Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessment of Biomarkers
2.3. Statistics
3. Results
3.1. Baseline Characteristics and Biomarker Levels
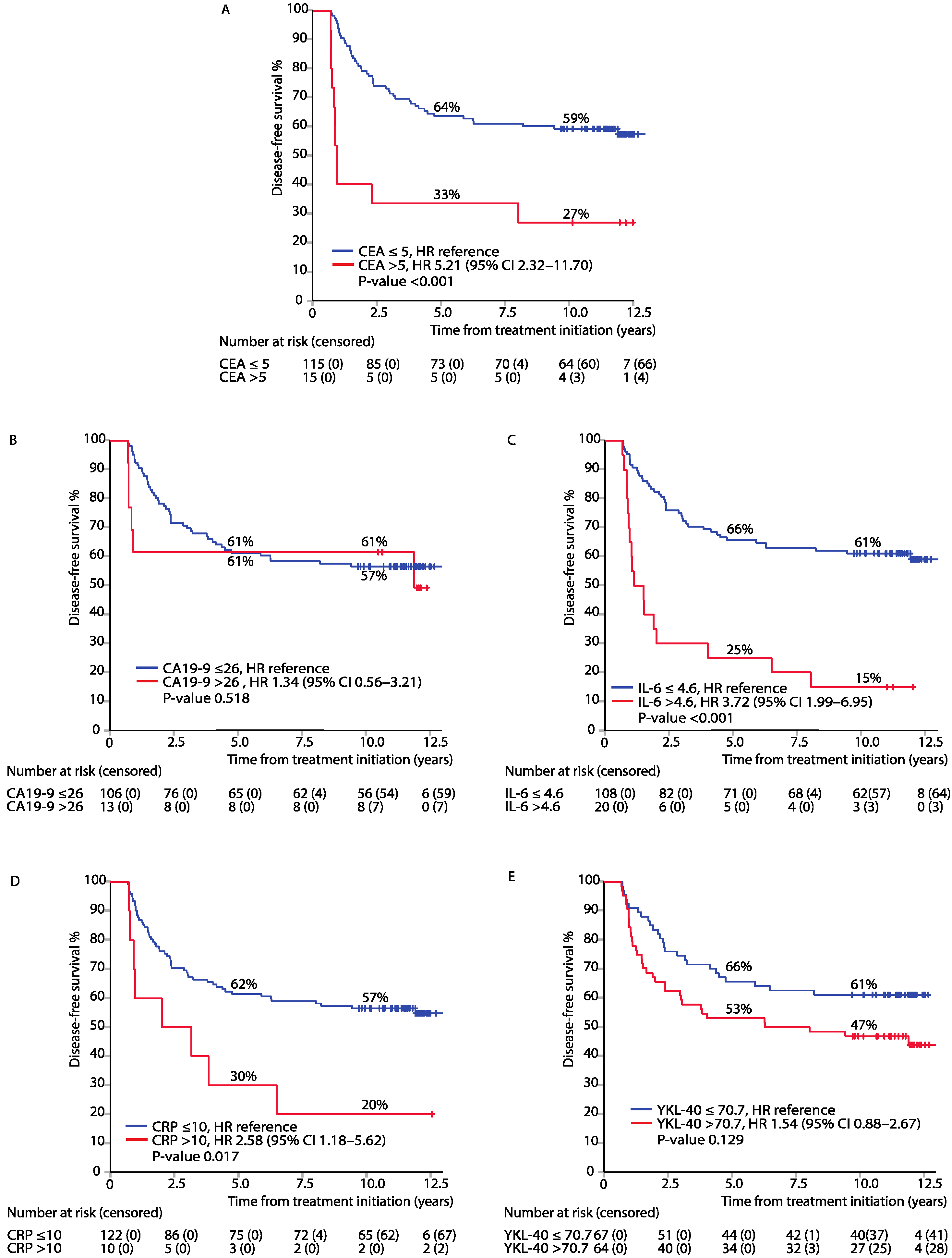
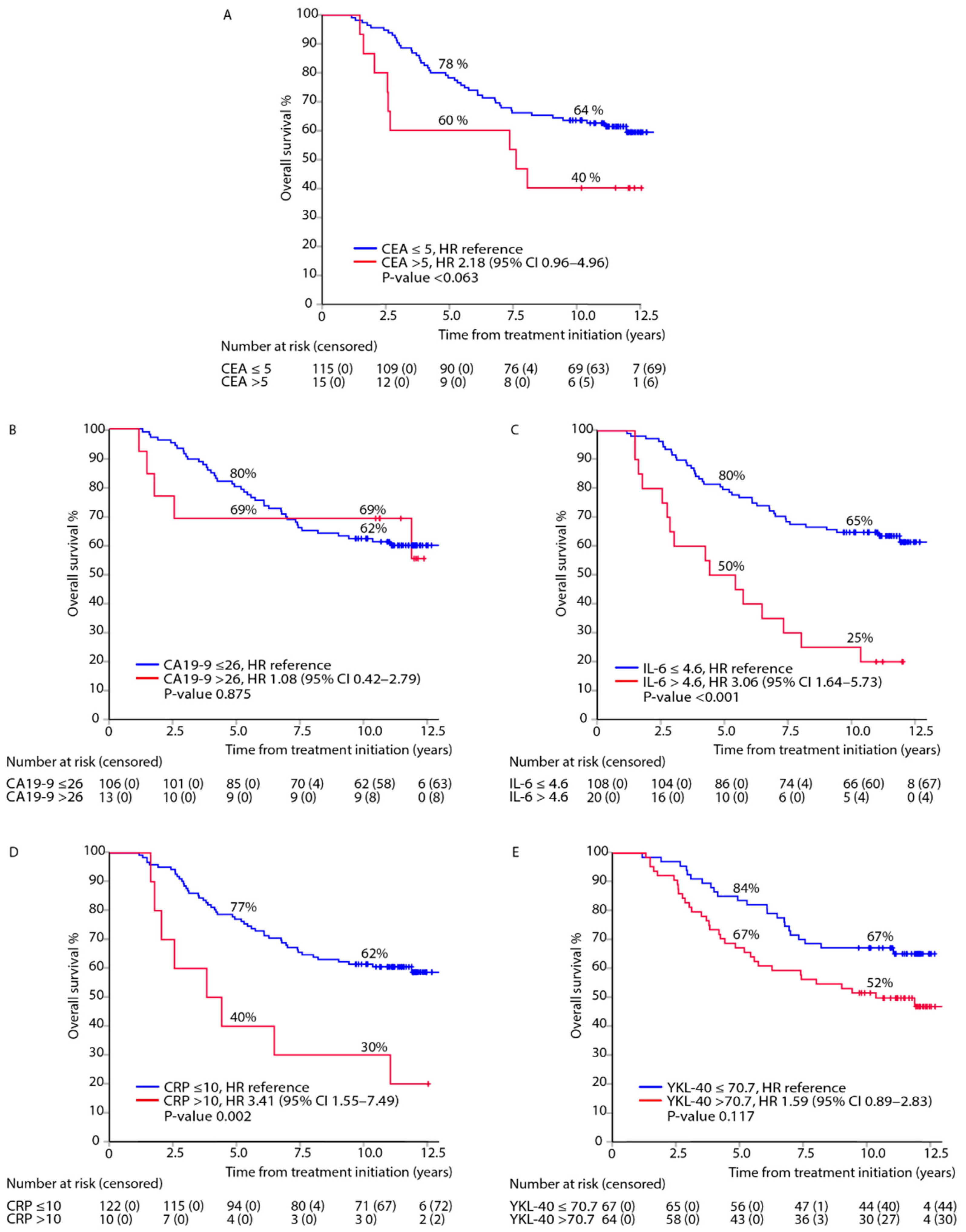
3.2. Association between Elevated Biomarkers in the Post-Adjuvant Setting, i.e., at 8 Months from Randomisation, and DFS or OS
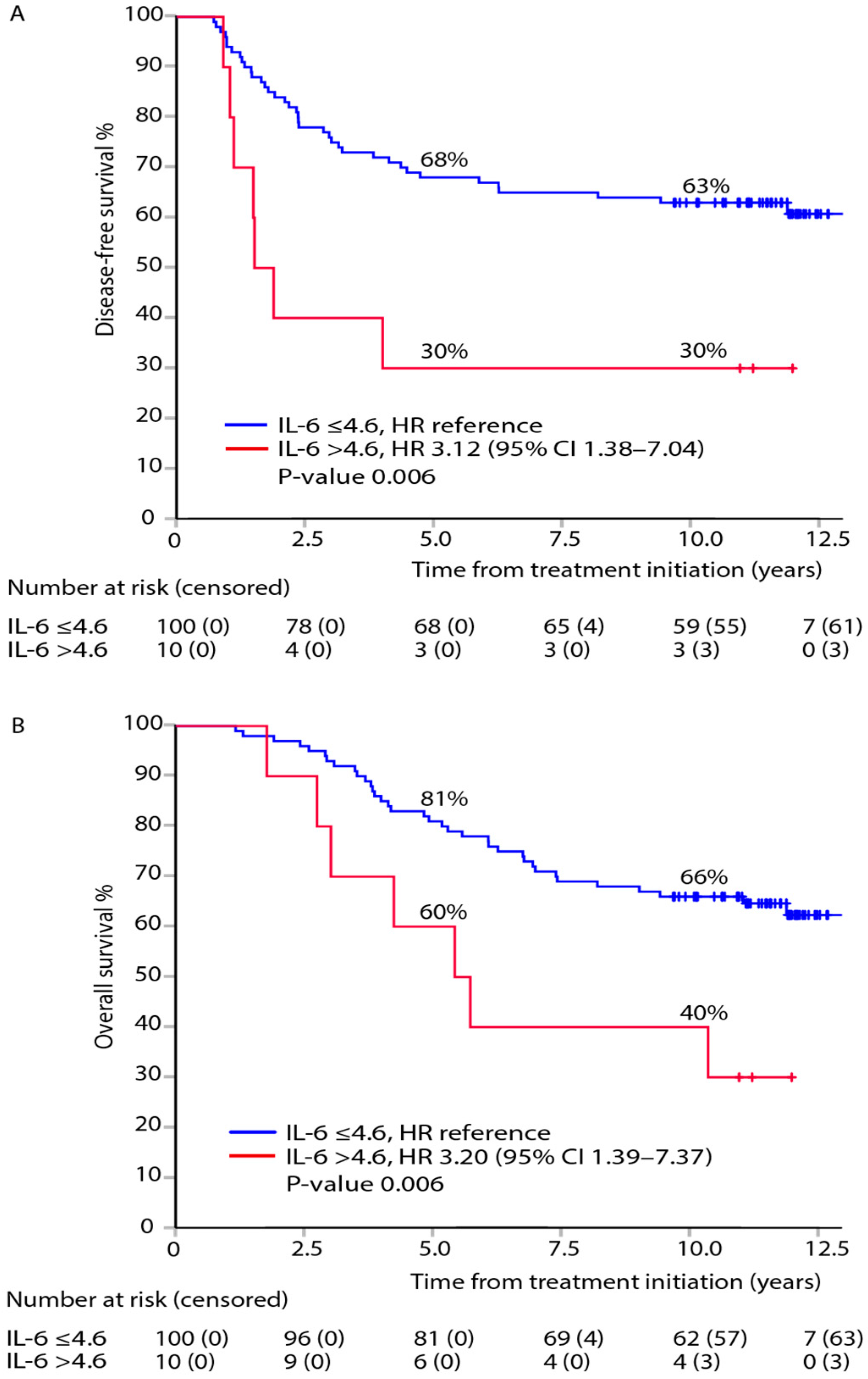
3.3. Association between Post-Adjuvant, i.e., at 8 Months after Randomisation, IL-6 and DFS or OS within TNM Stages
3.4. Mutually Adjusted Multivariable Model of DFS and OS for All Biomarkers Measured in the Post-Adjuvant Setting
3.5. Post-Adjuvant Normal CEA Combined with Elevated CA19-9, CRP, IL-6, and YKL-40
3.6. Diagnostic Accuracy for Postoperative Serum Biomarker Levels, i.e., before Adjuvant Treatment, and Diagnosis of Recurrence
3.7. Lead Times, i.e., Interval between Elevated Serum Biomarker and Diagnosis of Recurrence, during Surveillance
3.8. Time-Varying Serum Biomarkers during Surveillance in the Cox Regression Model and DFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Taieb, J.; André, T.; Auclin, E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat. Rev. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.A.; et al. NCCN Clinical Practice Guidelines in Oncology—Colon cancer Version 2.2021-Published January 21, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 15 April 2021).
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Gold, P.; Freedman, S.O. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J. Exp. Med. 1965, 121, 439–462. [Google Scholar] [CrossRef] [Green Version]
- Primrose, J.N.; Perera, R.; Gray, A.; Rose, P.; Fuller, A.; Corkhill, A.; George, S.; Mant, D. Effect of 3 to 5 Years of Scheduled CEA and CT Follow-up to Detect Recurrence of Colorectal Cancer. JAMA 2014, 311, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermunen, K.; Soveri, L.-M.; Boisen, M.K.; Mustonen, H.K.; Dehlendorff, C.; Haglund, C.H.; Johansen, J.S.; Osterlund, P. Postoperative serum CA19-9, YKL-40, CRP and IL-6 in combination with CEA as prognostic markers for recurrence and survival in colorectal cancer. Acta Oncol. (Madr.) 2020, 59, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Perera, R.; Primrose, J.N.; Mant, D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst. Rev. 2015, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.G.; Ahn, J.B.; Jung, M.; Beom, S.H.; Heo, S.J.; Kim, J.H.; Kim, Y.J.; Kim, N.K.; Min, B.S.; Koom, W.S.; et al. Preoperative Serum Carcinoembryonic Antigen Level as a Prognostic Factor for Recurrence and Survival After Curative Resection Followed by Adjuvant Chemotherapy in Stage III Colon Cancer. Ann. Surg. Oncol. 2017, 24, 227–235. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Auclin, E.; André, T.; Taieb, J.; Banzi, M.; Van Laethem, J.-L.; Tabernero, J.; Hickish, T.; de Gramont, A.; Vernerey, D. Association of post-operative CEA with survival and oxaliplatin benefit in patients with stage II colon cancer: A post hoc analysis of the MOSAIC trial. Br. J. Cancer 2019, 121, 312–317. [Google Scholar] [CrossRef]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsieh, M.-C.; Lai, C.-C.; Yeh, C.-Y.; Chen, J.-S.; Hsieh, P.-S.; Chiang, J.-M.; Tsai, W.-S.; Tang, R.; Changchien, C.-R.; et al. Lead time of carcinoembryonic antigen elevation in the postoperative follow-up of colorectal cancer did not affect the survival rate after recurrence. Int. J. Colorectal Dis. 2010, 25, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Mokhles, S.; Macbeth, F.; Farewell, V.; Fiorentino, F.; Williams, N.R.; Younes, R.N.; Takkenberg, J.J.M.; Treasure, T. Meta-analysis of colorectal cancer follow-up after potentially curative resection. Br. J. Surg. 2016, 103, 1259–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, M.; Hickey, B.E.; Hider, P.N. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst. Rev. 2019, 9, CD002200. [Google Scholar] [CrossRef] [PubMed]
- Colloca, G.A.; Venturino, A.; Guarneri, D. Carcinoembryonic antigen reduction after medical treatment in patients with metastatic colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2019, 34, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Miyamoto, Y.; Beppu, T.; Nitta, H.; Imai, K.; Hayashi, H.; Baba, Y.; Yoshida, N.; Chikamoto, A.; Baba, H. Post-chemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res. 2015, 35, 2359–2368. [Google Scholar]
- Conev, N.V.; Donev, I.S.; Konsoulova-Kirova, A.A.; Chervenkov, T.G.; Kashlov, J.K.; Ivanov, K.D. Serum expression levels of miR-17, miR-21, and miR-92 as potential biomarkers for recurrence after adjuvant chemotherapy in colon cancer patients. Biosci. Trends 2015, 9, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Shinkins, B.; Nicholson, B.D.; Primrose, J.; Perera, R.; James, T.; Pugh, S.; Mant, D. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: Results from the FACS trial. PLoS ONE 2017, 12, e0171810. [Google Scholar] [CrossRef]
- Jin, L.-J.; Chen, W.-B.; Zhang, X.-Y.; Bai, J.; Zhao, H.-C.; Wang, Z.-Y. Analysis of factors potentially predicting prognosis of colorectal cancer. World J. Gastrointest. Oncol. 2019, 11, 1206–1217. [Google Scholar] [CrossRef]
- Hou, H.; Gou, X.; Bu, J.; Su, Y.; Wei, X.; Wang, X.; Hou, B. Meprin α combined with CEA and CA19-9 improves prognostic prediction for surgically treated colorectal cancer patients. Int. J. Clin. Exp. Pathol. 2017, 10, 10441–10450. [Google Scholar]
- Turano, M.; Delrio, P.; Rega, D.; Cammarota, F.; Polverino, A.; Duraturo, F.; Izzo, P.; De Rosa, M. Promising Colorectal Cancer Biomarkers for Precision Prevention and Therapy. Cancers (Basel) 2019, 11, 1932. [Google Scholar] [CrossRef] [Green Version]
- Okamura, R.; Hasegawa, S.; Hida, K.; Hoshino, N.; Kawada, K.; Sugihara, K.; Sakai, Y.; Japanese Study Group for Postoperative Follow-up of Colorectal Cancer. The role of periodic serum CA19-9 test in surveillance after colorectal cancer surgery. Int. J. Clin. Oncol. 2017, 22, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; CHOI, G.-S.; Jun, S.H. Prognostic Value of Serum Tumor Antigen CA19-9 After Curative Resection of Colorectal Cancer. Anticancer Res. 2009, 29, 4303–4308. [Google Scholar]
- Nasr, R.; Salim Hammoud, M.; Nassar, F.; Mukherji, D.; Shamseddine, A.; Temraz, S. Inflammatory Markers and MicroRNAs: The Backstage Actors Influencing Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2018, 19, 1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S.; Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [Green Version]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Sato, M.; Takeyama, H. Preoperative Serum Interleukin-6 Is a Potential Prognostic Factor for Colorectal Cancer, including Stage II Patients. Gastroenterol. Res. Pract. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Nozoe, T.; Matsumata, T.; Kitamura, M.; Sugimachi, K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am. J. Surg. 1998, 176, 335–338. [Google Scholar] [CrossRef]
- Kersten, C.; Louhimo, J.; Ålgars, A.; Lahdesmaki, A.; Cvancerova, M.; Stenstedt, K.; Haglund, C.; Gunnarsson, U. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013, 52, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S. Serum YKL-40, A New Prognostic Biomarker in Cancer Patients? Cancer Epidemiol. Biomark. Prev. 2006, 15, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, I.; Lee, C.; Han, S.; Yun, J.; Hong, J. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef]
- Bian, B.; Li, L.; Yang, J.; Liu, Y.; Xie, G.; Zheng, Y.; Zeng, L.; Zeng, J.; Shen, L. Prognostic value of YKL-40 in solid tumors: A meta-analysis of 41 cohort studies. Cancer Cell Int. 2019, 19, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cintin, C.; Johansen, J.S.; Christensen, I.J.; Price, P.A.; Sørensen, S.; Nielsen, H.J. Serum YKL-40 and colorectal cancer. Br. J. Cancer 1999, 79, 1494–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Personalizing colon cancer adjuvant therapy: Selecting optimal treatments for individual patients. J. Clin. Oncol. 2015, 33, 1787–1796. [Google Scholar] [CrossRef]
- Dasari, A.; Morris, V.K.; Allegra, C.J.; Atreya, C.; Benson, A.B.; Boland, P.; Chung, K.; Copur, M.S.; Corcoran, R.B.; Deming, D.A.; et al. ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal-Anal Task Forces whitepaper. Nat. Rev. Clin. Oncol. 2020, 17, 757–770. [Google Scholar] [CrossRef]
- Lehtomaki, K.I.; Lahtinen, L.I.; Rintanen, N.; Kuopio, T.; Kholova, I.; Makela, R.; Rantala, J.K.; Kellokumpu-Lehtinen, P.-L.; Kononen, J. Clonal Evolution of MEK/MAPK Pathway Activating Mutations in a Metastatic Colorectal Cancer Case. Anticancer Res. 2019, 39, 5867–5877. [Google Scholar] [CrossRef] [Green Version]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.J.; Choi, G.-S.; Lim, K.H.; Kang, B.M.; Jun, S.H. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: Clinical significance of the preoperative level. Ann. Surg. Oncol. 2009, 16, 3087–3093. [Google Scholar] [CrossRef]
- Giessen, C.; Nagel, D.; Glas, M.; Spelsberg, F.; Lau-Werner, U.; Modest, D.P.; Michl, M.; Heinemann, V.; Stieber, P.; Schulz, C. Evaluation of preoperative serum markers for individual patient prognosis in stage I–III rectal cancer. Tumor Biol. 2014, 35, 10237–10248. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Xie, H.; Urrutia, R.; Mahipal, A. The Promise of Circulating Tumor DNA (ctDNA) in the Management of Early-Stage Colon Cancer: A Critical Review. Cancers (Basel) 2020, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, H.; Shimizu, Y.; Senda, Y.; Natsume, S.; Mizuno, N.; Hara, K.; Hijioka, S.; Hieda, N.; Tajika, M.; Tanaka, T.; et al. Post-adjuvant chemotherapy CA19-9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: A retrospective cohort study. Pancreatology 2016, 16, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, K.H.; Gangopadhyay, A.; Shoji, Y.; Nachman, A.P.; Thomas, P.; Jessup, J.M. In vivo induction of murine cytokine production by carcinoembryonic antigen. Cancer Res. 1997, 57, 4432–4436. [Google Scholar]
- Belluco, C.; Nitti, D.; Frantz, M.; Toppan, P.; Basso, D.; Plebani, M.; Lise, M.; Jessup, J.M. Interleukin-6 Blood Level Is Associated with Circulating Carcinoembryonic Antigen and Prognosis in Patients with Colorectal Cancer. Ann. Surg. Oncol. 2000, 7, 133–138. [Google Scholar] [CrossRef]
- Peltonen, R.; Gramkow, M.H.; Dehlendorff, C.; Osterlund, P.J.; Johansen, J.S.; Isoniemi, H. Elevated serum YKL-40, IL-6, CRP, CEA, and CA19-9 combined as a prognostic biomarker panel after resection of colorectal liver metastases. PLoS ONE 2020, 15, e0236569. [Google Scholar] [CrossRef] [PubMed]
- Pesta, M.; Kucera, R.; Topolcan, O.; Karlikova, M.; Houfkova, K.; Polivka, J.; Macanova, T.; Machova, I.; Slouka, D.; Kulda, V. Plasma microRNA Levels Combined with CEA and CA19-9 in the Follow-Up of Colorectal Cancer Patients. Cancers (Basel) 2019, 11, 864. [Google Scholar] [CrossRef] [Green Version]
- McCall, J.L.; Black, R.B.; Rich, C.A.; Harvey, J.R.; Baker, R.A.; Watts, J.M.; Toouli, J. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis. Colon Rectum 1994, 37, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Barillari, P.; Bolognese, A.; Chirletti, P.; Cardi, M.; Sammartino, P.; Stipa, V. Role of CEA, TPA, and Ca 19-9 in the early detection of localized and diffuse recurrent rectal cancer. Dis. Colon Rectum 1992, 35, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.B.; Jiban, M.J.H.; Specogna, A.V.; Vishnubhotla, P.; Lee, E.; Zhang, S. The association between post-treatment surveillance testing and survival in stage II and III colon cancer patients: An observational comparative effectiveness study. BMC Cancer 2019, 19, 418. [Google Scholar] [CrossRef]
- Sørensen, C.G.; Karlsson, W.K.; Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence e—A systematic review. Int. J. Surg. 2016, 25, 134–144. [Google Scholar] [CrossRef]
- Mäkelä, J.T.; Laitinen, S.O.; Kairaluoma, M.I. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch. Surg. 1995, 130, 1062–1067. [Google Scholar] [CrossRef]
- Thomsen, M.; Kersten, C.; Sorbye, H.; Skovlund, E.; Glimelius, B.; Pfeiffer, P.; Johansen, J.; Kure, E.; Ikdahl, T.; Tveit, K.; et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget 2016, 7, 5013–75022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.-H.; Pan, Y.-P.; Fan, C.-W.; Tseng, W.-K.; Huang, J.-S.; Wu, T.-H.; Chou, W.-C.; Wang, C.-H.; Yeh, K.-Y. Pretreatment serum interleukin-1β, interleukin-6, and tumor necrosis factor-α levels predict the progression of colorectal cancer. Cancer Med. 2016, 5, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Starr, M.D.; Bulusu, A.; Pang, H.; Wong, N.S.; Honeycutt, W.; Amara, A.; Hurwitz, H.I.; Nixon, A.B. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med. 2013, 2, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Cohen, J.D.; Kinde, I.; Ptak, J.; Popoli, M.; Schaefer, J.; Silliman, N.; Dobbyn, L.; Tie, J.; et al. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019, 5, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
| All Patients | No Relapse at 8 Months | ||||
|---|---|---|---|---|---|
| n = 147 | % | n = 135 | % | ||
| Age | Median years | 60.3 | 60.4 | ||
| Range | (31.1–75.9) | (31.1–75.9) | |||
| <70 | 131 | 89% | 119 | 88% | |
| ≥70 | 16 | 11% | 16 | 12% | |
| Sex | Male | 75 | 51% | 71 | 53% |
| Female | 72 | 49% | 64 | 47% | |
| Inflammatory disease * | No | 133 | 91% | 121 | 90% |
| Yes | 14 | 10% | 14 | 10% | |
| Chemotherapy regimen | 5FU + LV bolus inj. | 75 | 51% | 70 | 52% |
| 5FU + LV continuous inf. | 72 | 49% | 65 | 48% | |
| Primary location | Right colon | 41 | 28% | 39 | 29% |
| Left colon | 46 | 31% | 42 | 31% | |
| Rectal | 60 | 41% | 54 | 40% | |
| Radiotherapy for rectal primary | No | 9 | 15% | 8 | 15% |
| Preoperative | 8 | 13% | 8 | 15% | |
| Postoperative | 43 | 72% | 38 | 70% | |
| TNM stage | IIA-B | 38 | 26% | 38 | 28% |
| IIIA-C | 91 | 62% | 85 | 63% | |
| IV | 18 | 12% | 12 | 9% | |
| Relapse site | No relapse | 81 | 55% | 81 | 60% |
| Only local | 13 | 9% | 10 | 7% | |
| Distant metastases | 53 | 36% | 44 | 33% | |
| Postoperative | Post-Adjuvant | ||||||
|---|---|---|---|---|---|---|---|
| 0 Months | 8 Months from Randomisation | No Relapse | Relapse | Metastasectomy | Non-CRC Death | ||
| n = 147 | n = 135 | n = 81 | n = 54 | n = 23 | n = 12 | ||
| CEA | n | 132 | 130 | 79 | 51 | 22 | 11 |
| Median (range) (µg/L) | 1.9 (<1–305) | 2.5 (<1–111) | 2.3 (<1–7) | 2.6 (<1–111) | 2.3 (<1–15) | 2.1 (<1–8) | |
| Elevated (>5 μg/L), n (%) | 18 (14) | 15 (12) | 5 (6) | 10 (20) | 3 (14) | 2 (18) | |
| CA19-9 | n | 111 | 119 | 74 | 45 | 22 | 10 |
| Median (range) (kU/L) | 6.0 (<5–2003) | 6.0 (<5–902) | 6.5 (<5–108) | <5 (<5–902) | <5 (<5–27) | 7.0 (<5–27) | |
| Elevated (>26 kU/L), n (%) | 12 (11) | 13 (10) | 8 (11) | 5 (11) | 1 (5) | 1 (10) | |
| IL-6 | n | 143 | 128 | 76 | 52 | 22 | 11 |
| Median (range) (pg/mL) | 2.3 (0.4–36) | 1.9 (0.2–25) | 1.6 (0.2–10) | 2.4 (0.7–25) | 1.8 (0.7–25) | 2.2 (1–10) | |
| Elevated (>4.5 pg/mL), n (%) | 24 (17) | 20 (16) | 5 (7) | 15 (29) | 4 (18) | 4 (36) | |
| CRP | n | 146 | 132 | 79 | 53 | 22 | 11 |
| Median (range) (mg/L) | <5 (<5–174) | <5 (<5–175) | <5 (<5–15) | <5 (<5–175) | <5 (<5–14) | <5 (4–15) | |
| Elevated (>10 mg/L), n (%) | 17 (12) | 10 (8) | 4 (5) | 6 (11) | 2 (9) | 2 (18) | |
| YKL-40 | n | 144 | 131 | 78 | 53 | 23 | 11 |
| Median (range) (ng/mL) | 64.5 (20–1524) | 68.0 (20–1140) | 63.5 (20–230) | 89 (20–1140) | 66 (20–175) | 84 (34–203) | |
| Elevated (>70.7), n (%) | 63 (44) | 64 (49) | 34 (44) | 30 (57) | 10 (43) | 7 (64) | |
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Adjusted for TNM Stage n = 112 | ||||||
| CEA elevated vs. normal | 2.57 | 1.03–6.39 | 0.043 | 0.99 | 0.36–2.70 | 0.980 |
| CA19-9 elevated vs. normal | 1.17 | 0.47–2.92 | 0.741 | 1.01 | 0.38–2.70 | 0.988 |
| IL-6 elevated vs. normal | 3.09 | 1.39–6.86 | 0.006 | 2.88 | 1.29–6.42 | 0.010 |
| CRP elevated vs. normal | 2.12 | 0.79–5.72 | 0.137 | 3.16 | 1.20–8.27 | 0.019 |
| YKL-40 elevated vs. normal | 1.08 | 0.59–1.96 | 0.802 | 1.33 | 0.72–2.45 | 0.360 |
| n | Median (Months) | 95% CI | |
|---|---|---|---|
| CEA elevated | 29 | 7.8 | 5.7–9.8 |
| CA19-9 elevated | 14 | 10.0 | 6.7–13.3 |
| IL-6 elevated | 16 | 21.8 | 4.0–39.6 |
| CRP elevated | 12 | 10.2 | 5.4–15.1 |
| YKL-40 elevated | 27 | 53.1 | 27.1–79.1 |
| CEA, CA19-9, IL-6, CRP, or YKL-40 elevated | 42 | 27.3 | 17.1–37.5 |
| DFS | |||
|---|---|---|---|
| HR | 95% CI | p-Value | |
| Adjusted * | |||
| CEA 1 | 2.08 | 1.82–2.38 | <0.001 |
| CA19-9 2 | 1.39 | 1.22–1.58 | <0.001 |
| IL-6 3 | 1.48 | 1.24–1.76 | <0.001 |
| CRP 4 | 1.61 | 1.30–1.98 | <0.001 |
| YKL-40 5 | 1.30 | 1.07–1.57 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehtomäki, K.; Mustonen, H.; Kellokumpu-Lehtinen, P.-L.; Joensuu, H.; Hermunen, K.; Soveri, L.-M.; Boisen, M.K.; Dehlendorff, C.; Johansen, J.S.; Haglund, C.; et al. Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer. Cancers 2021, 13, 3892. https://doi.org/10.3390/cancers13153892
Lehtomäki K, Mustonen H, Kellokumpu-Lehtinen P-L, Joensuu H, Hermunen K, Soveri L-M, Boisen MK, Dehlendorff C, Johansen JS, Haglund C, et al. Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer. Cancers. 2021; 13(15):3892. https://doi.org/10.3390/cancers13153892
Chicago/Turabian StyleLehtomäki, Kaisa, Harri Mustonen, Pirkko-Liisa Kellokumpu-Lehtinen, Heikki Joensuu, Kethe Hermunen, Leena-Maija Soveri, Mogens Karsbøl Boisen, Christian Dehlendorff, Julia Sidenius Johansen, Caj Haglund, and et al. 2021. "Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer" Cancers 13, no. 15: 3892. https://doi.org/10.3390/cancers13153892
APA StyleLehtomäki, K., Mustonen, H., Kellokumpu-Lehtinen, P.-L., Joensuu, H., Hermunen, K., Soveri, L.-M., Boisen, M. K., Dehlendorff, C., Johansen, J. S., Haglund, C., & Osterlund, P. (2021). Lead Time and Prognostic Role of Serum CEA, CA19-9, IL-6, CRP, and YKL-40 after Adjuvant Chemotherapy in Colorectal Cancer. Cancers, 13(15), 3892. https://doi.org/10.3390/cancers13153892






