Exploring Differentially Methylated Genes in Vulvar Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
2.2. Tissue Procurement
2.3. Tissue Processing
2.4. Immunohistochemistry
2.5. DNA-Isolation
2.6. Methylation Assay
2.7. Statistical Analysis
3. Results
3.1. Clinico-Pathological Information
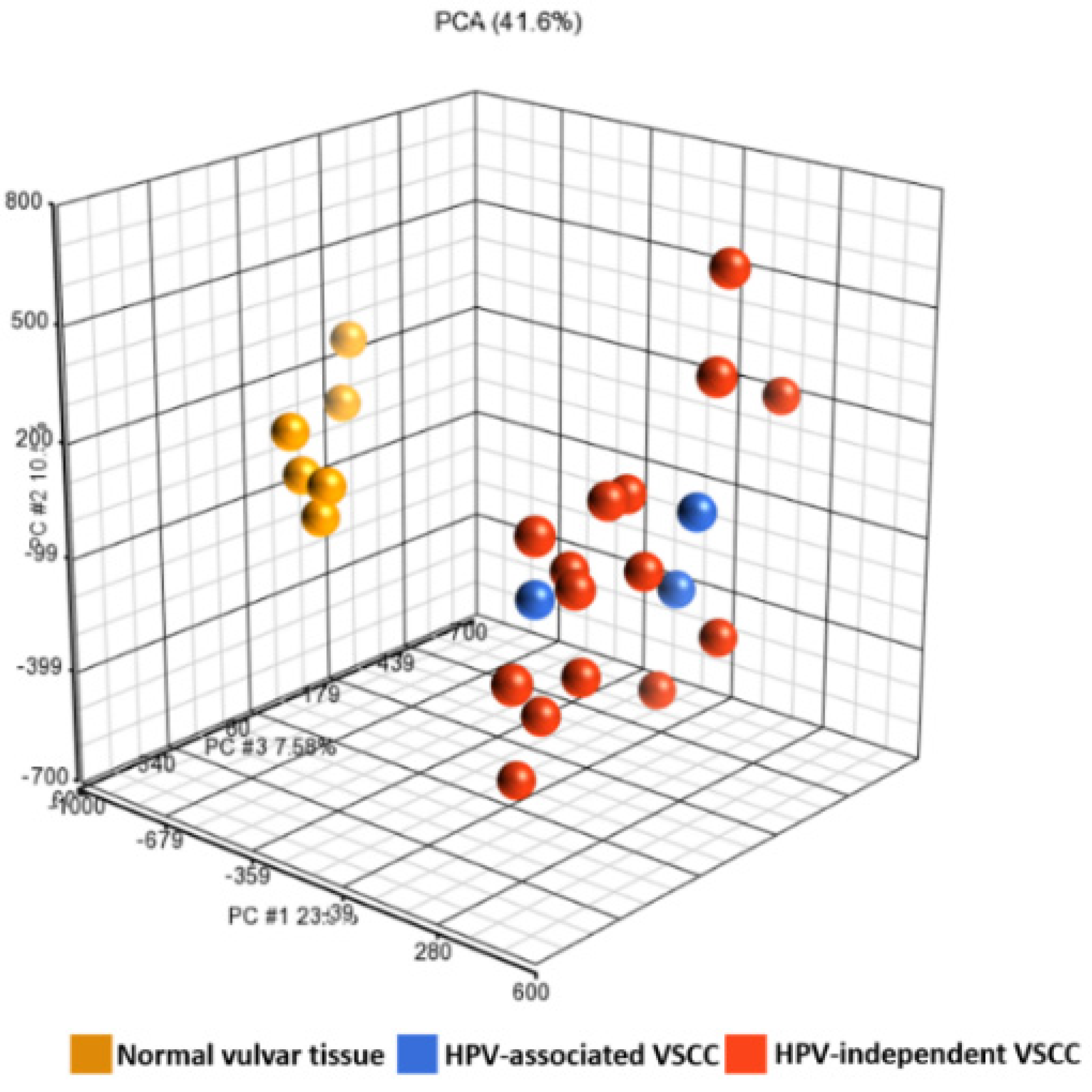
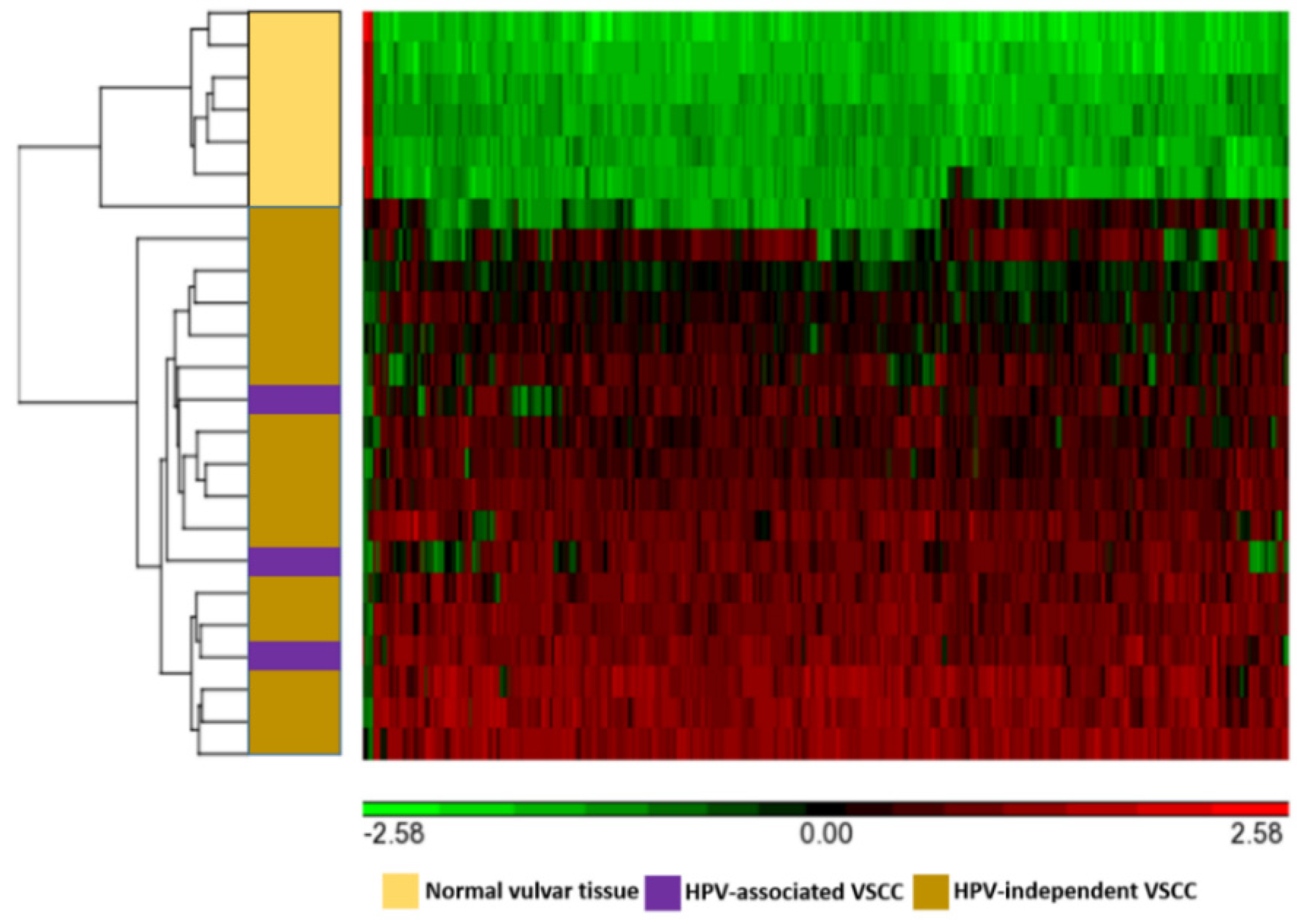
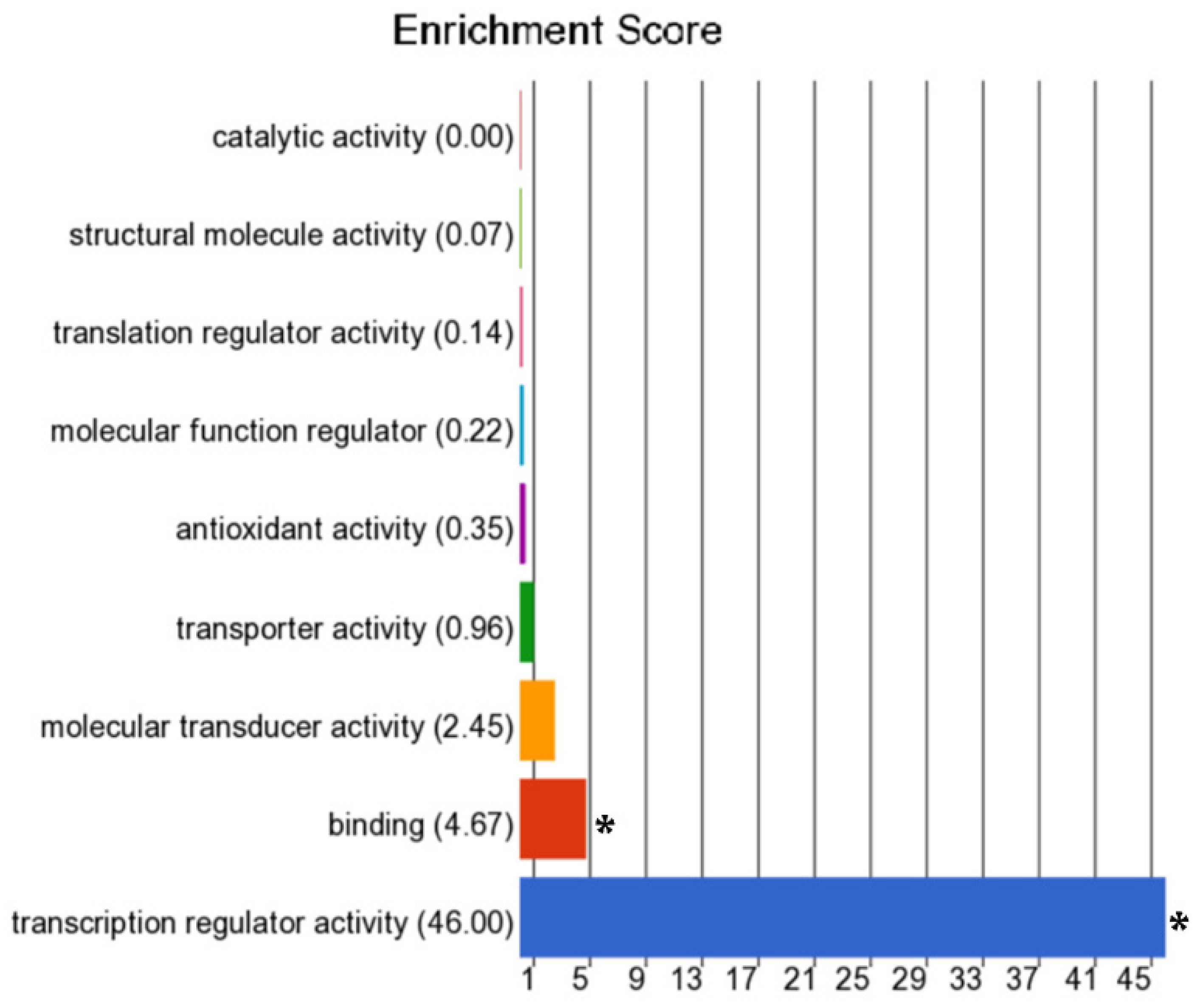
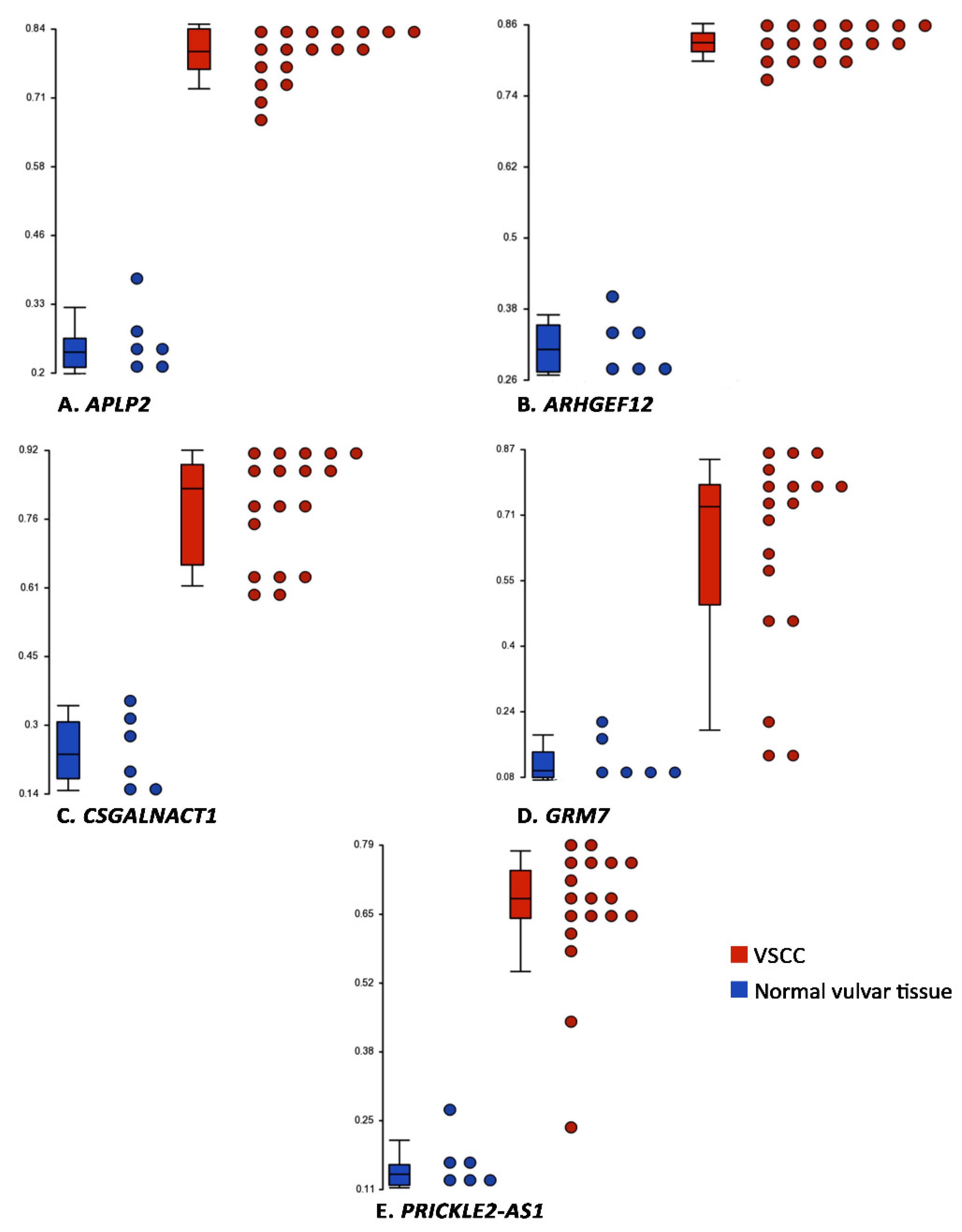
3.2. Differentially Methylated Genes
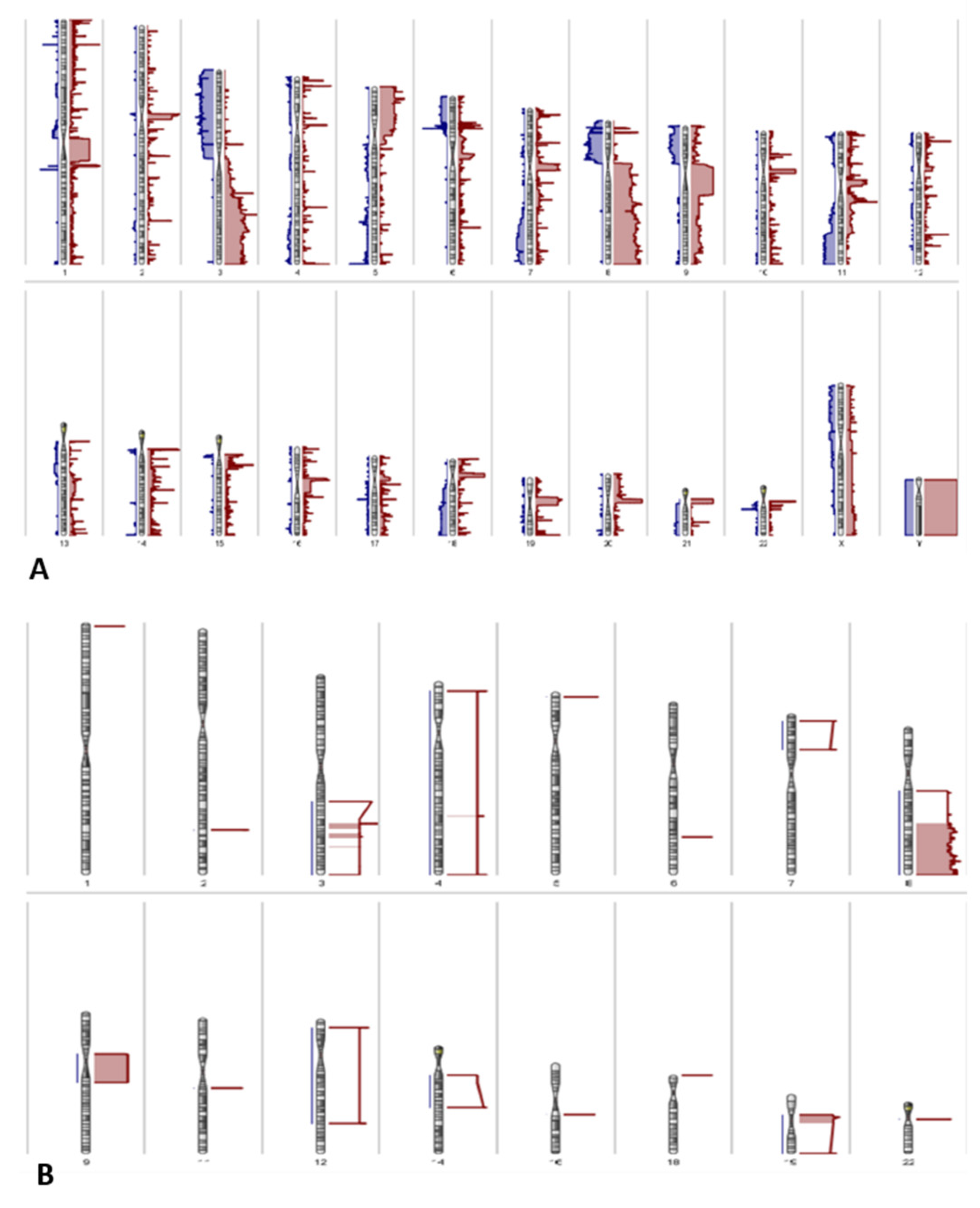
3.3. Copy Number Variations
3.4. Integration of Differential Methylation and Copy Number Variation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eva, L.J.; Sadler, L.; Fong, K.L.; Sahota, S.; Jones, R.W.; Bigby, S.M. Trends in HPV-dependent and HPV-independent vulvar cancers: The changing face of vulvar squamous cell carcinoma. Gynecol. Oncol. 2020, 157, 450–455. [Google Scholar] [CrossRef]
- Prieske, K.; Woelber, L.; Muallem, M.Z.; Eulenburg, C.; Jueckstock, J.K.; Hilpert, F.; de Gregorio, N.; Iborra, S.; Ignatov, A.; Hillemanns, P.; et al. Age, treatment and prognosis of patients with squamous cell vulvar cancer (VSCC)-analysis of the AGO-CaRE-1 study. Gynecol. Oncol. 2021, 161, 442–448. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.J.; Carcangiu, M.L.; Young, R.H.; Herrington, C.S. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Dasgupta, S.; Ewing-Graham, P.C.; Swagemakers, S.M.; van der Spek, P.J.; van Doorn, H.C.; Hegt, V.N.; Koljenović, S.; van Kemenade, F.J. Precursor lesions of vulvar squamous cell carcinoma—Histology and biomarkers: A systematic review. Crit. Rev. Oncol. 2020, 147, 102866. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gilks, C.B. Vulval squamous cell carcinoma and its precursors. Histopathology 2019, 76, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Prieske, K.; Alawi, M.; Oliveira-Ferrer, L.; Jaeger, A.; Eylmann, K.; Burandt, E.; Schmalfeldt, B.; Joosse, S.A.; Woelber, L. Genomic characterization of vulvar squamous cell carcinoma. Gynecol. Oncol. 2020, 158, 547–554. [Google Scholar] [CrossRef]
- Cohen, P.A.; Anderson, L.; Eva, L.; Scurry, J. Clinical and molecular classification of vulvar squamous pre-cancers. Int. J. Gynecol. Cancer 2019, 29, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.E.; Bastiaannet, E.; van Doorn, H.C.; van Steenwijk, P.J.; Ewing-Graham, P.C.; Creutzberg, C.L.; Akdeniz, K.; Nooij, L.S.; van der Burg, S.H.; Bosse, T.; et al. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol. Oncol. 2020, 159, 649–656. [Google Scholar] [CrossRef]
- Nooij, L.S.; Ter Haar, N.T.; Ruano, D.; Rakislova, N.; van Wezel, T.; Smit, V.; Trimbos, B.; Ordi, J.; van Poelgeest, M.I.E.; Bosse, T. Genomic Characterization of Vulvar (Pre)cancers Identifies Distinct Molecular Subtypes with Prognostic Significance. Clin. Cancer Res. 2017, 23, 6781–6789. [Google Scholar] [CrossRef] [Green Version]
- Weberpals, J.I.; Lo, B.; Duciaume, M.M.; Spaans, J.N.; Clancy, A.A.; Dimitroulakos, J.; Goss, G.D.; Sekhon, H.S. Vulvar Squamous Cell Carcinoma (VSCC) as Two Diseases: HPV Status Identifies Distinct Mutational Profiles Including Oncogenic Fibroblast Growth Factor Receptor 3. Clin. Cancer Res. 2017, 23, 4501–4510. [Google Scholar] [CrossRef] [Green Version]
- Han, M.R.; Shin, S.; Park, H.C.; Kim, M.S.; Lee, S.H.; Jung, S.H.; Song, S.Y.; Lee, S.H.; Chung, Y.J. Mutational signatures and chromosome alteration profiles of squamous cell carcinomas of the vulva. Exp. Mol. Med. 2018, 50, e442. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N.; et al. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology Version 3.2020—Vulvar Cancer (Squamous Cell Carcinoma). Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 18 May 2021).
- Zach, D.; Åvall-Lundqvist, E.; Falconer, H.; Hellman, K.; Johansson, H.; Flöter Rådestad, A. Patterns of recurrence and survival in vulvar cancer: A nationwide population-based study. Gynecol. Oncol. 2021, 161, 748–754. [Google Scholar] [CrossRef]
- Gaarenstroom, K.N.; Kenter, G.G.; Trimbos, J.B.; Agous, I.; Amant, F.; Peters, A.A.W.; Vergote, I. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int. J. Gynecol. Cancer 2003, 13, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Bader, J.S.; Braun, T.P.; Califano, A.; Clemons, P.A.; Druker, B.J.; Ewald, A.J.; Fu, H.; Jagu, S.; Kemp, C.J.; et al. An expanded universe of cancer targets. Cell 2021, 184, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Werth, A.J.; Sharaf, R.; Montension, M.; Sokol, E.S.; Pavlick, D.C.; McLaughlin-Drubin, M.; Erlich, R.; Toma, H.; Jon Williams, K.; et al. Vulvar Squamous Cell Carcinoma: Comprehensive Genomic Profling of HPV+ versus HPV− forms reveals distinct sets of potentially actionable targets. JCO Precis. Oncol. 2020, 4, 647–661. [Google Scholar] [CrossRef]
- Nagashima, M.; Miwa, N.; Hirasawa, H.; Katagiri, Y.; Takamatsu, K.; Morita, M. Genome-wide DNA methylation analysis in obese women predicts an epigenetic signature for future endometrial cancer. Sci. Rep. 2019, 9, 6469. [Google Scholar] [CrossRef] [Green Version]
- Matei, D.; Nephew, K.P. Epigenetic Attire in Ovarian Cancer: The Emperor’s New Clothes. Cancer Res. 2020, 80, 3775–3785. [Google Scholar] [CrossRef]
- Küster, M.M.; Schneider, M.A.; Richter, A.M.; Richtmann, S.; Winter, H.; Kriegsmann, M.; Pullamsetti, S.S.; Stiewe, T.; Savai, R.; Muley, T.; et al. Epigenetic Inactivation of the Tumor Suppressor IRX1 Occurs Frequently in Lung Adenocarcinoma and Its Silencing Is Associated with Impaired Prognosis. Cancers 2020, 12, 3528. [Google Scholar] [CrossRef]
- Buocikova, V.; Rios-Mondragon, I.; Pilalis, E.; Chatziioannou, A.; Miklikova, S.; Mego, M.; Pajuste, K.; Rucins, M.; Yama-ni, N.E.; Longhin, E.M.; et al. Epigenetics in Breast Cancer Therapy—New Strategies and Future Nanomedicine Perspectives. Cancers 2020, 12, 3622. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and Consensus Recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef] [PubMed]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, C.; Proctor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, K.E.; Solleveld-Westerink, N.; Tessier-Cloutier, B.; Rutten, T.A.; Van Poelgeest, M.I.E.; Gilks, C.B.; Hoang, L.N.; Bosse, T. Performance of the pattern-based interpretation of p53 immunohistochemistry as a surrogate for TP53 mutations in vulvar squamous cell carcinoma. Histopathology 2020, 77, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier-Cloutier, B.; Pors, J.; Thompson, E.; Ho, J.; Prentice, L.; McConechy, M.; Aguirre-Hernandez, R.; Miller, R.; Leung, S.; Proctor, L.; et al. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for morphologic diagnosis and outcome. Mod. Pathol. 2021, 34, 508–518. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar]
- Trietsch, M.D.; Nooij, L.S.; Gaarenstroom, K.N.; van Poelgeest, M.I. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: A review of the current literature. Gynecol. Oncol. 2015, 136, 143–157. [Google Scholar] [CrossRef]
- Pouwer, A.-F.W.; Einden, L.C.V.D.; Van Der Linden, M.; Hehir-Kwa, J.Y.; Yu, J.; Hendriks, K.M.; Kamping, E.J.; Eijkelenboom, A.; Massuger, L.F.; Bulten, J.; et al. Clonal Relationship Between Lichen Sclerosus, Differentiated Vulvar Intraepithelial Neoplasia and Non HPV-related Vulvar Squamous Cell Carcinoma. Cancer Genom. Proteom. 2020, 17, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Zięba, S.; Chechlińska, M.; Kowalik, A.; Kowalewska, M. Genes, pathways and vulvar carcinoma—New insights from next-generation sequencing studies. Gynecol. Oncol. 2020, 158, 498–506. [Google Scholar] [CrossRef]
- Dasgupta, S.; Koljenović, S.; van den Bosch, T.P.; Swagemakers, S.; van der Hoeven, N.; van Marion, R.; van der Spek, P.J.; van Doorn, H.C.; van Kemenade, F.J.; Ewing-Graham, P.C. Evaluation of immunohistochemical markers CK17 and SOX2 as adjuncts to p53 for the diagnosis of differentiated vulvar intraepithelial neoplasia. Pharmaceuticals 2021, 14, 324. [Google Scholar] [CrossRef]
- Swarts, D.R.; Voorham, Q.J.; van Splunter, A.P.; Wilting, S.M.; Sie, D.; Pronk, D.; van Beurden, M.; Heideman, D.A.; Snijders, P.J.; Meijer, C.; et al. Molecular heterogeneity in human papillomavirus-dependent and -independent vulvar carcinogenesis. Cancer Med. 2018, 7, 4542–4553. [Google Scholar] [CrossRef]
- Gasco, M.; Sullivan, A.; Repellin, C.; Brooks, L.; Farrell, P.J.; Tidy, J.A.; Dunne, B.; Gusterson, B.; Evans, D.J.; Crook, T. Coincident inactivation of 14-3-3sigma and p16INK4a is an early event in vulval squamous neoplasia. Oncogene 2002, 21, 1876–1881. [Google Scholar] [CrossRef] [Green Version]
- O’Nions, J.; Brooks, L.A.; Sullivan, A.; Bell, A.; Dunne, B.; Rozycka, M.; Reddy, A.; Tidy, J.A.; Evans, D.; Farrell, P.J.; et al. p73 is over-expressed in vulval cancer principally as the Delta 2 isoform. Br. J. Cancer 2001, 85, 1551–1556. [Google Scholar] [CrossRef]
- Oonk, M.H.; Eijsink, J.J.; Volders, H.H.; Hollema, H.; Wisman, G.B.; Schuuring, E.; Van Der Zee, A.G. Identification of inguinofemoral lymph node metastases by methylation markers in vulvar cancer. Gynecol. Oncol. 2012, 125, 352–357. [Google Scholar] [CrossRef]
- Leonard, S.; Pereira, M.; Fox, R.; Gordon, N.; Yap, J.; Kehoe, S.; Luesley, D.; Woodman, C.; Ganesan, R. Over-expression of DNMT3A predicts the risk of recurrent vulvar squamous cell carcinomas. Gynecol. Oncol. 2016, 143, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Setas, D.; Perez-Janices, N.; Ojer, A.; Blanco-Fernandez, L.; Guarch-Troyas, C.; Guarch, R. Differential gene hypermethylation in genital lichen sclerosus and cancer: A comparative study. Histopathology 2013, 63, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Soufir, N.; Queille, S.; Liboutet, M.; Thibaudeau, O.; Bachelier, F.; Delestaing, G.; Balloy, B.C.; Breuer, J.; Janin, A.; Dubertret, L.; et al. Inactivation of the CDKN2A and the p53 tumour suppressor genes in external genital carcinomas and their precursors. Br. J. Dermatol. 2006, 156, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Thuijs, N.B.; Berkhof, J.; Özer, M.; Duin, S.; van Splunter, A.P.; Snoek, B.C.; Heideman, D.A.M.; van Beurden, M.; Steenbergen, R.D.M.; Bleeker, M.C.G. DNA methylation markers for cancer risk prediction of vulvar intraepithelial neoplasia. Int. J. Cancer 2021, 148, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Verlaat, W.; Snijders, P.J.; Novianti, P.W.; Wilting, S.M.; De Strooper, L.M.; Trooskens, G.; Vandersmissen, J.; Van Criekinge, W.; Wisman, G.B.; Meijer, C.J.; et al. Genome-wide DNA Methylation Profiling Reveals Methylation Markers Associated with 3q Gain for Detection of Cervical Precancer and Cancer. Clin. Cancer Res. 2017, 23, 3813–3822. [Google Scholar] [CrossRef] [Green Version]
- Verlaat, W.; Snoek, B.C.; Heideman, D.A.; Wilting, S.; Snijders, P.J.; Novianti, P.W.; Van Splunter, A.P.; Peeters, C.F.; Van Trommel, N.E.; Massuger, L.F.; et al. Identification and Validation of a 3-Gene Methylation Classifier for HPV-Based Cervical Screening on Self-Samples. Clin. Cancer Res. 2018, 24, 3456–3464. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.F.; Chan, W.Y. The de novo DNA methyltransferase DNMT3A in development and cancer. Epigenetics 2014, 9, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Rotondo, J.C.; Borghi, A.; Selvatici, R.; Magri, E.; Bianchini, E.; Montinari, E.; Corazza, M.; Virgili, A.; Tognon, M.; Martini, F. Hypermethylation-Induced Inactivation of the IRF6 Gene as a Possible Early Event in Progression of Vulvar Squamous Cell Carcinoma Associated with Lichen Sclerosus. JAMA Derm. 2016, 152, 928–933. [Google Scholar] [CrossRef]
- Da Silva, M.L.R.; De Albuquerque, B.; Allyrio, T.A.; De Almeida, V.D.; Cobucci, R.N.O.; Bezerra, F.L.; Andrade, V.S.; Lan-za, D.C.F.; De Azevedo, J.C.V.; De Araújo, J.M.G.; et al. The role of HPV-induced epigenetic changes in cervical carcinogenesis (Review). Biomed. Rep. 2021, 15, 60. [Google Scholar] [CrossRef]
- Choudhury, J.H.; Ghosh, S.K. Promoter Hypermethylation Profiling Identifies Subtypes of Head and Neck Cancer with Distinct Viral, Environmental, Genetic and Survival Characteristics. PLoS ONE 2015, 10, e0129808. [Google Scholar] [CrossRef] [Green Version]
- Feber, A.; Guilhamon, P.; Lechner, M.; Fenton, T.; Wilson, G.A.; Thirlwell, C.; Morris, T.J.; Flanagan, A.M.; Teschendorff, A.E.; Kelly, J.D.; et al. Using high-density DNA methylation arrays to profile copy number alterations. Genome Biol. 2014, 15, R30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadlullah, M.Z.H.; Chiang, I.K.N.; Dionne, K.R.; Yee, P.S.; Gan, C.P.; Sam, K.K.; Tiong, K.H.; Ng, K.W.; Martin, D.; Lim, K.P.; et al. Genetically-defined novel oral squamous cell carcinoma cell lines for the development of molecular therapies. Oncotarget 2016, 7, 27802–27818. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Abba, M.C.; Molinolo, A.A.; Vitale-Cross, L.; Wang, Z.; Zaida, M.; Delic, N.C.; Samuels, Y.; Lyons, J.G.; Gutkind, J.S. The head and neck cancer cell oncogenome: A platform for the development of precision molecular therapies. Oncotarget 2014, 5, 8906–8923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Diagnoses | ||
|---|---|---|---|
| HPV-Independent VSCC (n = 15) | HPV-Associated VSCC (n = 3) | Normal Vulvar Tissue (n = 6) | |
| Median Age (Range) in years | 76 (52–90) | 73 (65–81) | 75 (52–90) |
| Location | |||
| Vulvar skin with adnexa | 8 (53) | 1 (33) | 1 (17) |
| Vulvar skin without adnexa | 7 (47) | 2 (67) | 5 (83) |
| Treatment | |||
| Excision with lymph node dissection/sentinel node procedure | 14 (94) | 3 (100) | 0 (0) |
| Excision only | 1 (6) | 0 (0) | 6 (100) |
| Follow-up | |||
| No evidence of VIN/VSCC | 7 (47) | 2 (67) | 6 (100) |
| VIN | 1 (6) | 0 (0) | 0 (0) |
| VSCC recurrence | 6 (41) | 1 (33) | 0 (0) |
| Died | 1 (6) | 0 (0) | 0 (0) |
| p16-expression | |||
| Block-type | 0 (0) | 3 (100) | 0 (0) |
| Non-block-type | 8 (53) | 0 (0) | 1 (17) |
| No expression | 7 (47) | 0 (0) | 5 (83) |
| p53-expression | |||
| Diffuse overexpression | 9 (60) | 1 (33) | 0 (0) |
| Basal overexpression | 1 (7) | 0 (0) | 0 (0) |
| Null pattern | 3 (20) | 0 (0) | 0 (0) |
| Wild-type expression (scattered) | 2 (13) | 0 (0) | 6 (100) |
| Wild-type expression (mid-epithelial) | 0 (0) | 2 (67) | 0 (0) |
| Symbol | Entrez Gene Name | Δβ-Values | Location | Type(s) |
|---|---|---|---|---|
| C1QTNF4 | C1q and TNF related 4 | 0.698 | Extracellular Space | other |
| TNR | tenascin R | 0.676 | Plasma Membrane | other |
| ZSCAN1 | zinc finger and SCAN domain containing 1 | 0.67 | Nucleus | transcription regulator |
| ZNF135 | zinc finger protein 135 | 0.662 | Nucleus | transcription regulator |
| ZNF471 | zinc finger protein 471 | 0.66 | Nucleus | transcription regulator |
| CCDC8 | coiled-coil domain containing 8 | 0.63 | Plasma Membrane | other |
| TBX3 | T-box transcription factor 3 | 0.626 | Nucleus | transcription regulator |
| LOC728392 | uncharacterized LOC728392 | 0.625 | Other | other |
| SSTR1 | somatostatin receptor 1 | 0.621 | Plasma Membrane | G-protein coupled receptor |
| MARCHF11 | membrane associated ring-CH-type finger 11 | 0.617 | Other | other |
| UNC79 | unc-79 homolog, NALCN channel complex subunit | 0.615 | Other | other |
| ACSF2 | acyl-CoA synthetase family member 2 | 0.614 | Cytoplasm | enzyme |
| ZIK1 | zinc finger protein interacting with K protein 1 | 0.613 | Other | other |
| KCNC2 | potassium voltage-gated channel subfamily C member 2 | 0.609 | Plasma Membrane | ion channel |
| PABPC5 | poly(A) binding protein cytoplasmic 5 | 0.607 | Cytoplasm | other |
| PPFIA3 | PTPRF interacting protein alpha 3 | 0.604 | Plasma Membrane | phosphatase |
| BARHL2 | BarH-like homeobox 2 | 0.603 | Nucleus | transcription regulator |
| BOLL | boule homolog, RNA binding protein | 0.603 | Cytoplasm | translation regulator |
| ONECUT2 | one cut homeobox 2 | 0.601 | Nucleus | transcription regulator |
| HAS1 | hyaluronan synthase 1 | 0.6 | Plasma Membrane | enzyme |
| CMTM2 | CKLF-like MARVEL transmembrane domain containing 2 | 0.596 | Extracellular Space | cytokine |
| ZIC4 | Zic family member 4 | 0.596 | Nucleus | transcription regulator |
| LBX2 | ladybird homeobox 2 | 0.595 | Nucleus | transcription regulator |
| SALL1 | spalt-like transcription factor 1 | 0.592 | Nucleus | transcription regulator |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, S.; Ewing-Graham, P.C.; Swagemakers, S.M.A.; van den Bosch, T.P.P.; Atmodimedjo, P.N.; Verbiest, M.M.P.J.; de Haan, M.; van Doorn, H.C.; van der Spek, P.J.; Koljenović, S.; et al. Exploring Differentially Methylated Genes in Vulvar Squamous Cell Carcinoma. Cancers 2021, 13, 3580. https://doi.org/10.3390/cancers13143580
Dasgupta S, Ewing-Graham PC, Swagemakers SMA, van den Bosch TPP, Atmodimedjo PN, Verbiest MMPJ, de Haan M, van Doorn HC, van der Spek PJ, Koljenović S, et al. Exploring Differentially Methylated Genes in Vulvar Squamous Cell Carcinoma. Cancers. 2021; 13(14):3580. https://doi.org/10.3390/cancers13143580
Chicago/Turabian StyleDasgupta, Shatavisha, Patricia C. Ewing-Graham, Sigrid M. A. Swagemakers, Thierry P. P. van den Bosch, Peggy N. Atmodimedjo, Michael M. P. J. Verbiest, Marit de Haan, Helena C. van Doorn, Peter J. van der Spek, Senada Koljenović, and et al. 2021. "Exploring Differentially Methylated Genes in Vulvar Squamous Cell Carcinoma" Cancers 13, no. 14: 3580. https://doi.org/10.3390/cancers13143580
APA StyleDasgupta, S., Ewing-Graham, P. C., Swagemakers, S. M. A., van den Bosch, T. P. P., Atmodimedjo, P. N., Verbiest, M. M. P. J., de Haan, M., van Doorn, H. C., van der Spek, P. J., Koljenović, S., & van Kemenade, F. J. (2021). Exploring Differentially Methylated Genes in Vulvar Squamous Cell Carcinoma. Cancers, 13(14), 3580. https://doi.org/10.3390/cancers13143580






