Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
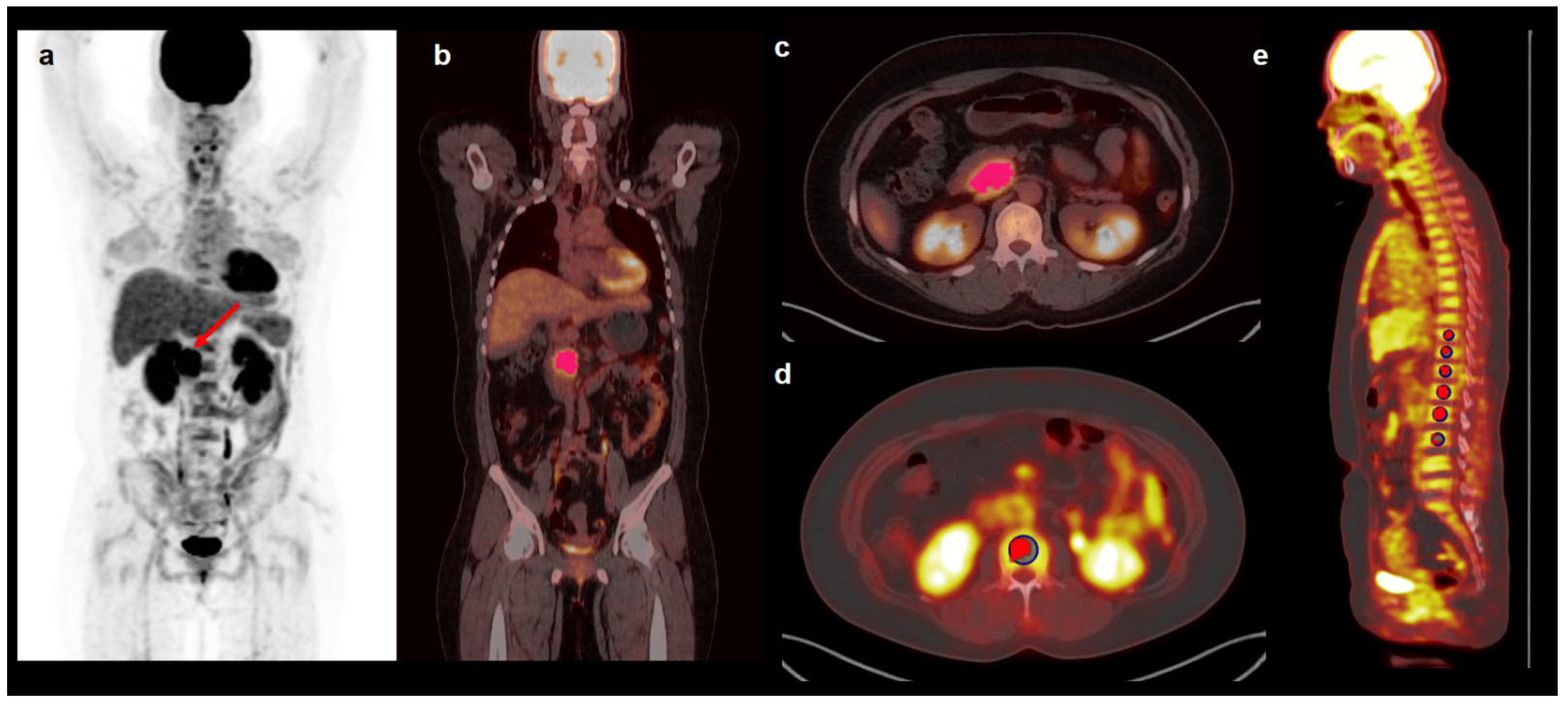
2.2. FDG PET/CT
2.3. PET/CT Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Survival Analysis
3.3. PET/CT Scoring System for Predicting Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Narayanan, S.; AlMasri, S.; Zenati, M.; Nassour, I.; Chopra, A.; Rieser, C.; Smith, K.; Oyefusi, V.; Daum, T.; Bahary, K.S.; et al. Predictors of early recurrence following neoadjuvant chemotherapy and surgical resection for localized pancreatic adenocarcinoma. J. Surg. Oncol. 2021. [Google Scholar] [CrossRef]
- Lau, S.C.; Cheung, W.Y. Evolving treatment landscape for early and advanced pancreatic cancer. World J. Gastrointest. Oncol. 2017, 9, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Belfiori, G.; Bissolati, M.; Partelli, S.; Pagnanelli, M.; Tamburrino, D.; Gasparini, G.; Rubini, C.; Zamboni, G.; Falconi, M. Recurrence after surgical resection of pancreatic cancer: The importance of postoperative complications beyond tumor biology. HPB 2021. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Hank, T.; Hinz, U.; Bergmann, F.; Schneider, L.; Springfeld, C.; Jäger, D.; Schirmacher, P.; Hackert, T.; Büchler, M.W. Pancreatic Cancer Surgery: The New R-status Counts. Ann. Surg 2016, 265, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Kim, H.S.; Choi, S.H.; Choi, D.W.; Lee, J.K.; Lee, K.H.; Park, J.O.; Lee, K.-H.; Kim, B.-T.; Choi, J.Y. Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1461–1468. [Google Scholar] [CrossRef]
- Osayi, S.N.; Bloomston, M.; Schmidt, C.M.; Ellison, E.C.; Muscarella, P. Biomarkers as predictors of recurrence following cu-rative resection for pancreatic ductal adenocarcinoma: A review. BioMed Res. Int. 2014, 2014, 468959. [Google Scholar] [CrossRef]
- Thyagarajan, A.; AlShehri, M.S.A.; Miller, K.L.; Sherwin, C.M.; Travers, J.B.; Sahu, R.P. Myeloid-Derived Suppressor Cells and Pancreatic Cancer: Implications in Novel Therapeutic Approaches. Cancers 2019, 11, 1627. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, T.; Huang, L.; Wang, H.; Zhang, L.; Wang, Z.; Cui, Y. Neutrophils infiltrating pancreatic ductal adenocar-cinoma indicate higher malignancy and worse prognosis. Biochem. Biophys. Res. Commun. 2018, 501, 313–319. [Google Scholar] [CrossRef]
- Iwai, N.; Okuda, T.; Sakagami, J.; Harada, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Hara, T.; Tsuji, T.; et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci. Rep. 2020, 10, 18758. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, M.; Choi, J.Y. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics 2020, 10, 952. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M.; Chung, Y.A. Prognostic value of CT attenuation and FDG uptake of adipose tissue in patients with pan-creatic adenocarcinoma. Clin. Radiol. 2018, 73, 1056.e1–1056.e10. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, C.M.; Choi, H.J.; Lee, W.J.; Song, S.Y.; Lee, J.H.; Lee, J.D. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J. Nucl. Med. 2014, 55, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Osipov, A.; Fraass, B.; Sandler, H.; Zhang, X.; Nissen, N.; Hendifar, A.; Tuli, R. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J. Gastrointest. Oncol. 2017, 8, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Na, J.O.; Kang, D.Y.; Lee, S.Y.; Lee, S.M. Prognostic significance of FDG uptake of bone marrow on pet/ct in patients with non-small-cell lung cancer after curative surgical resection. Clin. Lung Cancer 2017, 18, 198–206. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Lee, H.J.; Heo, N.H.; Lee, S.M. [18F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimura, K.; Mabuchi, S.; Komura, N.; Yokoi, E.; Kozasa, K.; Sasano, T.; Kawano, M.; Matsumoto, Y.; Watabe, T.; Kodama, M.; et al. Prognostic significance of bone marrow FDG uptake in patients with gynecological cancer. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; Lapi, S.E. Positron emission tomography imaging of macrophages in cancer. Cancers (Basel) 2021, 13, 1921. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ban, M.J.; Park, J.H.; Lee, S.M. Effect of F-18 Fluorodeoxyglucose Uptake by Bone Marrow on the Prognosis of Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A. Paget’s "Seed and Soil" theory of cancer metastasis: An idea whose time has come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.G.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, U.; Kremp, S.; Schaefer-Schuler, A.; Sebastian-Welsch, C.; Hellwig, D.; Rübe, C.; Kirsch, C.-M. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J. Nucl. Med. 2005, 46, 1342–1348. [Google Scholar]
- Maisonobe, J.-A.; Garcia, C.A.; Necib, H.; Vanderlinden, B.; Hendlisz, A.; Flamen, P.; Buvat, I. Comparison of PET metabolic indices for the early assessment of tumour response in metastatic colorectal cancer patients treated by polychemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Ha, S.; Kwon, S.J.; Park, S.Y.; Kim, J.; Yoo, I.R. Prognostic value of tumor metabolic imaging phenotype by FDG PET radiomics in HNSCC. Ann. Nucl. Med. 2021, 35, 370–377. [Google Scholar] [CrossRef]
- Ha, S.; Choi, H.; Paeng, J.C.; Cheon, G.J. Radiomics in Oncological PET/CT: A Methodological Overview. Nucl. Med. Mol. Imaging 2019, 53, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2006, 165, 710–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Seo, K.H.; Kim, E.-S.; Lee, S.M. The role of 18F-fluorodeoxyglucose uptake of bone marrow on PET/CT in predicting clinical outcomes in non-small cell lung cancer patients treated with chemoradiotherapy. Eur. Radiol. 2016, 27, 1912–1921. [Google Scholar] [CrossRef]
- Murata, Y.; Kubota, K.; Yukihiro, M.; Ito, K.; Watanabe, H.; Shibuya, H. Correlations between 18F-FDG uptake by bone marrow and hematological parameters: Measurements by PET/CT. Nucl. Med. Biol. 2006, 33, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, M.-S.; Chung, I.K.; Son, M.W.; Cho, Y.S.; Lee, S.M. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J. Gastroenterol. 2017, 23, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Wang, L.L.; Liu, B.L.; Hong, J.J.; Xu, N.N.; Tang, K.; Zheng, X.W. Association between bone marrow fluorodeoxy-glucose uptake and recurrence after curative surgical resection in patients with T1-2N0M0 lung adenocarcinoma: A retro-spective cohort study. Quant. Imaging Med. Surg. 2020, 10, 2285–2296. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, J.S.; Lyu, J.; Lee, S.M. Prognostic significance of 18 F-fluorodeoxyglucose uptake of bone marrow measured on positron emission tomography in patients with small cell lung cancer. Lung Cancer 2018, 118, 41–47. [Google Scholar] [CrossRef]
- Lee, J.W.; Baek, M.-J.; Ahn, T.S.; Lee, S.M. Fluorine-18-fluorodeoxyglucose uptake of bone marrow on PET/CT can predict prognosis in patients with colorectal cancer after curative surgical resection. Eur. J. Gastroenterol. Hepatol. 2018, 30, 187–194. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeon, S.; Mun, S.T.; Lee, S.M. Prognostic value of fluorine-18 fluorodeoxyglucose uptake of bone marrow on positron emission tomography/computed tomography for prediction of disease progression in cervical cancer. Int. J. Gynecol. Cancer 2017, 27, 776–783. [Google Scholar] [CrossRef]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Iwamoto, C.; Ohuchida, K.; Shinkawa, T.; Okuda, S.; Otsubo, Y.; Okumura, T.; Sagara, A.; Koikawa, K.; Ando, Y.; Shindo, K.; et al. Bone marrow-derived macrophages converted into cancer-associated fibroblast-like cells promote pancreatic cancer progression. Cancer Lett. 2021, 512, 15–27. [Google Scholar] [CrossRef]
- Oweida, A.J.; Mueller, A.C.; Piper, M.; Milner, D.; Van Court, B.; Bhatia, S.; Phan, A.; Bickett, T.; Jordan, K.; Proia, T.; et al. Response to radiotherapy in pancreatic ductal adenocarcinoma is enhanced by inhibition of myeloid-derived suppressor cells using STAT3 anti-sense oligonucleotide. Cancer Immunol. Immunother. 2021, 70, 989–1000. [Google Scholar] [CrossRef]
- Wang, W.; Yan, L.; Guan, X.; Dong, B.; Zhao, M.; Wu, J.; Tian, X.; Hao, C. Identification of an Immune-Related Signature for Predicting Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021, 10, 618215. [Google Scholar] [CrossRef]
- Leijenaar, R.T.H.; Carvalho, S.; Velazquez, E.R.; Van Elmpt, W.J.C.; Parmar, C.; Hoekstra, O.S.; Hoekstra, C.J.; Boellaard, R.; Dekker, A.L.A.J.; Gillies, R.; et al. Stability of FDG-PET Radiomics features: An integrated analysis of test-retest and inter-observer variability. Acta Oncol. 2013, 52, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Chu-Shern, J.L.; Loi, H.Y.; Khor, L.K.; Sinha, A.K.; Quek, S.T.; Tham, I.W.; Townsend, D. Impact of image recon-struction settings on texture features in 18F-FDG PET. J. Nucl. Med. 2015, 56, 1667–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited-The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwa, T.; Kobayashi, M.; Nam, J.-M.; Harada, H. Tumor microenvironment and radioresistance. Exp. Mol. Med. 2021, 53, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Mattonen, S.A.; Davidzon, G.A.; Benson, J.; Leung, A.N.C.; Vasanawala, M.; Horng, G.; Shrager, J.B.; Napel, S.; Nair, V.S. Bone Marrow and Tumor Radiomics at 18F-FDG PET/CT: Impact on Outcome Prediction in Non–Small Cell Lung Cancer. Radiol. 2019, 293, 451–459. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number of Patients (%) | |
|---|---|---|
| Age (years) | 66 (40–89) * | |
| Sex | Men | 34 (52.3%) |
| Women | 31 (47.7%) | |
| Tumor location | Head/neck | 37 (56.9%) |
| Body | 13 (20.0%) | |
| Tail | 15 (23.1%) | |
| Tumor size (cm) | 3.7 (1.9–8.7) * | |
| T classification | T1–T2 | 9 (13.8%) |
| T3–T4 | 56 (86.1%) | |
| N classification | N0 | 35 (53.8%) |
| N1 | 30 (46.2%) | |
| M classification | M0 | 53 (81.5%) |
| M1 | 12 (18.5%) | |
| Clinical TNM stage | I | 6 (9.2%) |
| II | 28 (43.1%) | |
| III | 19 (29.2%) | |
| IV | 12 (18.5%) | |
| Serum CEA (ng/mL) | 4.32 (0.56–100,000.00) * | |
| Serum CA19-9 (U/mL) | 138.5 (0.6–4628.0) * | |
| NLR | 2.26 (0.80–21.74) * | |
| PLR | 150.22 (56.96–1574.51) * | |
| PET/CT parameters | Maximum SUV of primary tumor | 7.01 (3.55–22.47) * |
| MTV of primary tumor (cm3) | 15.58 (1.91–77.37) * | |
| TLG of primary tumor (g) | 55.54 (10.11–845.87) * | |
| BM SUV | 1.74 (1.13–2.91) * | |
| BLR | 0.86 (0.49–1.86) * | |
| Treatment | Surgical resection | 37 (56.9%) |
| Concurrent chemotherapy | 13 (20.0%) | |
| Chemotherapy alone | 11 (16.9%) | |
| Radiotherapy alone | 4 (6.2%) | |
| Variables | p-Value | Hazard Ratio (95% Confidence Interval) | C-Index | |
|---|---|---|---|---|
| Age (≤65 years vs. >65 years) | 0.020 | 2.26 (1.14–4.48) | 0.632 | |
| Sex (women vs. men) | 0.346 | 0.73 (0.38–1.41) | 0.574 | |
| Clinical TNM stage | Stage I–II vs. stage III | 0.025 | 2.41 (1.12–5.20) | |
| Stage I–II vs. stage IV | <0.001 | 5.07 (2.18–11.78) | 0.690 | |
| Serum CEA (≤5.00 ng/mL vs. >5.00 ng/mL) | 0.002 | 2.93 (1.49–5.74) | 0.663 | |
| CA19-9 (≤103.0 U/mL vs. >103.0 U/mL) | 0.021 | 2.28 (1.13–4.59) | 0.632 | |
| NLR (≤4.17 vs. >4.17) | 0.047 | 2.03 (1.01–4.10) | 0.642 | |
| PLR (≤217.54 vs. >217.54) | 0.068 | 1.85 (0.96–3.57) | 0.625 | |
| Treatment (surgery vs. others treatments) | 0.048 | 1.91 (1.02–3.70) | 0.602 | |
| BM imaging parameters | BM SUV (≤1.53 vs. >1.53) | 0.002 | 4.47 (1.73–11.51) | 0.678 |
| BLR (≤0.79 vs. >0.79) | 0.003 | 4.76 (1.68–13.54) | 0.658 | |
| Conventional PET/CT parameters | Peak SUV (≤6.65 vs. >6.65) | 0.048 | 2.90 (1.01–6.53) | 0.622 |
| MTV (≤15.60 cm3 vs. >15.60 cm3) | 0.021 | 2.18 (1.12–4.21) | 0.619 | |
| TLG (≤41.40 g vs. >41.40 g) | 0.002 | 3.80 (1.65–8.72) | 0.655 | |
| First-order textural parameter | Entropy (≤3.40 vs. >3.40) | 0.002 | 2.87 (1.47–5.61) | 0.650 |
| Higher-order textural parameters | GLCM energy (≤0.012 vs. >0.012) | 0.011 | 0.43 (0.22–0.82) | 0.648 |
| GLCM entropy (≤6.45 vs. >6.45) | 0.027 | 2.10 (1.09–4.05) | 0.640 | |
| GLZLM zone length nonuniformity (≤22.03 vs. >22.03) | 0.035 | 2.03 (1.05–3.91) | 0.640 | |
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | |
| Clinical TNM stage | ||||
| Stage III | 0.042 | 1.98 (1.02–4.69) | 0.177 | 1.82 (0.76–4.36) |
| Stage IV | 0.005 | 4.71 (1.59–13.95) | 0.001 | 5.66 (2.26–15.64) |
| NLR | 0.835 | 1.11 (0.41–2.98) | 0.751 | 1.17 (0.44–3.10) |
| BM SUV | 0.005 | 4.30 (1.57–11.76) | 0.003 | 5.17 (1.76–15.16) |
| TLG | 0.037 | 2.37 (1.08–5.97) | 0.028 | 2.78 (1.12–6.95) |
| First-order entropy | 0.013 | 2.89 (1.29–6.01) | - | - |
| GLCM energy | - | - | 0.379 | 0.84 (0.25–1.81) |
| GLZLM zone length nonuniformity | - | - | 0.751 | 1.89 (0.55–6.47) |
| Score | Number of Events (%) | p-Value | Hazard Ratio (95% CI) | Median Overall Survival (95% CI) |
|---|---|---|---|---|
| Score 0–2 (n = 28) | 8 (28.6%) | - | 1.00 | 42.1 months (23.0–42.1 months) |
| Score 3 (n = 21) | 14 (66.7%) | 0.004 | 3.71 (1.54–8.98) | 14.4 months (9.2–25.3 months) |
| Score 4 (n = 16) | 15 (93.8%) | <0.001 | 14.52 (5.46–38.64) | 7.6 months (6.9–16.8 months) |
| Score | Clinical TNM Stage | Treatment | |||
|---|---|---|---|---|---|
| Stage I–II | Stage III–IV | Surgical Resection | Other Treatments | ||
| Score 0–2 | p-value | - | - | - | - |
| Hazard ratio (95% CI) | 1.00 | 1.00 | 1.00 | 1.00 | |
| Median overall survival (months) | 42.1 | 34.5 | 42.1 | 34.5 | |
| Score 3 | p-value | 0.046 | 0.008 | 0.017 | 0.043 |
| Hazard ratio (95% CI) | 2.96 (1.05–9.24) | 6.38 (1.62–25.06) | 5.03 (1.34–18.87) | 3.18 (1.08–12.04) | |
| Median overall survival (months) | 15.2 | 8.0 | 14.4 | 15.2 | |
| Score 4 | p-value | 0.002 | <0.001 | <0.001 | <0.001 |
| Hazard ratio (95% CI) | 18.54 (2.93–117.33) | 12.04 (3.00–48.39) | 27.40 (5.11–146.92) | 11.05 (2.82–43.34) | |
| Median overall survival (months) | 9.2 | 6.9 | 9.2 | 6.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Park, S.-H.; Ahn, H.; Lee, S.M.; Jang, S.J. Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT. Cancers 2021, 13, 3563. https://doi.org/10.3390/cancers13143563
Lee JW, Park S-H, Ahn H, Lee SM, Jang SJ. Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT. Cancers. 2021; 13(14):3563. https://doi.org/10.3390/cancers13143563
Chicago/Turabian StyleLee, Jeong Won, Sang-Heum Park, Hyein Ahn, Sang Mi Lee, and Su Jin Jang. 2021. "Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT" Cancers 13, no. 14: 3563. https://doi.org/10.3390/cancers13143563
APA StyleLee, J. W., Park, S.-H., Ahn, H., Lee, S. M., & Jang, S. J. (2021). Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT. Cancers, 13(14), 3563. https://doi.org/10.3390/cancers13143563






