Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Healthy Volunteer Recruitment
2.2. ELISA Assay
2.3. Soluble Immune Checkpoint Measurement
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Patient Characteristics
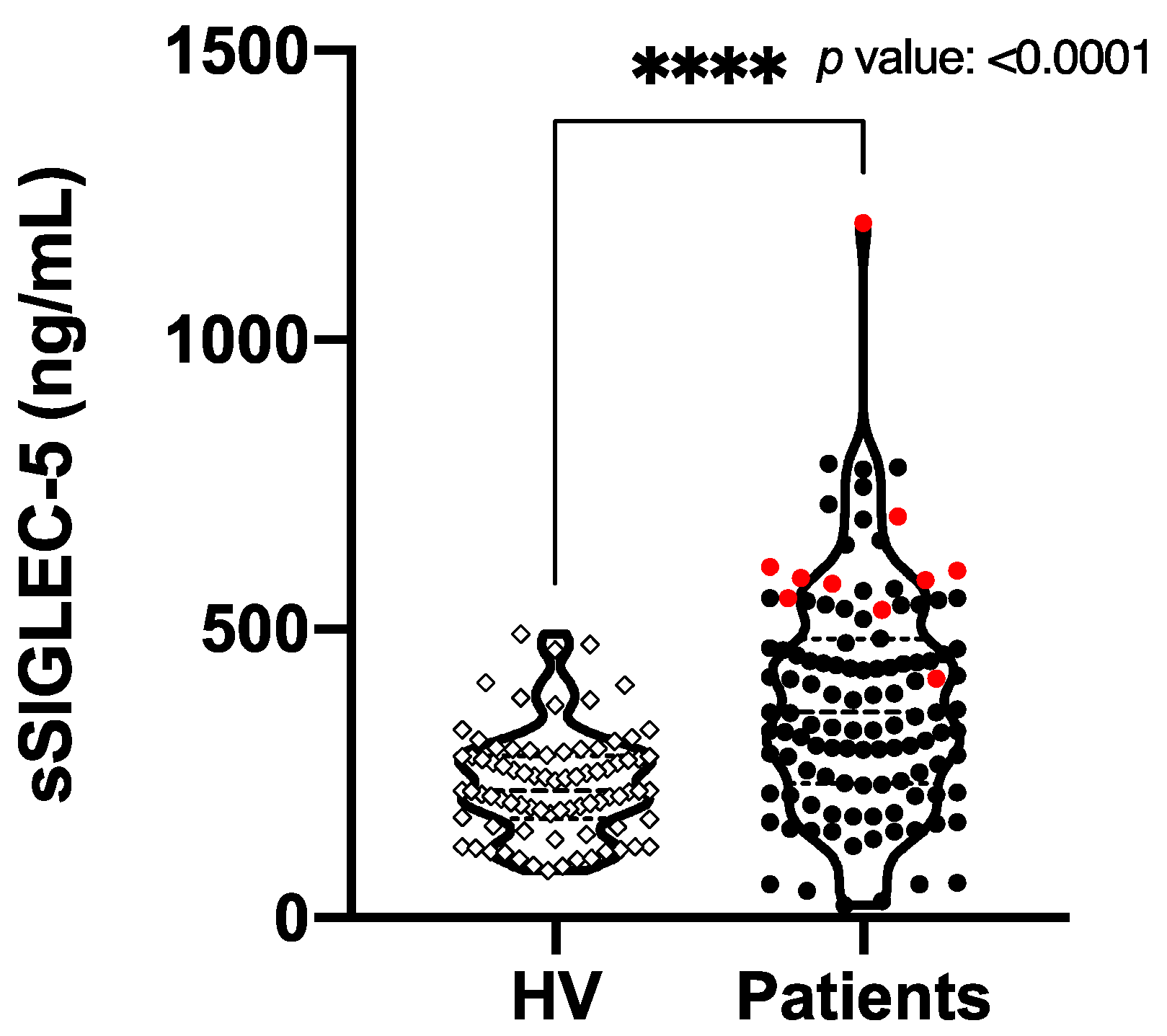
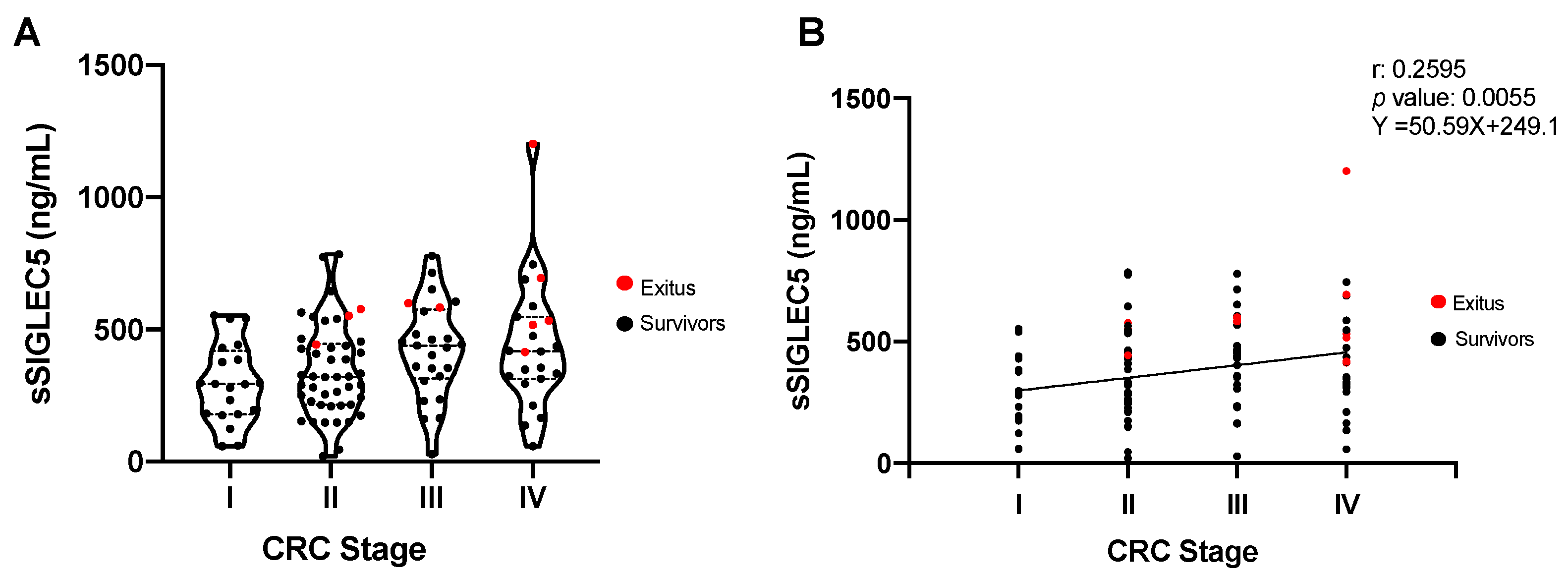
3.2. sSIGLEC5 Is a Prognosis Biomarker in Colorectal Cancer Patients
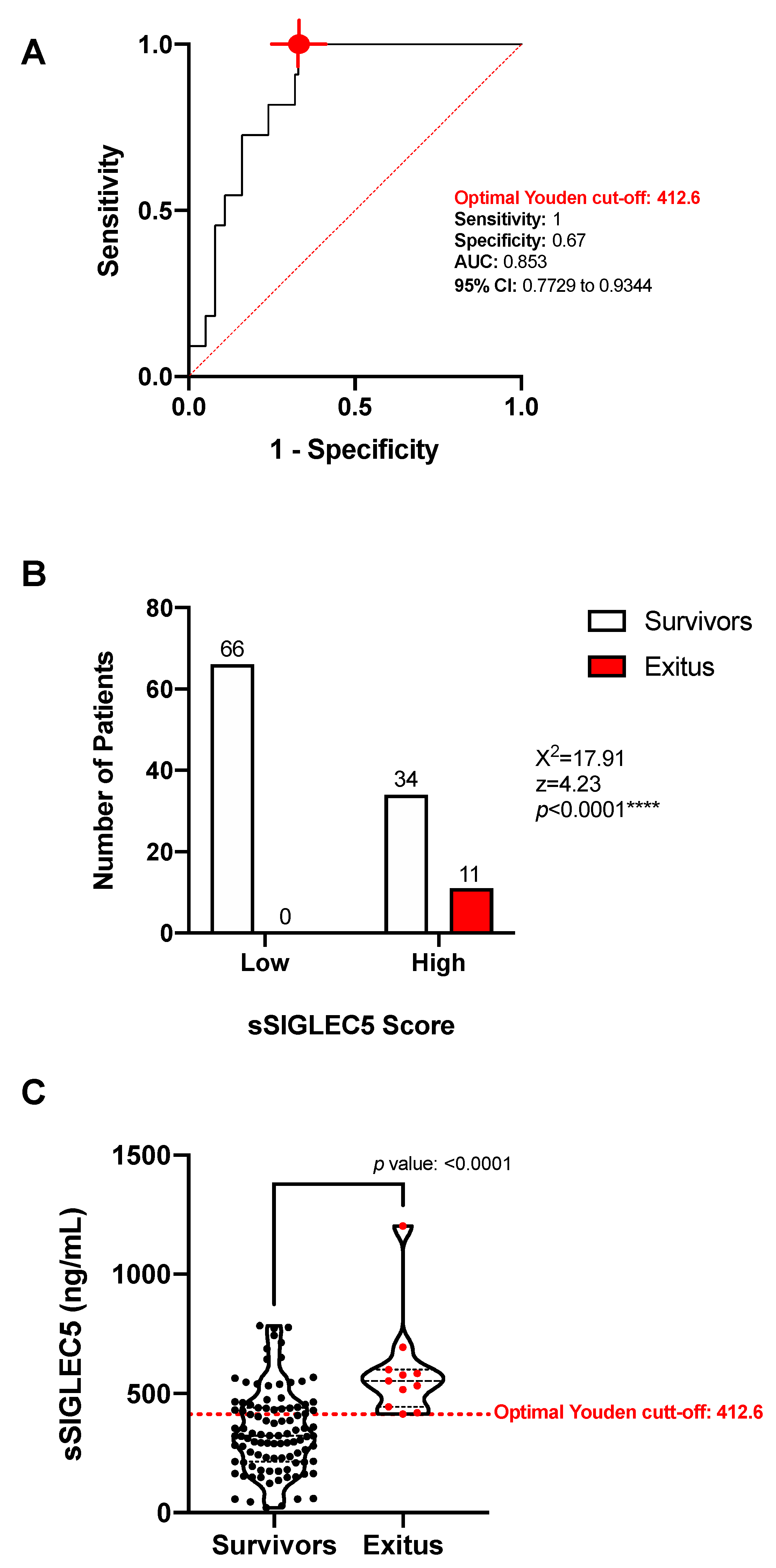
3.3. sSIGLEC5 Is an Exitus Predictor in Patients with Colorectal Cancer
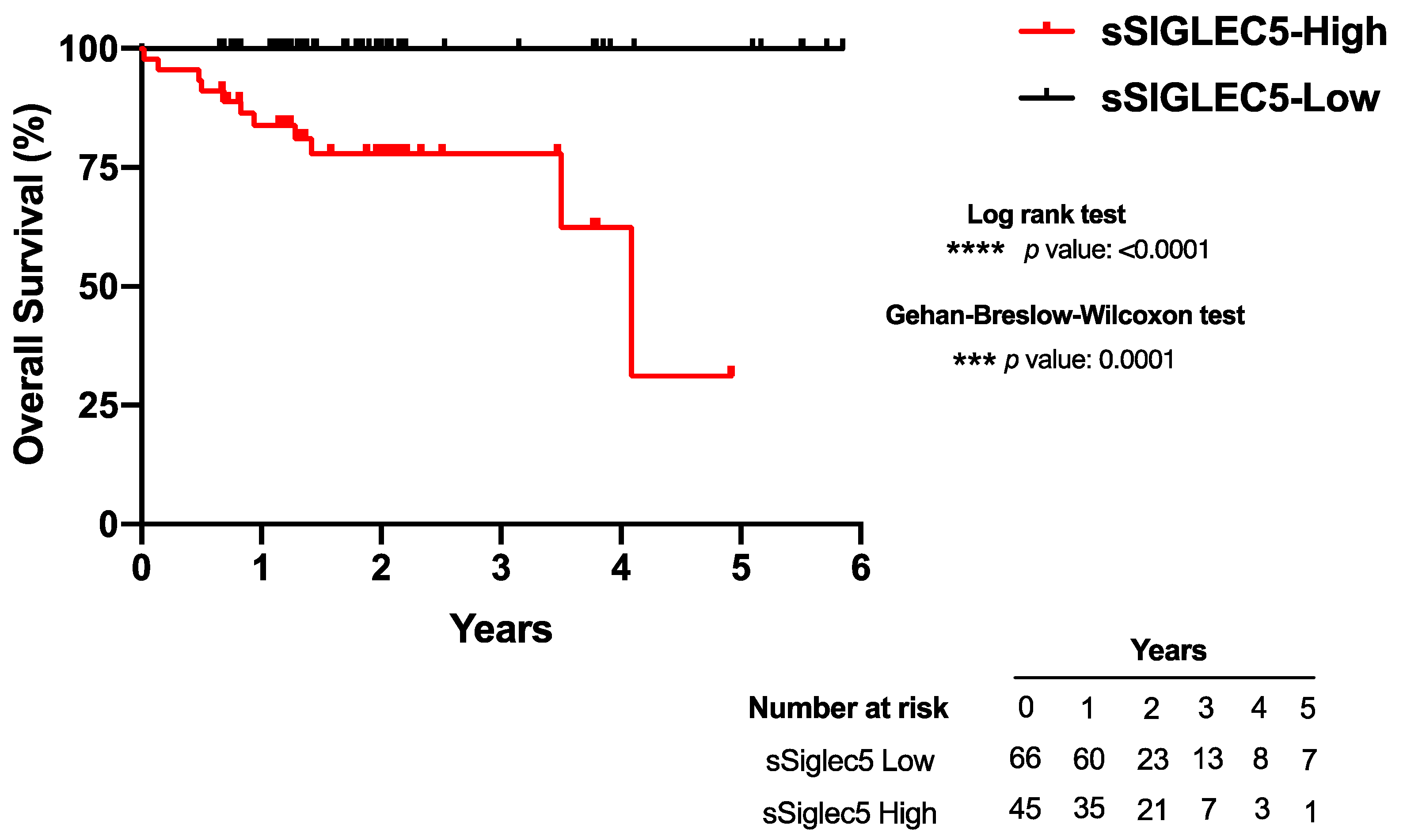
3.4. High sSIGLEC5 Concentration Is Associated with Decreased Overall Survival in Patients with Colorectal Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alves Martins, B.A.; de Bulhões, G.F.; Cavalcanti, I.N.; Martins, M.M.; de Oliveira, P.G.; Martins, A.M.A. Biomarkers in Colorectal Cancer: The Role of Translational Proteomics Research. Front. Oncol. 2019, 9, 1284. [Google Scholar] [CrossRef]
- Markman, J.L.; Shiao, S.L. Impact of the Immune System and Immunotherapy in Colorectal Cancer. J. Gastrointest Oncol. 2015, 6, 208–223. [Google Scholar] [CrossRef]
- Műzes, G.; Molnár, B.; Sipos, F. Regulatory T Cells in Inflammatory Bowel Diseases and Colorectal Cancer. World J. Gastroenterol. 2012, 18, 5688–5694. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Beckhove, P.; Op den Winkel, J.; Autenrieth, D.; Wagner, P.; Nummer, D.; Specht, S.; Antolovic, D.; Galindo, L.; Schmitz-Winnenthal, F.H.; et al. Tumor Infiltrating T Lymphocytes in Colorectal Cancer: Tumor-Selective Activation and Cytotoxic Activity in Situ. Ann. Surg. 2006, 244, 986–992. [Google Scholar] [CrossRef]
- Laghi, L.; Bianchi, P.; Miranda, E.; Balladore, E.; Pacetti, V.; Grizzi, F.; Allavena, P.; Torri, V.; Repici, A.; Santoro, A.; et al. CD3+ Cells at the Invasive Margin of Deeply Invading (PT3-T4) Colorectal Cancer and Risk of Post-Surgical Metastasis: A Longitudinal Study. Lancet Oncol. 2009, 10, 877–884. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Rajput, A.; Jin, N.; Wang, J. Mechanisms of Immunosuppression in Colorectal Cancer. Cancers 2020, 12, 3850. [Google Scholar] [CrossRef]
- Advani, S.; Kopetz, S. Ongoing and Future Directions in the Management of Metastatic Colorectal Cancer: Update on Clinical Trials. J. Surg. Oncol. 2019, 119, 642–652. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef]
- Sgroi, D.; Varki, A.; Braesch-Andersen, S.; Stamenkovic, I. CD22, a B Cell-Specific Immunoglobulin Superfamily Member, Is a Sialic Acid-Binding Lectin. J. Biol. Chem. 1993, 268, 7011–7018. [Google Scholar] [CrossRef]
- Fraschilla, I.; Pillai, S. Viewing Siglecs through the Lens of Tumor Immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Li, R.E.; van Vliet, S.J.; van Kooyk, Y. Using the Glycan Toolbox for Pathogenic Interventions and Glycan Immunotherapy. Curr. Opin. Biotechnol. 2018, 51, 24–31. [Google Scholar] [CrossRef]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Pascolutti, R.; Sun, X.; Kao, J.; Maute, R.; Ring, A.M.; Bowman, G.R.; Kruse, A.C. Structure and Dynamics of PD-L1 and an Ultra High-Affinity PD-1 Receptor Mutant. Structure 2016, 24, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Press, O.W.; Farr, A.G.; Borroz, K.I.; Anderson, S.K.; Martin, P.J. Endocytosis and Degradation of Monoclonal Antibodies Targeting Human B-Cell Malignancies. Cancer Res. 1989, 49, 4906–4912. [Google Scholar]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.M.; Schetters, S.T.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.M.; et al. Sialic Acid-Modified Antigens Impose Tolerance via Inhibition of T-Cell Proliferation and de Novo Induction of Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster, M.M.; Esko, J.D. The Sweet and Sour of Cancer: Glycans as Novel Therapeutic Targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Büll, C.; den Brok, M.H.; Adema, G.J. Sweet Escape: Sialic Acids in Tumor Immune Evasion. Biochim. Biophys. Acta 2014, 1846, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.; Mezouar, S.; Pegon, J.; Muczynski, V.; Adam, F.; Bianchini, E.P.; Bazaa, A.; Proulle, V.; Rupin, A.; Paysant, J.; et al. Soluble Siglec-5 Associates to PSGL-1 and Displays Anti-Inflammatory Activity. Sci. Rep. 2016, 6, 37953. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, R.; Carrette, F.; Barraza, M.L.; Otero, D.C.; Magaña, J.; Bosenberg, M.W.; Swain, S.L.; Bradley, L.M. PSGL-1 Is an Immune Checkpoint Regulator That Promotes T Cell Exhaustion. Immunity 2016, 44, 1190–1203. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, D.; Ao, X.; Yang, Y.; Chen, Z.; Xu, X. Soluble Immune Checkpoints in Cancer: Production, Function and Biological Significance. J. Immunother. Cancer 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, K.; Toiyama, Y.; Tanaka, K.; Saigusa, S.; Hiro, J.; Uchida, K.; Inoue, Y.; Kusunoki, M. Soluble CXCL16 in Preoperative Serum Is a Novel Prognostic Marker and Predicts Recurrence of Liver Metastases in Colorectal Cancer Patients. Ann. Surg. Oncol. 2012, 19 (Suppl. 3), S518–S527. [Google Scholar] [CrossRef]
- Wilmanns, C.; Grossmann, J.; Steinhauer, S.; Manthey, G.; Weinhold, B.; Schmitt-Gräff, A.; von Specht, B.U. Soluble Serum E-Cadherin as a Marker of Tumour Progression in Colorectal Cancer Patients. Clin. Exp. Metastasis 2004, 21, 75–78. [Google Scholar] [CrossRef]
- Del Fresno, C.; García-Rio, F.; Gómez-Piña, V.; Soares-Schanoski, A.; Fernández-Ruíz, I.; Jurado, T.; Kajiji, T.; Shu, C.; Marín, E.; Gutierrez del Arroyo, A.; et al. Potent Phagocytic Activity with Impaired Antigen Presentation Identifying Lipopolysaccharide-Tolerant Human Monocytes: Demonstration in Isolated Monocytes from Cystic Fibrosis Patients. J. Immunol. 2009, 182, 6494–6507. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Tolba, M.F. Revolutionizing the Landscape of Colorectal Cancer Treatment: The Potential Role of Immune Checkpoint Inhibitors. Int. J. Cancer 2020, 147, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of Colorectal Cancer: Challenges for Therapeutic Efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Rodriguez, R.; Avendano-Ortiz, J.; Montalban-Hernandez, K.; Ruiz-Rodriguez, J.C.; Ferrer, R.; Martin-Quiros, A.; Maroun-Eid, C.; Gonzalez-Lopez, J.J.; Fabrega, A.; Terron, V.; et al. SIGLEC5: An Immune Checkpoint Ligand in Sepsis. medRxiv 2020. [Google Scholar] [CrossRef]
| Characteristic | Healthy Volunteers n = 67 | All Patients n = 114 | Stage I n = 20 | Stage II n = 47 | Stage III n = 25 | Stage IV n = 22 | p-Value |
|---|---|---|---|---|---|---|---|
| Sex | 0.804 | ||||||
| Male | 29 (43) | 71 (56) | 11 (55) | 26 (55) | 16 (64) | 11 (50) | |
| Female | 38 (56) | 54 (43) | 9 (45) | 21 (44) | 9 (36) | 11 (50) | |
| Age | 0.268 | ||||||
| Median Range | 59 (50–75) | 71 (50–92) | 70,5 (50–86) | 75,5 (52–91) | 70 (59–92) | 69,5 (51–88) | |
| Metastasis | 35 (30) | 0 (0) | 2 (5) | 5 (20) | 22 (100) | <0.0001 (****) | |
| Synchronic | 22 (62) | 0 (0) | 0 (0) | 0 (0) | 22 (100) | <0.0001 (****) | |
| Metachronic | 13 (37) | 0 (0) | 2 (100) | 5 (100) | 6 (26) | 0.006 (**) | |
| Site of Metastasis | |||||||
| Liver | 25 (71) | 0 (0) | 1 (4) | 3 (12) | 21 (84) | <0.0001 (****) | |
| Lung | 9 (25) | 0 (0) | 1 (12) | 2 (25) | 6 (66) | 0.001 (**) | |
| Peritoneum | 2 (5) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0.036 (*) | |
| Exitus | 11 (10) | 0 (0) | 3 (27) | 2 (18) | 6 (54) | 0.013 (*) | |
| Tumor Site | |||||||
| Caecum | 18 (15) | 6 (33) | 9 (50) | 2 (11) | 1 (5) | 0.083 | |
| Ascending Colon | 30 (26) | 6 (20) | 13 (43) | 7 (23) | 4 (13) | 0.808 | |
| Transverse Colon | 9 (7) | 2 (22) | 5 (55) | 1 (11) | 1 (11) | 0.689 | |
| Descending Colon | 45 (39) | 4 (8) | 17 (37) | 10 (22) | 14 (31) | 0.032 (*) | |
| Rectum | 11 (9) | 2 (18) | 3 (27) | 5 (45) | 1 (9) | 0.230 | |
| Comorbidities | |||||||
| Smoker | 51 (44) | 5 (9) | 24 (47) | 11 (21) | 11 (21) | 0.244 | |
| Arterial Hypertension | 64 (56) | 12 (18) | 28 (43) | 14 (21) | 10 (15) | 0.713 | |
| Dyslipidaemia | 42 (36) | 7 (16) | 22 (52) | 9 (21) | 4 (9) | 0.148 | |
| Diabetes Mellitus | 27 (23) | 5 (18) | 8 (29) | 10 (37) | 4 (14) | 0.156 | |
| BMI | |||||||
| Median | 25.9 | 27.1 | 25.5 | 25.95 | 23.45 | ||
| Range | (18.4–43.7) | (18.4–43.7) | (22.3–43.7) | (18.4–37) | (19.4–32) | 0.041 (*) | |
| ASA Score | |||||||
| I | 4 (3) | 1 (25) | 2 (50) | 0 (0) | 1 (25) | 0.756 | |
| II | 43 (37) | 9 (20) | 14 (32) | 9 (20) | 11 (25) | 0.368 | |
| III | 63 (55) | 10 (15) | 29 (46) | 16 (25) | 8 (12) | 0.174 | |
| IV | 4 (4) | 0 (0) | 2 (50) | 0 (0) | 2 (50) | 0.291 |
| Variable | B | SD | Wald | p-Value | OR | OR CI 95 | |
|---|---|---|---|---|---|---|---|
| Low | High | ||||||
| Dedifferentiation | 3.977 | 1.335 | 8.880 | 0.00288 | 53.343 | 3.901 | 729.508 |
| sSIGLEC5 | 0.010 | 0.004 | 6.303 | 0.01205 | 1.009 | 1.002 | 1.017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalbán-Hernández, K.; Cantero-Cid, R.; Lozano-Rodríguez, R.; Pascual-Iglesias, A.; Avendaño-Ortiz, J.; Casalvilla-Dueñas, J.C.; Bonel Pérez, G.C.; Guevara, J.; Marcano, C.; Barragán, C.; et al. Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients. Cancers 2021, 13, 3896. https://doi.org/10.3390/cancers13153896
Montalbán-Hernández K, Cantero-Cid R, Lozano-Rodríguez R, Pascual-Iglesias A, Avendaño-Ortiz J, Casalvilla-Dueñas JC, Bonel Pérez GC, Guevara J, Marcano C, Barragán C, et al. Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients. Cancers. 2021; 13(15):3896. https://doi.org/10.3390/cancers13153896
Chicago/Turabian StyleMontalbán-Hernández, Karla, Ramón Cantero-Cid, Roberto Lozano-Rodríguez, Alejandro Pascual-Iglesias, José Avendaño-Ortiz, José Carlos Casalvilla-Dueñas, Gloria Cristina Bonel Pérez, Jenny Guevara, Cristóbal Marcano, Cristina Barragán, and et al. 2021. "Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients" Cancers 13, no. 15: 3896. https://doi.org/10.3390/cancers13153896
APA StyleMontalbán-Hernández, K., Cantero-Cid, R., Lozano-Rodríguez, R., Pascual-Iglesias, A., Avendaño-Ortiz, J., Casalvilla-Dueñas, J. C., Bonel Pérez, G. C., Guevara, J., Marcano, C., Barragán, C., Valentín, J., del Fresno, C., Aguirre, L. A., & López Collazo, E. (2021). Soluble SIGLEC5: A New Prognosis Marker in Colorectal Cancer Patients. Cancers, 13(15), 3896. https://doi.org/10.3390/cancers13153896





