Factors Associated with the Incidence of Xerostomia in Nasopharyngeal Carcinoma Patients Receiving Parotid-Sparing Helical Tomotherapy. Sci. Rep. 2015, 5, 13165. [Google Scholar] [CrossRef] [PubMed]
- Sharabiani, M.; Clementel, E.; Andratschke, N.; Hurkmans, C. Generalizability assessment of head and neck cancer NTCP models based on the TRIPOD criteria. Radiother. Oncol. 2020, 146, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Van Luijk, P.; Pringle, S.; Deasy, J.O.; Moiseenko, V.V.; Faber, H.; Hovan, A.; Baanstra, M.; van der Laan, H.P.; Kierkels, R.G.; van der Schaaf, A.; et al. Sparing the Region of the Salivary Gland Containing Stem Cells Preserves Saliva Production after Radiotherapy for Head and Neck Cancer. Sci. Transl. Med. 2015, 7, 305ra147. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Lakshminarayanan, P.; Hui, X.; Han, P.; Cheng, Z.; Bowers, M.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; Quon, H.; et al. Machine Learning Methods Uncover Radiomorphologic Dose Patterns in Salivary Glands That Predict Xerostomia in Patients with Head and Neck Cancer. Adv. Radiat. Oncol. 2019, 4, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Jellema, A.P.; Doornaert, P.; Slotman, B.J.; Leemans, C.R.; Langendijk, J.A. Does Radiation Dose to the Salivary Glands and Oral Cavity Predict Patient-Rated Xerostomia and Sticky Saliva in Head and Neck Cancer Patients Treated with Curative Radiotherapy? Radiother. Oncol. 2005, 77, 164–171. [Google Scholar] [CrossRef]
- Lee, T.F.; Chao, P.J.; Ting, H.M.; Chang, L.; Huang, Y.J.; Wu, J.M.; Wang, H.Y.; Horng, M.F.; Chang, C.M.; Lan, J.H.; et al. Using Multivariate Regression Model with Least Absolute Shrinkage and Selection Operator (LASSO) to Predict the Incidence of Xerostomia after Intensity-Modulated Radiotherapy for Head and Neck Cancer. PLoS ONE 2014, 9, e89700. [Google Scholar] [CrossRef] [PubMed]
- Buettner, F.; Miah, A.B.; Gulliford, S.L.; Hall, E.; Harrington, K.J.; Webb, S.; Partridge, M.; Nutting, C.M. Novel Approaches to Improve the Therapeutic Index of Head and Neck Radiotherapy: An Analysis of Data from the PARSPORT Randomised Phase III Trial. Radiother. Oncol. 2012, 103, 82–87. [Google Scholar] [CrossRef]
- Beetz, I.; Steenbakkers, R.J.; Chouvalova, O.; Leemans, C.R.; Doornaert, P.; van der Laan, B.F.; Christianen, M.E.; Vissink, A.; Bijl, H.P.; van Luijk, P.; et al. The QUANTEC Criteria for Parotid Gland Dose and Their Efficacy to Prevent Moderate to Severe Patient-Rated Xerostomia. Acta Oncol. 2014, 53, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Gabryś, H.S.; Buettner, F.; Sterzing, F.; Hauswald, H.; Bangert, M. Design and Selection of Machine Learning Methods Using Radiomics and Dosiomics for Normal Tissue Complication Probability Modelling of Xerostomia. Front. Oncol. 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Onjukka, E.; Mercke, C.; Björgvinsson, E.; Embring, A.; Berglund, A.; von Döbeln, G.A.; Friesland, S.; Gagliardi, G.; Lenneby Helleday, C.; Sjödin, H.; et al. Modelling of Xerostomia After Radiotherapy for Head and Neck Cancer: A Registry Study. Front. Oncol. 2020, 10, 1647. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Iacovelli, N.A.; Rancati, T.; Cicchetti, A.; Bossi, P.; Pignoli, E.; Bergamini, C.; Licitra, L.; Fallai, C.; Valdagni, R.; et al. Multivariable Model for Predicting Acute Oral Mucositis during Combined IMRT and Chemotherapy for Locally Advanced Nasopharyngeal Cancer Patients. Oral Oncol. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Leal, S.C.; Bittar, J.; Portugal, A.; Falcão, D.P.; Faber, J.; Zanotta, P. Medication in Elderly People: Its Influence on Salivary Pattern, Signs and Symptoms of Dry Mouth. Gerodontology 2010, 27, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, A.; Avuzzi, B.; Palorini, F.; Ballarini, F.; Stucchi, C.; Fellin, G.; Gabriele, P.; Vavassori, V.; Esposti, C.D.; Cozzarini, C.; et al. Predicting Late Fecal Incontinence Risk After Radiation Therapy for Prostate Cancer: New Insights From External Independent International. J. Radiat. Oncol. Biol. Phys. 2018, 102, 127–136. [Google Scholar] [CrossRef]
- Rancati, T.; Fiorino, C.; Fellin, G.; Vavassori, V.; Cagna, E.; Casanova Borca, V.; Girelli, G.; Menegotti, L.; Monti, A.F.; Tortoreto, F.; et al. Inclusion of clinical risk factors into NTCP modelling of late rectal toxicity after high dose radiotherapy for prostate cancer. Radiother. Oncol. 2011, 100, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, K.; Lee, S.H.; Cheng, Z.; Lakshminarayanan, P.; Peng, L.; Han, P.; McNutt, T.R.; Quon, H.; Lee, J. Predicting acute radiation-induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands. Radiat. Oncol. 2019, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, H.; Huang, S.; Chen, X.; Zhou, H.; Chang, H.; Xia, Y.; Wang, G.; Yang, X. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images. Quant. Imaging Med. Surg. 2019, 9, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Pota, M.; Scalco, E.; Sanguineti, G.; Farneti, A.; Cattaneo, G.M.; Rizzo, G.; Esposito, M. Early prediction of radiotherapy-induced parotid shrinkage and toxicity based on CT radiomics and fuzzy classification. Artif. Intell. Med. 2017, 81, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.S.; Hawkins, P.G.; Polan, D.F.; Balter, J.M.; Brock, K.K.; Kamp, J.D.; Lockhart, C.M.; Eisbruch, A.; Mierzwa, M.L.; Ten Haken, R.K.; et al. Early Changes in Serial CBCT-Measured Parotid Gland Biomarkers Predict Chronic Xerostomia After Head and Neck Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, X.; Yang, X.; Tao, Y.; Xia, Y.; Deng, X.; Zheng, C.; Robbins, J.; Schultz, C.; Li, X.A. Early Prediction of Acute Xerostomia During Radiation Therapy for Head and Neck Cancer Based on Texture Analysis of Daily CT. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
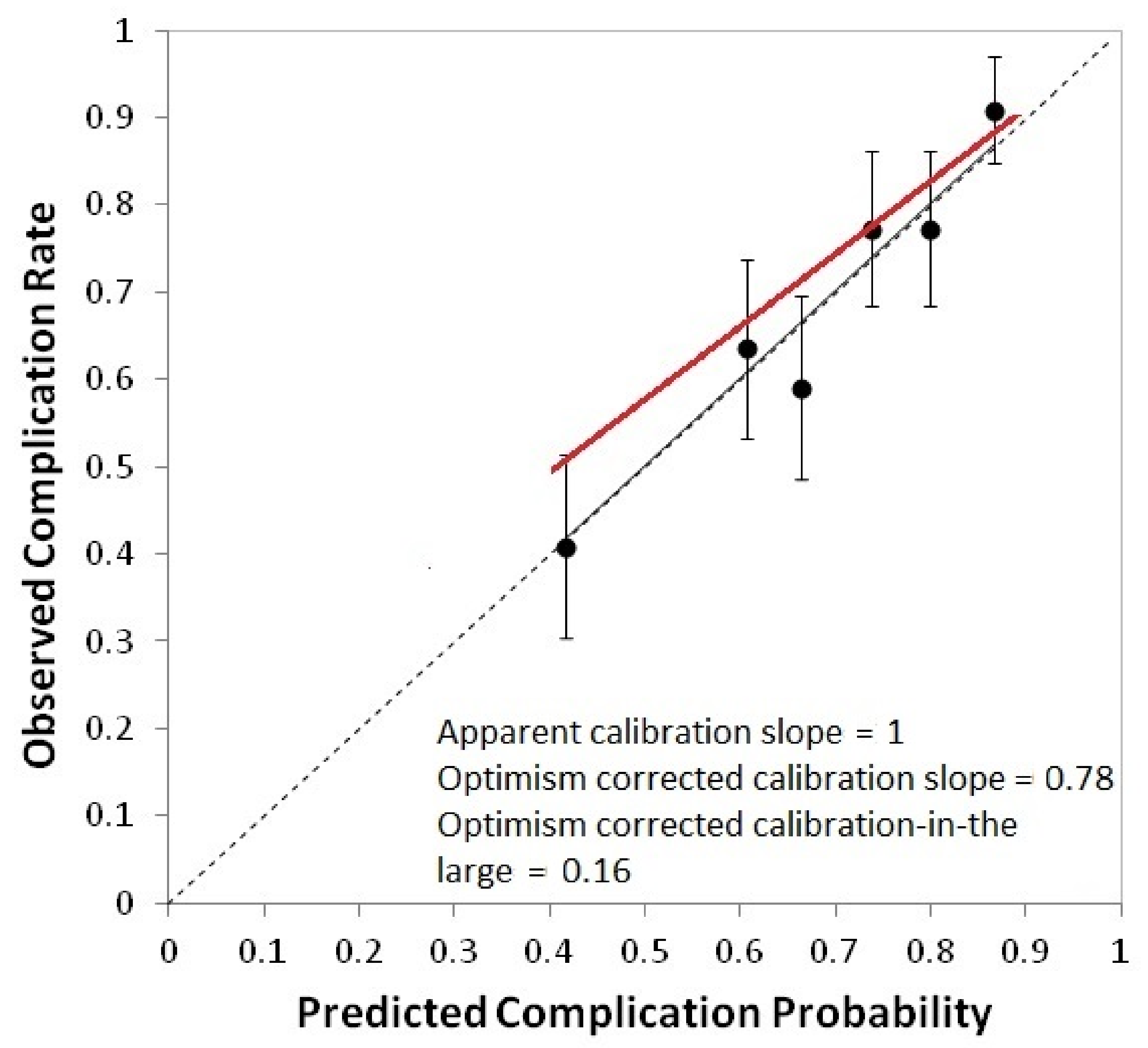
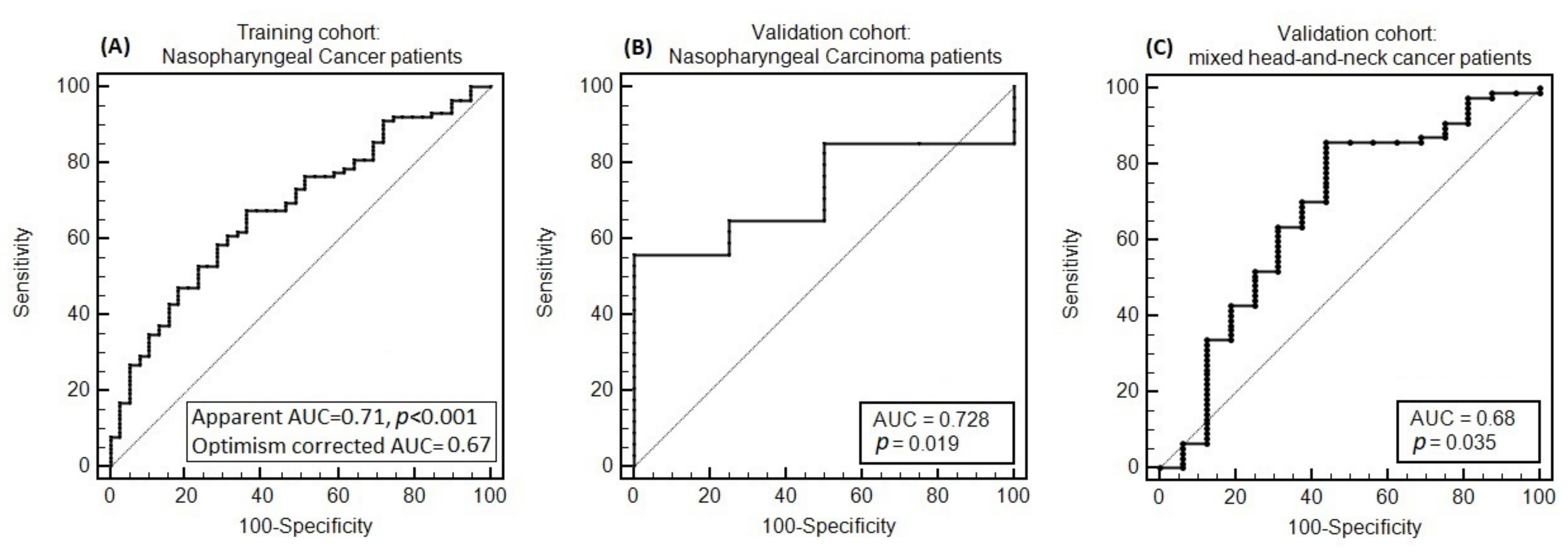
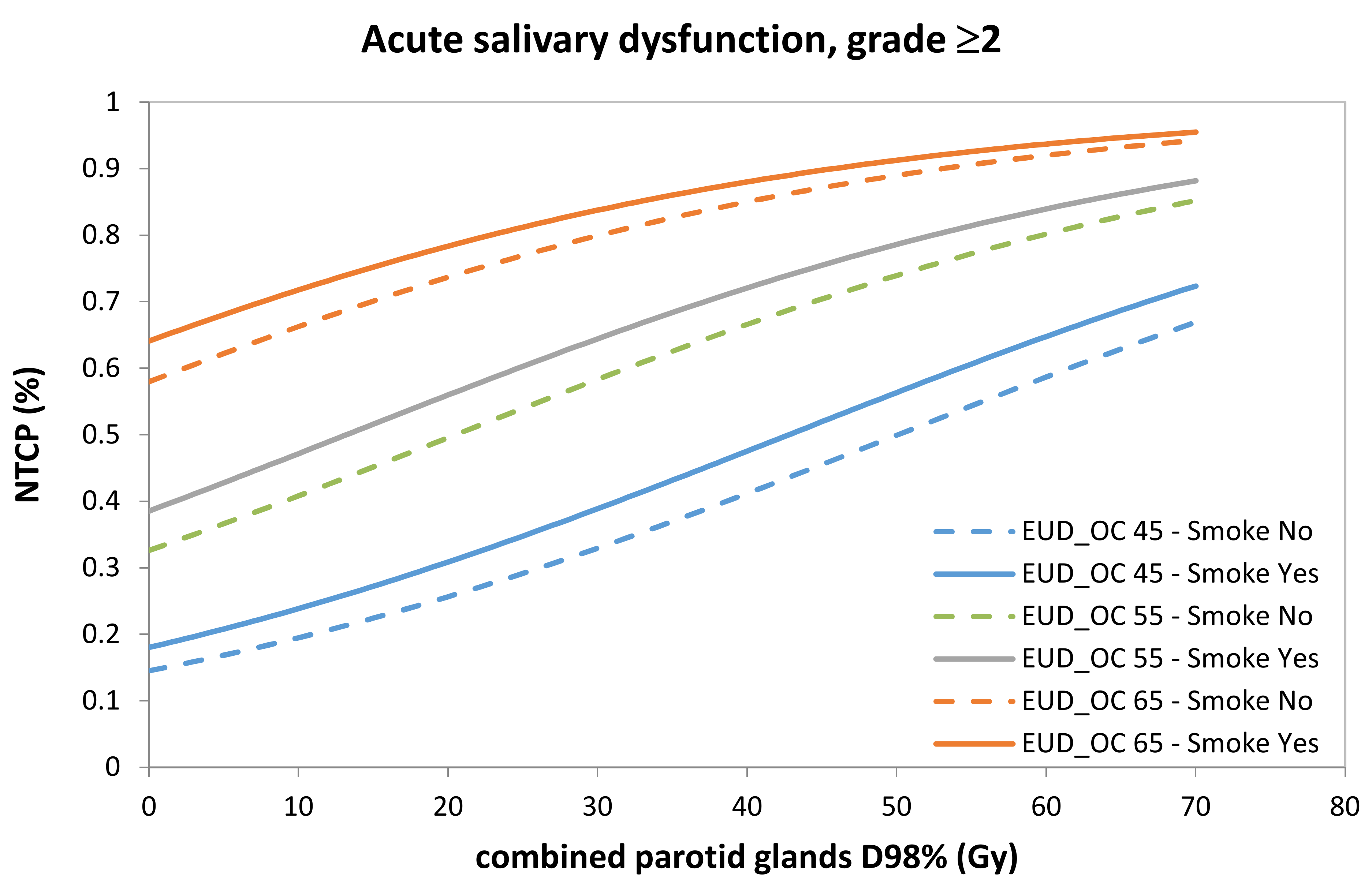
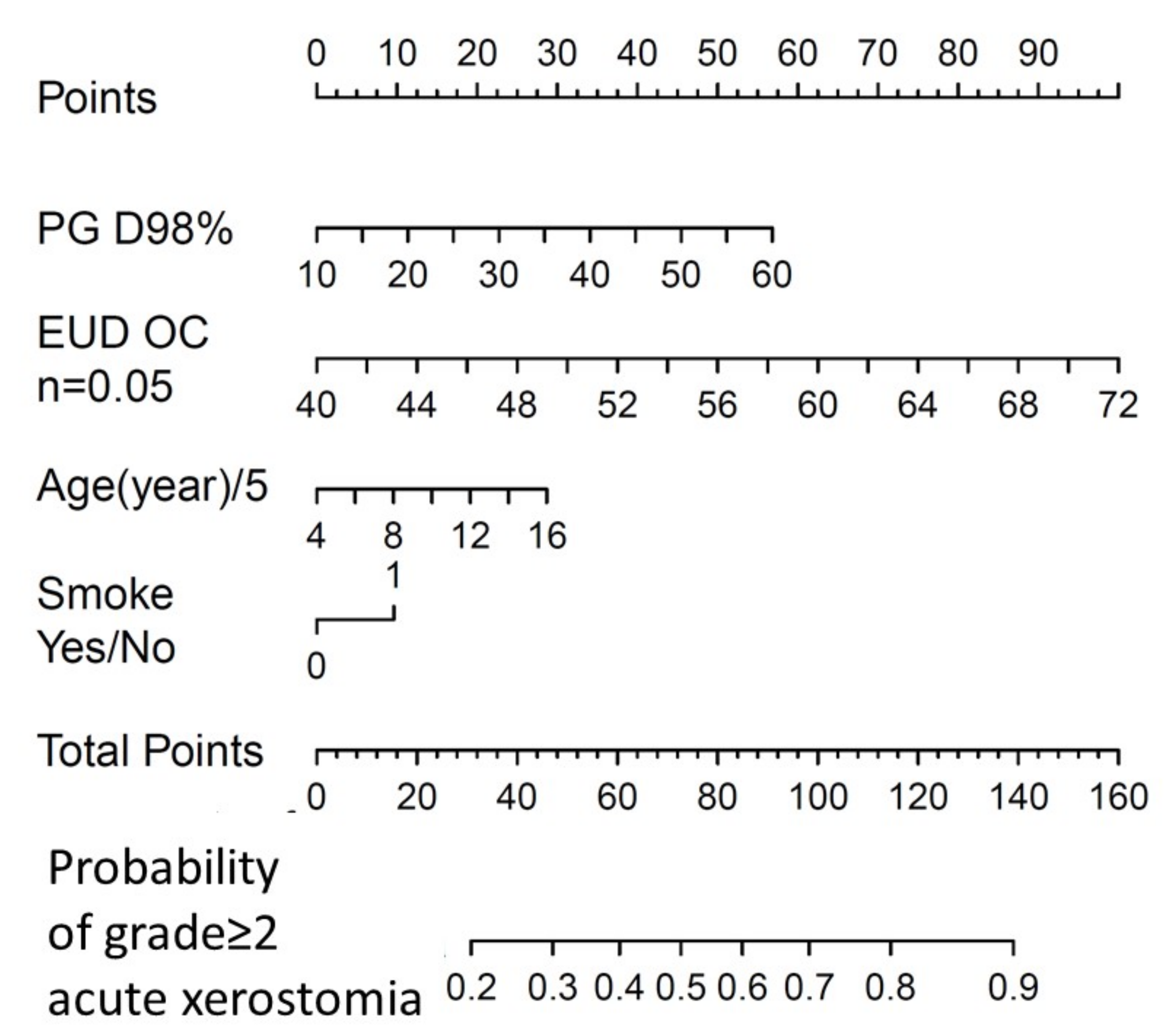

| Patient Characteristics | (a) NPC Development (n = 132 pts) (%) | (b) NPC Validation (n = 38 pts) (%) | (c) HNC Validation (n = 93 pts) (%) | |
|---|---|---|---|---|
| Gender | Female | 40 (30.3) | 8 (21.1) | 25 (26.9) |
| Male | 92 (69.7) | 30 (78.9) | 68 (73.1) | |
| Age median {range} (years) | 49 {18–81} | 52 {24–72} | 62 {23–83} | |
| BMI median {range} kg/m2 | 25.9 {16.6–42.9} | 25.8 {18.2–32.9} | 24.8 {14.7–36.4} | |
| Smoke (Yes) | 24 (18.2) | 20 (52.6) | 63 (67.7) | |
| Comorbidities (Yes) | Hypertension | 19 (14.4) | 10 (26.3) | 37 (39.8) |
| Diabetes mellitus | 4 (3.0) | 1 (2.6) | 4 (4.3) | |
| Cardiological | 6 (4.5) | 3 (7.9) | 20 (21.5) | |
| Haematological | 7 (5.3) | - | 2 (2.1) | |
| Oncological | 5 (3.8) | 3 (7.9) | 15 (16.1) | |
| Histology (WHO tumour classification) | Undifferentiated | 119 (90.2) | 34 (89.5) | - |
| SCC | 13 (9.8) | 4 (10.5) | 84 (90.3) | |
| Other | - | 9 (9.7) | ||
| Staging procedures | MRI | 129 (97.7) | 38 (100) | 86 (92.5) |
| (18F)FDG-PET | 123 (93.2) | 38 (100) | 88 (94.6) | |
| Stage (Edge 2010) | II | 20 (15.1) | 6 (15.8) | 7 (7.5) |
| III | 38 (28.8) | 12 (31.6) | 28 (30.1) | |
| IVA | 29 (22.0) | 9 (23.7) | 45 (48.4) | |
| IVB | 45 (34.1) | 11 (28.9) | 13 (14) | |
| Treatment | RT-CHT | 30 (22.7) | 19 (50.0) | 56 (60.2) |
| iCHT + RT-CHT | 102 (77.3) | 16 (42.1) | 11 (11.8) | |
| RT alone | 3 (7.9) | 26 (28.0) | ||
| RT technique | IMRT | 70 (53.0) | - | - |
| VMAT | 62 (47.0) | 38 (100) | 93 (100) | |
| Fractionation | 2 Gy/fraction | 101 (76.5) | 1 (2.6) | 32 (34.4) |
| ≥2.12 Gy/fraction | 31 (23.5) | 37 (97.4) | 61 (65.6) | |
| Organ at Risk | Variable | Median | Mean ± Standard Deviation |
|---|---|---|---|
| Combined parotid glands (cPG) | Volume (cc) | 51.3 | 53.3 ± 19.3 |
| Mean dose (Gy) | 47.5 | 47.2 ± 9.5 | |
| Maximum dose (Gy) | 74.0 | 74.2 ± 3.5 | |
| D98% (Gy) | 25.5 | 26.8 ± 9.5 | |
| Oral cavity (OC) | Volume (cc) | 63.3 | 64.8 ± 20.4 |
| Mean dose (Gy) | 45.9 | 46.7 ± 6.4 | |
| Maximum dose (Gy) | 72.6 | 71.9 ± 3.7 |
| Variable | Coeff | Standard Error | Odds Ratio | 95% Confidence Interval for OR |
|---|---|---|---|---|
| Acute salivary dysfunction, Grade ≥ 2 | ||||
| Combined parotid glands D98% (Gy) | 0.038 | 0.023 | 1.04 | 0.99–1.09 |
| Oral cavity EUD (n = 0.05) (Gy) | 0.103 | 0.043 | 1.11 | 1.02–1.21 |
| Age (5-year interval) | 0.079 | 0.074 | 1.08 | 0.94–1.25 |
| Smoking history (Yes) | 0.318 | 0.540 | 1.37 | 0.48–3.96 |
| Constant | −7.233 | 2.620 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, A.; Iacovelli, N.A.; Facchinetti, N.; Rancati, T.; Alfieri, S.; Giandini, T.; Cicchetti, A.; Fallai, C.; Ingargiola, R.; Licitra, L.; et al. Modelling Radiation-Induced Salivary Dysfunction during IMRT and Chemotherapy for Nasopharyngeal Cancer Patients. Cancers 2021, 13, 3983. https://doi.org/10.3390/cancers13163983
Cavallo A, Iacovelli NA, Facchinetti N, Rancati T, Alfieri S, Giandini T, Cicchetti A, Fallai C, Ingargiola R, Licitra L, et al. Modelling Radiation-Induced Salivary Dysfunction during IMRT and Chemotherapy for Nasopharyngeal Cancer Patients. Cancers. 2021; 13(16):3983. https://doi.org/10.3390/cancers13163983
Chicago/Turabian StyleCavallo, Anna, Nicola Alessandro Iacovelli, Nadia Facchinetti, Tiziana Rancati, Salvatore Alfieri, Tommaso Giandini, Alessandro Cicchetti, Carlo Fallai, Rossana Ingargiola, Lisa Licitra, and et al. 2021. "Modelling Radiation-Induced Salivary Dysfunction during IMRT and Chemotherapy for Nasopharyngeal Cancer Patients" Cancers 13, no. 16: 3983. https://doi.org/10.3390/cancers13163983
APA StyleCavallo, A., Iacovelli, N. A., Facchinetti, N., Rancati, T., Alfieri, S., Giandini, T., Cicchetti, A., Fallai, C., Ingargiola, R., Licitra, L., Locati, L., Cavalieri, S., Pignoli, E., Romanello, D. A., Valdagni, R., & Orlandi, E. (2021). Modelling Radiation-Induced Salivary Dysfunction during IMRT and Chemotherapy for Nasopharyngeal Cancer Patients. Cancers, 13(16), 3983. https://doi.org/10.3390/cancers13163983






