BRCA2 Promotes Spontaneous Homologous Recombination In Vivo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Lines
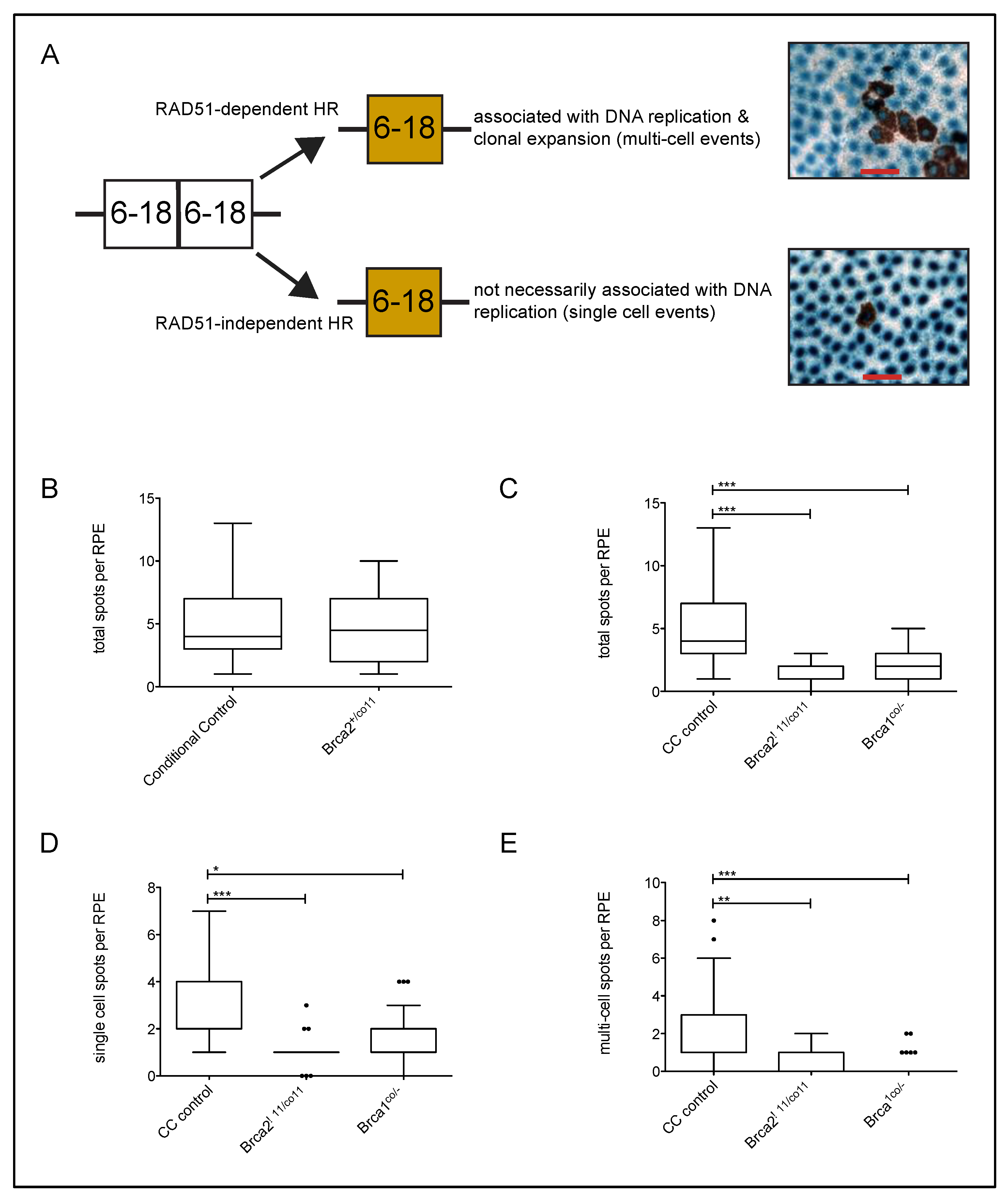
2.2. Obtaining and Scoring Reversion Events of the Retinal Pigment Epithelium
2.3. RNA Isolation and Reverse Transcription-PCR (RT-PCR)
2.4. Protein Isolation and Immunoprecipitation
2.5. Statistical Analysis
3. Results
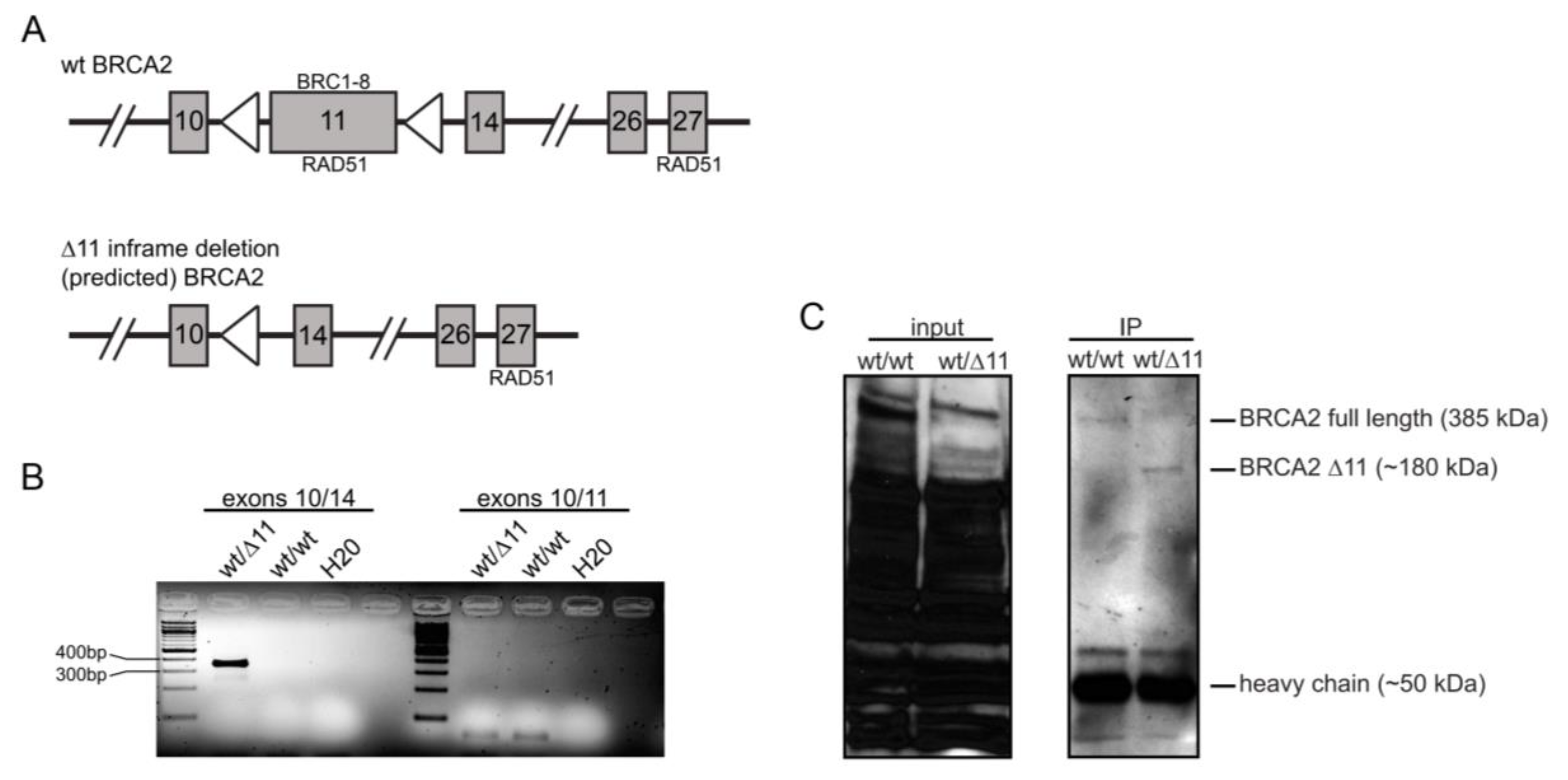
3.1. Establishment of the Constitutive pun Brca2 Cohort
3.2. Brca2 Delta 11 Conditional Heterozygosity Does Not Alter HR Frequency
3.3. Conditional Loss of Full-Length BRCA2 Results in Decreased HR Frequency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonoda, E.; Sasaki, M.S.; Buerstedde, J.M.; Bezzubova, O.; Shinohara, A.; Ogawa, H.; Takata, M.; Yamaguchi-Iwai, Y.; Takeda, S. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998, 17, 598–608. [Google Scholar] [CrossRef]
- Lim, D.S.; Hasty, P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 1996, 16, 7133–7143. [Google Scholar] [CrossRef]
- Suzuki, A.; de la Pompa, J.L.; Hakem, R.; Elia, A.; Yoshida, R.; Mo, R.; Nishina, H.; Chuang, T.; Wakeham, A.; Itie, A.; et al. Brca2 is required for embryonic cellular proliferation in the mouse. Genes Dev. 1997, 11, 1242–1252. [Google Scholar] [CrossRef]
- Sharan, S.K.; Morimatsu, M.; Albrecht, U.; Lim, D.S.; Regel, E.; Dinh, C.; Sands, A.; Eichele, G.; Hasty, P.; Bradley, A. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature 1997, 386, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Connor, F.; Bertwistle, D.; Mee, P.J.; Ross, G.M.; Swift, S.; Grigorieva, E.; Tybulewicz, V.L.; Ashworth, A. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat. Genet. 1997, 17, 423–430. [Google Scholar] [CrossRef]
- Friedman, L.S.; Thistlethwaite, F.C.; Patel, K.J.; Yu, V.P.; Lee, H.; Venkitaraman, A.R.; Abel, K.J.; Carlton, M.B.; Hunter, S.M.; Colledge, W.H.; et al. Thymic lymphomas in mice with a truncating mutation in Brca2. Cancer Res. 1998, 58, 1338–1343. [Google Scholar]
- Rajan, J.V.; Wang, M.; Marquis, S.T.; Chodosh, L.A. Brca2 is coordinately regulated with Brca1 during proliferation and differentiation in mammary epithelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 13078–13083. [Google Scholar] [CrossRef]
- Wong, A.K.; Pero, R.; Ormonde, P.A.; Tavtigian, S.V.; Bartel, P.L. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. J. Biol. Chem. 1997, 272, 31941–31944. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.S.; Lee, S.Y.; Chen, G.; Song, M.; Tomlinson, G.E.; Lee, E.Y. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999, 59, 3547–3551. [Google Scholar] [PubMed]
- Moynahan, M.E.; Pierce, A.J.; Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 2001, 7, 263–272. [Google Scholar] [CrossRef]
- Tutt, A.; Bertwistle, D.; Valentine, J.; Gabriel, A.; Swift, S.; Ross, G.; Griffin, C.; Thacker, J.; Ashworth, A. Mutation in Brca2 stimu-lates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 2001, 20, 4704–4716. [Google Scholar] [CrossRef] [PubMed]
- JStark, M.; Pierce, A.J.; Oh, J.; Pastink, A.; Jasin, M. Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol. Cell. Biol. 2004, 24, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Howlett, N.G.; Taniguchi, T.; Olson, S.; Cox, B.; Waisfisz, Q.; de Die-Smulders, C.; Persky, N.; Grompe, M.; Joenje, H.; Pals, G.; et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 2002, 297, 606–609. [Google Scholar] [CrossRef]
- Brown, A.D.; Claybon, A.D.B.; Bishop, A.J. A conditional mouse model for measuring the frequency of homologous recombination events in vivo in the absence of essential genes. Mol. Cell. Biol. 2011, 31, 3593–3602. [Google Scholar] [CrossRef]
- Bishop, A.J.; Hollander, M.C.; Kosaras, B.; Sidman, R.L.; Fornace, A.J., Jr.; Schiestl, R.H. Atm-, p53-, and Gadd45a-deficient mice show an increased frequency of homologous recombination at different stages during development. Cancer Res. 2003, 63, 5335–5343. [Google Scholar]
- Aubrecht, J.; Secretan, M.B.; Bishop, A.J.; Schiestl, R.H. Involvement of p53 in X-ray induced intrachromosomal recombination in mice. Carcinogenesis 1999, 20, 2229–2236. [Google Scholar] [CrossRef][Green Version]
- Claybon, A.; Karia, B.; Bruce, C.; Bishop, A.J. PARP1 suppresses homologous recombination events in mice in vivo. Nucleic Acids Res. 2010, 38, 7538–7545. [Google Scholar] [CrossRef]
- Galli, A.; Schiestl, R.H. On the mechanism of UV and gamma-ray-induced intrachromosomal recombination in yeast cells synchronized in different stages of the cell cycle. Mol. Gen. Genet. 1995, 248, 301–310. [Google Scholar] [CrossRef]
- Jonkers, J.; Meuwissen, R.; van der Gulden, H.; Peterse, H.; van der Valk, M.; Berns, A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 2001, 29, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Metzger, D.; Garnier, J.M.; Chambon, P.; Mark, M. Site-specific somatic mutagenesis in the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1384–1388. [Google Scholar]
- Farago, A.F.; Awatramani, R.B.; Dymecki, S.M. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron 2006, 50, 205–218. [Google Scholar] [CrossRef]
- Evers, B.; Drost, R.; Schut, E.; de Bruin, M.; van der Burg, E.; Derksen, P.W.; Holstege, H.; Liu, X.; van Drunen, E.; Beverloo, H.B.; et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 2008, 14, 3916–3925. [Google Scholar] [CrossRef]
- Liu, Y.; West, S.C. Distinct functions of BRCA1 and BRCA2 in double-strand break repair. Breast Cancer Res. 2002, 4, 9–13. [Google Scholar] [CrossRef][Green Version]
- Dooley, T.P.; Miranda, M.; Jones, N.C.; DePamphilis, M.L. Transactivation of the adenovirus EIIa promoter in the absence of adenovirus E1A protein is restricted to mouse oocytes and preimplantation embryos. Development 1989, 107, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Gayther, S.A.; Mangion, J.; Russell, P.; Seal, S.; Barfoot, R.; Ponder, B.A.; Stratton, M.R.; Easton, D. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat. Genet. 1997, 15, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Osorio, A.; de la Hoya, M.; Rodriguez-Lopez, R.; Martinez-Ramirez, A.; Cazorla, A.; Granizo, J.J.; Esteller, M.; Rivas, C.; Caldes, T.; Benitez, J. Loss of heterozygosity analysis at the BRCA loci in tumor samples from patients with familial breast cancer. Int. J. Cancer 2002, 99, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, M.E.; Chiu, J.W.; Koller, B.H.; Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell 1999, 4, 511–518. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Cui, T.Y.; Jasin, M. Homology-directed dna repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 2001, 61, 4842–4850. [Google Scholar]
- Chen, J.; Silver, D.P.; Walpita, D.; Cantor, S.B.; Gazdar, A.F.; Tomlinson, G.; Couch, F.J.; Weber, B.L.; Ashley, T.; Livingston, D.M.; et al. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol. Cell 1998, 2, 317–328. [Google Scholar] [CrossRef]
- Bodenstein, L.; Sidman, R.L. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev. Biol. 1987, 121, 192–204. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Li, S. Inhibition of poly(ADP-ribose) polymerase in BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 1707, author reply 1707–1708. [Google Scholar]
- Tannenbaum, B.; Mofunanya, T.; Schoenfeld, A.R. DNA damage repair is unaffected by mimicked heterozygous levels of BRCA2 in HT-29 cells. Int. J. Biol. Sci. 2007, 3, 402–407. [Google Scholar] [CrossRef]
- Saeki, H.; Siaud, N.; Christ, N.; Wiegant, W.W.; van Buul, P.P.; Han, M.; Zdzienicka, M.Z.; Stark, J.M.; Jasin, M. Suppression of the DNA repair defects of BRCA2-deficient cells with heterologous protein fusions. Proc. Natl. Acad. Sci. USA 2006, 103, 8768–8773. [Google Scholar] [CrossRef]
- Shivji, M.K.; Davies, O.R.; Savill, J.M.; Bates, D.L.; Pellegrini, L.; Venkitaraman, A.R. A region of human BRCA2 containing multiple BRC repeats promotes RAD51-mediated strand exchange. Nucleic Acids Res. 2006, 34, 4000–4011. [Google Scholar] [CrossRef]
- Davies, A.A.; Masson, J.Y.; McIlwraith, M.J.; Stasiak, A.Z.; Stasiak, A.; Venkitaraman, A.R.; West, S.C. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell 2001, 7, 273–282. [Google Scholar] [CrossRef]
- Ayoub, N.; Rajendra, E.; Su, X.; Jeyasekharan, A.D.; Mahen, R.; Venkitaraman, A.R. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr. Biol. 2009, 19, 1075–1085. [Google Scholar] [CrossRef]
- Edwards, S.L.; Brough, R.; Lord, C.J.; Natrajan, R.; Vatcheva, R.; Levine, D.A.; Boyd, J.; Reis-Filho, J.S.; Ashworth, A. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 2008, 451, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Larminat, F.; Germanier, M.; Papouli, E.; Defais, M. Deficiency in BRCA2 leads to increase in non-conservative homologous recombination. Oncogene 2002, 21, 5188–5192. [Google Scholar] [CrossRef][Green Version]
- Baumann, P.; West, S.C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci. 1998, 23, 247–251. [Google Scholar] [CrossRef]
- Ivanov, E.L.; Sugawara, N.; Fishman-Lobell, J.; Haber, J.E. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics 1996, 142, 693–704. [Google Scholar] [CrossRef]
- Kang, L.E.; Symington, L.S. Aberrant double-strand break repair in rad51 mutants of Saccharomyces cerevisiae. Mol. Cell. Biol. 2000, 20, 9162–9172. [Google Scholar] [CrossRef][Green Version]
- Zdzienicka, M.Z.; Simons, J.W. Mutagen-sensitive cell lines are obtained with a high frequency in V79 Chinese hamster cells. Mutat. Res. 1987, 178, 235–244. [Google Scholar] [CrossRef]
- Chaung, W.; Mi, L.J.; Boorstein, R.J. The p53 status of Chinese hamster V79 cells frequently used for studies on DNA damage and DNA repair. Nucleic Acids Res. 1997, 25, 992–994. [Google Scholar] [CrossRef]
- Dominguez-Bendala, J.; Priddle, H.; Clarke, A.; McWhir, J. Elevated expression of exogenous Rad51 leads to identical increases in gene-targeting frequency in murine embryonic stem (ES) cells with both functional and dysfunctional p53 genes. Exp. Cell Res. 2003, 286, 298–307. [Google Scholar] [CrossRef]
- Aladjem, M.I.; Spike, B.T.; Rodewald, L.W.; Hope, T.J.; Klemm, M.; Jaenisch, R.; Wahl, G.M. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol. 1998, 8, 145–155. [Google Scholar] [CrossRef]
- Schmidt-Kastner, P.K.; Jardine, K.; Cormier, M.; McBurney, M.W. Absence of p53-dependent cell cycle regulation in pluripotent mouse cell lines. Oncogene 1998, 16, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Berrozpe, G.; Schaeffer, J.; Peinado, M.A.; Real, F.X.; Perucho, M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int. J. Cancer 1994, 58, 185–191. [Google Scholar] [CrossRef]
- Roy, S.; Tomaszowski, K.H.; Luzwick, J.W.; Park, S.; Li, J.; Murphy, M.; Schlacher, K. p53 orchestrates DNA replication restart homeostasis by suppressing mutagenic RAD52 and POLθ pathways. eLIFE 2018, 7, e31723. [Google Scholar] [CrossRef]
- Onaka, A.T.; Su, J.; Katahira, Y.; Tang, C.; Zafar, F.; Aoki, K.; Kagawa, W.; Niki, H.; Iwasaki, H.; Nakagawa, T. DNA replication machinery prevents Rad52-dependent single-strand annealing that leads to gross chromosomal rearrangements at centromeres. Commun. Biol. 2020, 3, 202. [Google Scholar] [CrossRef]
- Schlacher, K.; Christ, N.; Siaud, N.; Egashira, A.; Wu, H.; Jasin, M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 2011, 145, 529–542. [Google Scholar] [CrossRef]
| Genotype | Number of RPE | Avg. # of Eye Spots per RPE |
|---|---|---|
| Conditional control | 31 | 4.81 |
| * CC control | 41 | 4.76 |
| Brca2wt/co11 | 10 | 4.6 |
| Brca2∆11/co11 | 15 | 1.53 |
| Brca2co/- | 29 | 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, A.D.; Greenman, S.; Claybon, A.B.; Bishop, A.J.R. BRCA2 Promotes Spontaneous Homologous Recombination In Vivo. Cancers 2021, 13, 3663. https://doi.org/10.3390/cancers13153663
Brown AD, Greenman S, Claybon AB, Bishop AJR. BRCA2 Promotes Spontaneous Homologous Recombination In Vivo. Cancers. 2021; 13(15):3663. https://doi.org/10.3390/cancers13153663
Chicago/Turabian StyleBrown, Adam D., Scott Greenman, Alison B. Claybon, and Alexander J. R. Bishop. 2021. "BRCA2 Promotes Spontaneous Homologous Recombination In Vivo" Cancers 13, no. 15: 3663. https://doi.org/10.3390/cancers13153663
APA StyleBrown, A. D., Greenman, S., Claybon, A. B., & Bishop, A. J. R. (2021). BRCA2 Promotes Spontaneous Homologous Recombination In Vivo. Cancers, 13(15), 3663. https://doi.org/10.3390/cancers13153663






