Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor TF, VTE, and Survival in Cancer Patients
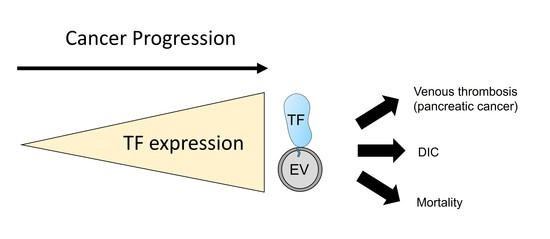
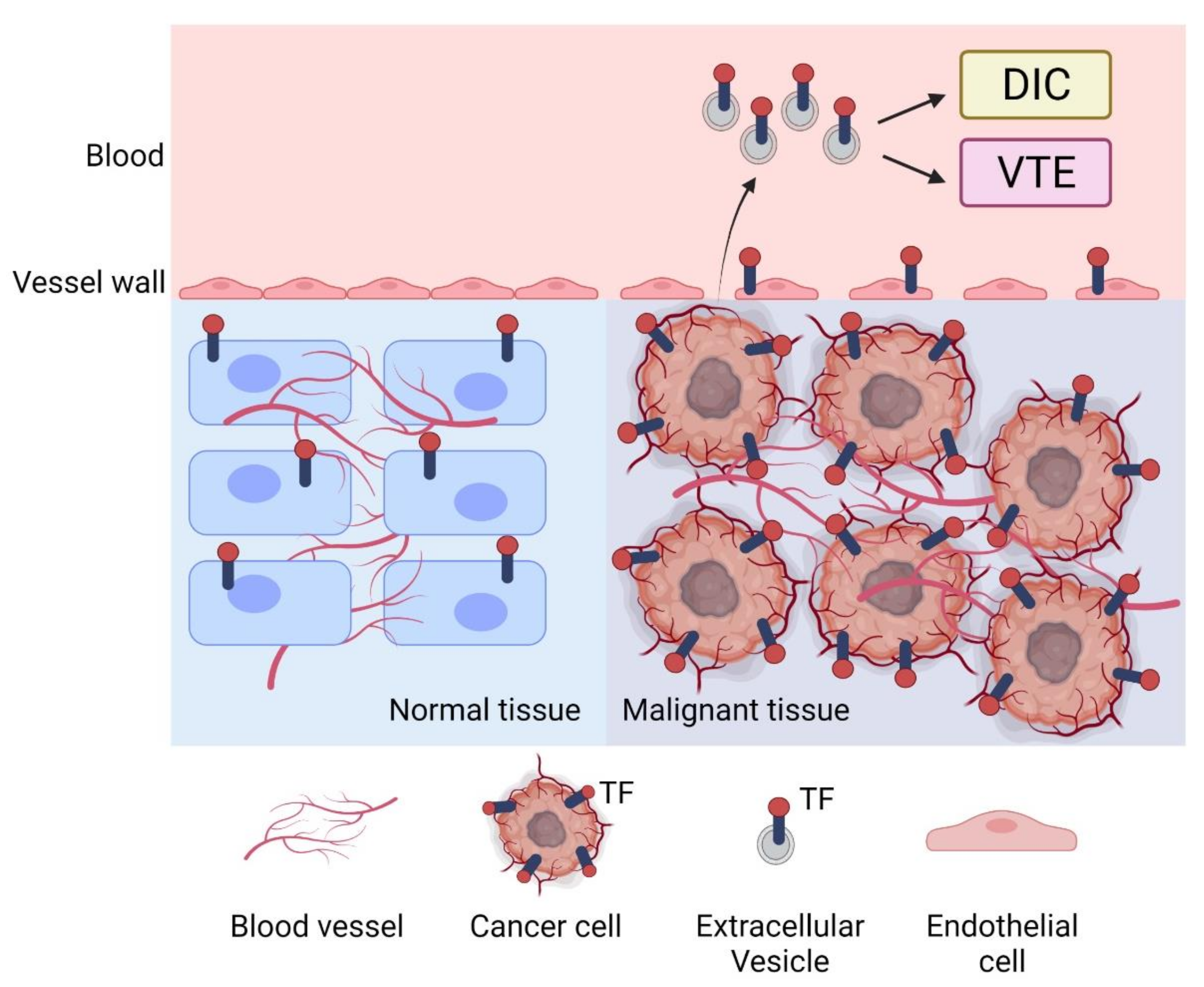
2.1. Tumor TF and Progression
2.2. Tumor TF Expression and Tumor Gene Mutations
2.3. Tumor TF and VTE in Cancer Patients
2.4. Tumor TF and Survival in Cancer Patients
2.5. Targeting Tumor TF to Kill Tumors
3. TF-Positive Extracellular Vesicles in Cancer Patients
3.1. Measurement of TF + EVs in Plasma
3.2. Association between EVTF Activity and VTE in Cancer Patients
3.3. Association between TF Antigen and EVTF Activity and DIC in Cancer Patients
3.4. Association between EVTF Activity and Survival in Cancer Patients
4. Role of TF-Positive Extracellular Vesicles in Mice
4.1. Choice of Mouse Models
4.2. Studies of TF + EVs in Tumor Bearing Mice
4.3. Injection of TF + EVs Increases Thrombosis
4.4. Tumor-Derived TF + EVs Increase Thrombosis
4.5. Pro-angiogenic and Pro-inflammatory Functions of TF + EVs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Horvath-Puho, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Buller, H.R.; Sorensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, Y.D.; Kim, C.H. Incidence and Risk of Various Types of Arterial Thromboembolism in Patients with Cancer. Mayo. Clin. Proc. 2021, 96, 592–600. [Google Scholar] [CrossRef]
- Sorensen, H.T.; Mellemkjaer, L.; Olsen, J.H.; Baron, J.A. Prognosis of cancers associated with venous thromboembolism. N. Engl. J. Med. 2000, 343, 1846–1850. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Culakova, E.; Poniewierski, M.S.; Kuderer, N.M. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb. Res. 2018, 164 (Suppl. 1), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Geddings, J.E.; Ay, C.; Mackman, N. Venous thrombosis and cancer: From mouse models to clinical trials. J. Thromb. Haemost. 2015, 13, 1372–1382. [Google Scholar] [CrossRef]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017, 130, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Levi, M. Disseminated Intravascular Coagulation in Cancer: An Update. Semin. Thromb. Hemost. 2019, 45, 342–347. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.K.; Lemoine, N.R.; Scully, M.F.; Tebbutt, S.; Williamson, R.C. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br. J. Surg. 1995, 82, 1101–1104. [Google Scholar] [CrossRef]
- Khorana, A.A.; Ahrendt, S.A.; Ryan, C.K.; Francis, C.W.; Hruban, R.H.; Hu, Y.C.; Hostetter, G.; Harvey, J.; Taubman, M.B. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin. Cancer Res. 2007, 13, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, C.; Gou, J.; Yi, T.; Qian, Y.; Du, X.; Zhao, X. Expression of tissue factor in human cervical carcinoma tissue. Exp. Ther. Med. 2018, 16, 4075–4081. [Google Scholar] [CrossRef]
- Nitori, N.; Ino, Y.; Nakanishi, Y.; Yamada, T.; Honda, K.; Yanagihara, K.; Kosuge, T.; Kanai, Y.; Kitajima, M.; Hirohashi, S. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2005, 11, 2531–2539. [Google Scholar] [CrossRef]
- El-Telbany, A.; Ma, P.C. Cancer genes in lung cancer: Racial disparities: Are there any? Genes Cancer 2012, 3, 467–480. [Google Scholar] [CrossRef]
- Dearden, S.; Stevens, J.; Wu, Y.L.; Blowers, D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 2013, 24, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.L.; Mitchell, P.; Dobrovic, A.; John, T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin. Epidemiol. 2014, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Regina, S.; Rollin, J.; Blechet, C.; Iochmann, S.; Reverdiau, P.; Gruel, Y. Tissue factor expression in non-small cell lung cancer: Relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J. Thorac. Oncol. 2008, 3, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Regina, S.; Valentin, J.B.; Lachot, S.; Lemarie, E.; Rollin, J.; Gruel, Y. Increased tissue factor expression is associated with reduced survival in non-small cell lung cancer and with mutations of TP53 and PTEN. Clin. Chem. 2009, 55, 1834–1842. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Wu, Y.; Zhang, C.; Wang, S.; Ma, N.; Wang, L.; Wang, Q. Anaplastic lymphoma kinase rearrangement may increase the incidence of venous thromboembolism by increasing tissue factor expression in advanced lung adenocarcinoma. Ann. Transl. Med. 2020, 8, 1307. [Google Scholar] [CrossRef]
- Dunbar, A.; Bolton, K.L.; Devlin, S.M.; Sanchez-Vega, F.; Gao, J.; Mones, J.V.; Wills, J.; Kelly, D.; Farina, M.; Cordner, K.B.; et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood 2021, 137, 2103–2113. [Google Scholar] [CrossRef]
- Unruh, D.; Schwarze, S.R.; Khoury, L.; Thomas, C.; Wu, M.; Chen, L.; Chen, R.; Liu, Y.; Schwartz, M.A.; Amidei, C.; et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016, 132, 917–930. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Petersen, H.H.; Ahamed, J.; Felding-Habermann, B.; Takada, Y.; Mueller, B.M.; Ruf, W. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008, 111, 190–199. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Ellies, L.G.; Andrade-Gordon, P.; Mueller, B.M.; Ruf, W. Protease-activated receptor (PAR) 2, but not PAR1, signaling promotes the development of mammary adenocarcinoma in polyoma middle T mice. Cancer Res. 2008, 68, 7219–7227. [Google Scholar] [CrossRef]
- Yu, J.L.; May, L.; Lhotak, V.; Shahrzad, S.; Shirasawa, S.; Weitz, J.I.; Coomber, B.L.; Mackman, N.; Rak, J.W. Oncogenic events regulate tissue factor expression in colorectal cancer cells: Implications for tumor progression and angiogenesis. Blood 2005, 105, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.M.; Reisfeld, R.A.; Edgington, T.S.; Ruf, W. Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. USA 1992, 89, 11832–11836. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Zarpellon, A.; Chakrabarty, S.; Bogdanov, V.Y.; Gruber, A.; Castellino, F.J.; Mackman, N.; Ellies, L.G.; Weiler, H.; Ruggeri, Z.M.; et al. Contributions of thrombin targets to tissue factor-dependent metastasis in hyperthrombotic mice. J. Thromb. Haemost. 2014, 12, 71–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thaler, J.; Preusser, M.; Ay, C.; Kaider, A.; Marosi, C.; Zielinski, C.; Pabinger, I.; Hainfellner, J.A. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb. Res. 2013, 131, 162–165. [Google Scholar] [CrossRef]
- Seto, S.; Onodera, H.; Kaido, T.; Yoshikawa, A.; Ishigami, S.; Arii, S.; Imamura, M. Tissue factor expression in human colorectal carcinoma: Correlation with hepatic metastasis and impact on prognosis. Cancer 2000, 88, 295–301. [Google Scholar] [CrossRef]
- Yamashita, H.; Kitayama, J.; Ishikawa, M.; Nagawa, H. Tissue factor expression is a clinical indicator of lymphatic metastasis and poor prognosis in gastric cancer with intestinal phenotype. J. Surg. Oncol. 2007, 95, 324–331. [Google Scholar] [CrossRef]
- Chen, L.; Luo, G.; Tan, Y.; Wei, J.; Wu, C.; Zheng, L.; Zhang, X.; Xu, N. Immunolocalisation of tissue factor in esophageal cancer is correlated with intratumoral angiogenesis and prognosis of the patient. Acta Histochem. 2010, 112, 233–239. [Google Scholar] [CrossRef]
- Ueno, T.; Toi, M.; Koike, M.; Nakamura, S.; Tominaga, T. Tissue factor expression in breast cancer tissues: Its correlation with prognosis and plasma concentration. Br. J. Cancer 2000, 83, 164–170. [Google Scholar] [CrossRef]
- Akashi, T.; Furuya, Y.; Ohta, S.; Fuse, H. Tissue factor expression and prognosis in patients with metastatic prostate cancer. Urology 2003, 62, 1078–1082. [Google Scholar] [CrossRef]
- Patry, G.; Hovington, H.; Larue, H.; Harel, F.; Fradet, Y.; Lacombe, L. Tissue factor expression correlates with disease-specific survival in patients with node-negative muscle-invasive bladder cancer. Int. J. Cancer 2008, 122, 1592–1597. [Google Scholar] [CrossRef]
- Stampfli, S.F.; Akhmedov, A.; Hausladen, S.; Varga, Z.; Dedes, K.J.; Hellermann, J.; Luscher, T.F.; Kristiansen, G.; Tanner, F.C.; Breitenstein, A. Tissue Factor Expression Does Not Predict Mortality in Breast Cancer Patients. Anticancer Res. 2017, 37, 3259–3264. [Google Scholar]
- Huang, X.; Molema, G.; King, S.; Watkins, L.; Edgington, T.S.; Thorpe, P.E. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997, 275, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Gao, B.; Duffy, S.; Watkins, L.; Rote, N.; Thorpe, P.E. Infarction of solid Hodgkin’s tumors in mice by antibody-directed targeting of tissue factor to tumor vasculature. Cancer Res. 1998, 58, 4646–4653. [Google Scholar] [PubMed]
- Liu, C.; Huang, H.; Donate, F.; Dickinson, C.; Santucci, R.; El-Sheikh, A.; Vessella, R.; Edgington, T.S. Prostate-specific membrane antigen directed selective thrombotic infarction of tumors. Cancer Res. 2002, 62, 5470–5475. [Google Scholar] [PubMed]
- El-Sheikh, A.; Borgstrom, P.; Bhattacharjee, G.; Belting, M.; Edgington, T.S. A selective tumor microvasculature thrombogen that targets a novel receptor complex in the tumor angiogenic microenvironment. Cancer Res. 2005, 65, 11109–11117. [Google Scholar] [CrossRef][Green Version]
- Hu, Z.; Sun, Y.; Garen, A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc. Natl. Acad. Sci. USA 1999, 96, 8161–8166. [Google Scholar] [CrossRef]
- Hu, Z.; Garen, A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2000, 97, 9221–9225. [Google Scholar] [CrossRef]
- Hu, Z.; Garen, A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 12180–12185. [Google Scholar] [CrossRef]
- Cocco, E.; Hu, Z.; Richter, C.E.; Bellone, S.; Casagrande, F.; Bellone, M.; Todeschini, P.; Krikun, G.; Silasi, D.A.; Azodi, M.; et al. hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor for immunotherapy of uterine serous papillary carcinoma. Br. J. Cancer 2010, 103, 812–819. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, R.; Campbell, A.; McMichael, E.; Yu, L.; Ramaswamy, B.; London, C.A.; Xu, T.; Carson, W.E., 3rd. Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON. Cancer Immunol. Res. 2018, 6, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hyodo, I.; Koga, Y.; Tsumura, R.; Sato, R.; Obonai, T.; Fuchigami, H.; Furuya, F.; Yasunaga, M.; Harada, M.; et al. Enhanced antitumor effect of anti-tissue factor antibody-conjugated epirubicin-incorporating micelles in xenograft models. Cancer Sci. 2015, 106, 627–634. [Google Scholar] [CrossRef]
- Koga, Y.; Manabe, S.; Aihara, Y.; Sato, R.; Tsumura, R.; Iwafuji, H.; Furuya, F.; Fuchigami, H.; Fujiwara, Y.; Hisada, Y.; et al. Antitumor effect of antitissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. Int. J. Cancer 2015, 137, 1457–1466. [Google Scholar] [CrossRef]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Q.; Zhao, H.; Ma, L.; Meng, T.; Qian, J.; Jin, R.; Shen, J.; Yu, K. Pathological expression of tissue factor confers promising antitumor response to a novel therapeutic antibody SC1 in triple negative breast cancer and pancreatic adenocarcinoma. Oncotarget 2017, 8, 59086–59102. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, J.W.; Cai, A.G.; Bhatti, M.M.; Cooper, A.B.; Avery, A.D.; Dorfman, R.; Guelman, S.; Levashova, Z.; Migone, T.S. Treating Tissue Factor-Positive Cancers with Antibody-Drug Conjugates That Do Not Affect Blood Clotting. Mol. Cancer Ther. 2018, 17, 2412–2426. [Google Scholar] [CrossRef]
- de Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.P.; Arkenau, H.T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Quay, S.C.; Orenstein, N.S.; Dvorak, A.M.; Hahn, P.; Bitzer, A.M.; Carvalho, A.C. Tumor shedding and coagulation. Science 1981, 212, 923–924. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Van DeWater, L.; Bitzer, A.M.; Dvorak, A.M.; Anderson, D.; Harvey, V.S.; Bach, R.; Davis, G.L.; DeWolf, W.; Carvalho, A.C. Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 1983, 43, 4434–4442. [Google Scholar] [PubMed]
- Bastida, E.; Ordinas, A.; Escolar, G.; Jamieson, G.A. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood 1984, 64, 177–184. [Google Scholar] [CrossRef]
- Yu, J.L.; Rak, J.W. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J. Thromb. Haemost. 2004, 2, 2065–2067. [Google Scholar] [CrossRef]
- Lee, R.D.; Barcel, D.A.; Williams, J.C.; Wang, J.G.; Boles, J.C.; Manly, D.A.; Key, N.S.; Mackman, N. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb. Res. 2012, 129, 80–85. [Google Scholar] [CrossRef]
- Claussen, C.; Rausch, A.V.; Lezius, S.; Amirkhosravi, A.; Davila, M.; Francis, J.L.; Hisada, Y.M.; Mackman, N.; Bokemeyer, C.; Schmalfeldt, B.; et al. Microvesicle-associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb. Res. 2016, 141, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.; Spath, B.; Haubold, K.; Holstein, K.; Marx, G.; Wierecky, J.; Brummendorf, T.H.; Dierlamm, J.; Bokemeyer, C.; Eifrig, B. Tissue factor procoagulant activity of plasma microparticles in patients with cancer-associated disseminated intravascular coagulation. Ann. Hematol. 2008, 87, 451–457. [Google Scholar] [CrossRef]
- Ay, C.; Mackman, N. Tissue Factor: Catch Me If You Can! J. Clin. Oncol. 2017, 35, 1128–1130. [Google Scholar] [CrossRef]
- Parhami-Seren, B.; Butenas, S.; Krudysz-Amblo, J.; Mann, K.G. Immunologic quantitation of tissue factors. J. Thromb. Haemost. 2006, 4, 1747–1755. [Google Scholar] [CrossRef]
- Key, N.S.; Mackman, N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin. Thromb. Hemost. 2010, 36, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Menzies, K.E.; Wang, J.G.; Hyrien, O.; Hathcock, J.; Mackman, N.; Taubman, M.B. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J. Thromb. Haemost. 2008, 6, 1983–1985. [Google Scholar] [CrossRef]
- Tesselaar, M.E.; Romijn, F.P.; Van Der Linden, I.K.; Prins, F.A.; Bertina, R.M.; Osanto, S. Microparticle-associated tissue factor activity: A link between cancer and thrombosis? J. Thromb. Haemost. 2007, 5, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Ay, C.; Mackman, N.; Bertina, R.M.; Kaider, A.; Marosi, C.; Key, N.S.; Barcel, D.A.; Scheithauer, W.; Kornek, G.; et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J. Thromb. Haemost. 2012, 10, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Antoniak, S.; Monroe, D.M., 3rd; Khorana, A.A.; Mackman, N. Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1932–1934. [Google Scholar] [CrossRef]
- Tesselaar, M.E.; Romijn, F.P.; van der Linden, I.K.; Bertina, R.M.; Osanto, S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J. Thromb. Haemost. 2009, 7, 1421–1423. [Google Scholar] [CrossRef]
- Manly, D.A.; Wang, J.; Glover, S.L.; Kasthuri, R.; Liebman, H.A.; Key, N.S.; Mackman, N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb. Res. 2010, 125, 511–512. [Google Scholar] [CrossRef]
- Bharthuar, A.; Khorana, A.A.; Hutson, A.; Wang, J.G.; Key, N.S.; Mackman, N.; Iyer, R.V. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb. Res. 2013, 132, 180–184. [Google Scholar] [CrossRef]
- Woei, A.J.F.J.; Tesselaar, M.E.; Garcia Rodriguez, P.; Romijn, F.P.; Bertina, R.M.; Osanto, S. Tissue factor-bearing microparticles and CA19.9: Two players in pancreatic cancer-associated thrombosis? Br. J. Cancer 2016, 115, 332–338. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Hisada, Y.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Mahe, I.; Otten, H.M.; Kamphuisen, P.W.; Berckmans, R.J.; Buller, H.R.; et al. Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: A prospective cohort study. Thromb. Res. 2018, 166, 54–59. [Google Scholar] [CrossRef]
- van Doormaal, F.; Kleinjan, A.; Berckmans, R.J.; Mackman, N.; Manly, D.; Kamphuisen, P.W.; Richel, D.J.; Buller, H.R.; Sturk, A.; Nieuwland, R. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb. Haemost. 2012, 108, 160–165. [Google Scholar] [CrossRef]
- Gezelius, E.; Flou Kristensen, A.; Bendahl, P.O.; Hisada, Y.; Risom Kristensen, S.; Ek, L.; Bergman, B.; Wallberg, M.; Falkmer, U.; Mackman, N.; et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: A sub-study of RASTEN-A randomized trial with low molecular weight heparin. PLoS ONE 2018, 13, e0207387. [Google Scholar] [CrossRef]
- Auwerda, J.J.; Yuana, Y.; Osanto, S.; de Maat, M.P.; Sonneveld, P.; Bertina, R.M.; Leebeek, F.W. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb. Haemost. 2011, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.G.; Prendergast, E.; Geddings, J.E.; Walts, A.E.; Agadjanian, H.; Hisada, Y.; Karlan, B.Y.; Mackman, N.; Walsh, C.S. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Bae-Jump, V.; Mackman, N. UNC Blood Research Center, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. unpublished data. 2021. [Google Scholar]
- Kasthuri, R.S.; Hisada, Y.; Ilich, A.; Key, N.S.; Mackman, N. Effect of chemotherapy and longitudinal analysis of circulating extracellular vesicle tissue factor activity in patients with pancreatic and colorectal cancer. Res. Pract. Thromb. Haemost. 2020, 4, 636–643. [Google Scholar] [CrossRef]
- Reitter, E.M.; Kaider, A.; Prager, G.; Ay, C.; Pabinger, I.; Thaler, J. Longitudinal analysis of extracellular vesicle-associated tissue factor activity in cancer patients. Thromb Res. 2020, 195, 215–218. [Google Scholar] [CrossRef]
- Osterud, B.; Bjorklid, E. The tissue factor pathway in disseminated intravascular coagulation. Semin. Thromb. Hemost. 2001, 27, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Andoh, K.; Sadakata, H.; Tanaka, H.; Kobayashi, N. Tissue factor released from leukemic cells. Thromb. Haemost. 1991, 65, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Satoh, N.; Wada, K.; Takakuwa, E.; Seki, Y.; Shibata, A. Tissue factor in plasma of patients with disseminated intravascular coagulation. Am. J. Hematol. 1994, 46, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Kamikubo, Y.; Goto, A.; Shiratori, Y.; Yamazaki, M.; Jokaji, H.; Saito, M.; Uotani, C.; Kumabashiri, I.; Morishita, E.; et al. Role of tissue factor in disseminated intravascular coagulation. Thromb. Res. 1995, 80, 217–224. [Google Scholar] [CrossRef]
- Thaler, J.; Pabinger, I.; Sperr, W.R.; Ay, C. Clinical evidence for a link between microparticle-associated tissue factor activity and overt disseminated intravascular coagulation in patients with acute myelocytic leukemia. Thromb. Res. 2014, 133, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Hell, L.; Daullary, T.; Burghart, V.; Mauracher, L.M.; Grilz, E.; Moser, B.; Kramer, G.; Schmid, J.A.; Ay, C.; Pabinger, I.; et al. Extracellular Vesicle-Associated Tissue Factor Activity in Prostate Cancer Patients with Disseminated Intravascular Coagulation. Cancers 2021, 13, 1487. [Google Scholar] [CrossRef]
- Hernandez, C.; Orbe, J.; Roncal, C.; Alvarez-Hernandez, M.; Martinez de Lizarrondo, S.; Alves, M.T.; Garcia Mata, J.; Paramo, J.A. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb. Haemost. 2013, 110, 598–608. [Google Scholar] [CrossRef]
- Hisada, Y.; Thalin, C.; Lundstrom, S.; Wallen, H.; Mackman, N. Comparison of microvesicle tissue factor activity in non-cancer severely ill patients and cancer patients. Thromb. Res. 2018, 165, 1–5. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Mackman, N.; Metz-Schimmerl, S.; Stift, J.; Kaider, A.; Mullauer, L.; Gnant, M.; Scheithauer, W.; Pabinger, I. Microparticle-associated tissue factor activity in patients with pancreatic cancer: Correlation with clinicopathological features. Eur. J. Clin. Investig. 2013, 43, 277–285. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Mouse models of cancer-associated thrombosis. Thromb. Res. 2018, 164 (Suppl. 1), S48–S53. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.A.; Obi, A.T.; Myers, D.D., Jr.; Wrobleski, S.K.; Henke, P.K.; Mackman, N.; Wakefield, T.W. Critical review of mouse models of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef]
- Davila, M.; Amirkhosravi, A.; Coll, E.; Desai, H.; Robles, L.; Colon, J.; Baker, C.H.; Francis, J.L. Tissue factor-bearing microparticles derived from tumor cells: Impact on coagulation activation. J. Thromb. Haemost. 2008, 6, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Geddings, J.E.; Aleman, M.M.; Cardenas, J.C.; Chantrathammachart, P.; Williams, J.C.; Kirchhofer, D.; Bogdanov, V.Y.; Bach, R.R.; Rak, J.; et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012, 119, 5543–5552. [Google Scholar] [CrossRef] [PubMed]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. 2016, 14, 153–166. [Google Scholar] [CrossRef]
- Hisada, Y.; Ay, C.; Auriemma, A.C.; Cooley, B.C.; Mackman, N. Human pancreatic tumors grown in mice release tissue factor-positive microvesicles that increase venous clot size. J. Thromb. Haemost. 2017, 15, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Tawil, N.; Bassawon, R.; Meehan, B.; Nehme, A.; Montermini, L.; Gayden, T.; De Jay, N.; Spinelli, C.; Chennakrishnaiah, S.; Choi, D.; et al. Glioblastoma cell populations with distinct oncogenic programs release podoplanin as procoagulant extracellular vesicles. Blood Adv. 2021, 5, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.M.; Panicot-Dubois, L.; Lacroix, R.; Dignat-George, F.; Lombardo, D.; Dubois, C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J. Exp. Med. 2009, 206, 1913–1927. [Google Scholar] [CrossRef]
- Stark, K.; Schubert, I.; Joshi, U.; Kilani, B.; Hoseinpour, P.; Thakur, M.; Grunauer, P.; Pfeiler, S.; Schmidergall, T.; Stockhausen, S.; et al. Distinct Pathogenesis of Pancreatic Cancer Microvesicle-Associated Venous Thrombosis Identifies New Antithrombotic Targets In Vivo. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 772–786. [Google Scholar] [CrossRef]
- Thomas, G.M.; Brill, A.; Mezouar, S.; Crescence, L.; Gallant, M.; Dubois, C.; Wagner, D.D. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J. Thromb. Haemost. 2015, 13, 1310–1319. [Google Scholar] [CrossRef]
- Sasano, T.; Cho, S.M.; Rodriguez-Aguayo, C.; Bayraktar, E.; Taki, M.; Afshar-Kharghan, V.; Sood, A.K. Role of tissue-factor bearing extracellular vesicles released from ovarian cancer cells in platelet aggregation in vitro and venous thrombosis in mice. Thromb. Update 2021, 2, 100020. [Google Scholar] [CrossRef]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Skold, S.; Lofstedt, T.; Johansson, M.C.; Morgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef] [PubMed]
- Che, S.P.Y.; Park, J.Y.; Stokol, T. Tissue Factor-Expressing Tumor-Derived Extracellular Vesicles Activate Quiescent Endothelial Cells via Protease-Activated Receptor-1. Front. Oncol. 2017, 7, 261. [Google Scholar] [CrossRef] [PubMed]
| Study | Tumor Type | No. of Patients with VTE/Total No. of Patients | % of VTE | Association between EVTF Activity and VTE |
|---|---|---|---|---|
| Single time point studies | ||||
| van Doormaal et al. [76] | More than 6 different types of cancer | 5/43 | 11.6 | Yes * |
| van Es et al. [75] | 9 different types of cancer | 40/648 | 6.2 | Yes ** |
| Bharthuar et al. [73] | Pancreaticobiliary | 52/117 | 44.4 | Yes |
| Woei A-Jin et al. [74] | Pancreatic | 14/79 | 17.7 | Yes |
| Thaler et al. [69] | Pancreatic | 12/60 | 20 | No |
| Brain | 6/43 | 14 | No | |
| Colorectal | 12/126 | 9.5 | No | |
| Stomach | 19/119 | 16 | No | |
| Gezelius et al. [77] | Small cell lung carcinoma | 15/235 | 6.3 | No |
| Cohen et al. [79] | Epithelial ovarian | 19/59 | 32.2 | No |
| Hisada et al. [80] | Ovarian | 4/84 | 4.8 | No |
| Auwerda et al. [78] | Multiple myeloma | 15/122 | 12.3 | No |
| Longitudinal studies | ||||
| Khorana et al. [67] | Pancreatic | 2/10 | 20 | 2 patients with serial increases in EVTF activity had VTE |
| Kasthuri et al. [81] | Pancreatic | 1/13 | 7.7 | 1 patient with increased EVTF activity had VTE |
| Colorectal | 4/22 | 18.2 | All VTE patients did not have increased EVTF activity | |
| Reitter et al. [82] | 4 different types of cancer | 12/38 | 31.6 | No |
| Study | Tumor Type | Total No. of Patients | Association between EVTF and Mortality |
|---|---|---|---|
| Tesselaar et al. [68] | Pancreatic | 23 | Yes |
| Tesselaar et al. [71] | 13 different types of cancer | 100 | Yes |
| Thaler et al. [69] | Pancreatic | 60 | Yes |
| Brain | 43 | No | |
| Colorectal | 126 | No | |
| Stomach | 119 | Yes * | |
| Thaler et al. [91] | Pancreatic | 73 | Yes |
| Bharthuar et al. [73] | Pancreaticobiliary | 117 | Yes |
| Hernandez et al. [89] | Stomach | 25 | Yes ** |
| Colorectal | 96 | ||
| Pancreatic | 9 | ||
| Lung | 18 | ||
| Breast | 42 | ||
| Non-Hodgkin lymphoma | 62 | ||
| Woei et al. [74] | Pancreatic | 79 | Yes |
| Hisada et al. [90] | 17 different types of cancer | 60 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisada, Y.; Mackman, N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers 2021, 13, 3839. https://doi.org/10.3390/cancers13153839
Hisada Y, Mackman N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers. 2021; 13(15):3839. https://doi.org/10.3390/cancers13153839
Chicago/Turabian StyleHisada, Yohei, and Nigel Mackman. 2021. "Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer" Cancers 13, no. 15: 3839. https://doi.org/10.3390/cancers13153839
APA StyleHisada, Y., & Mackman, N. (2021). Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers, 13(15), 3839. https://doi.org/10.3390/cancers13153839