Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment and Ethics Approval
2.2. Blood Sampling
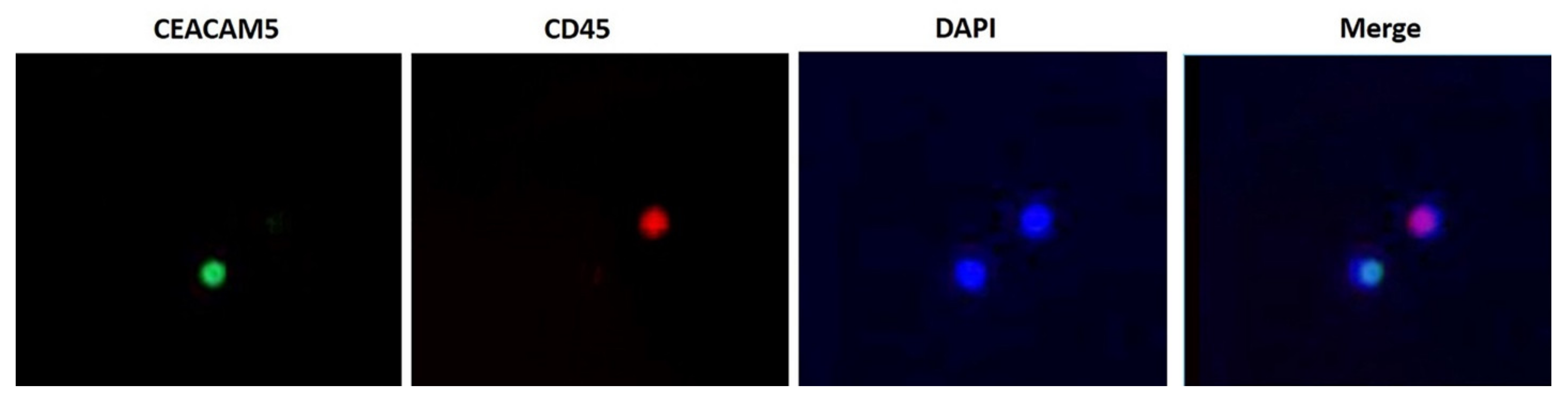
2.3. Double Immunofluorescence Assay (IFAT)
2.4. Reverse Transcription-Quantitative PCR (RT-qPCR)
2.5. Microbial DNA Amplification by PCR
2.6. Study Design and Statistical Analysis
3. Results
3.1. Patient’s Characteristics
3.2. Detection of CTCs
3.2.1. Double Immunofluorescence Assay (IFAT)
3.2.2. Reverse Transcription—Quantitative PCR
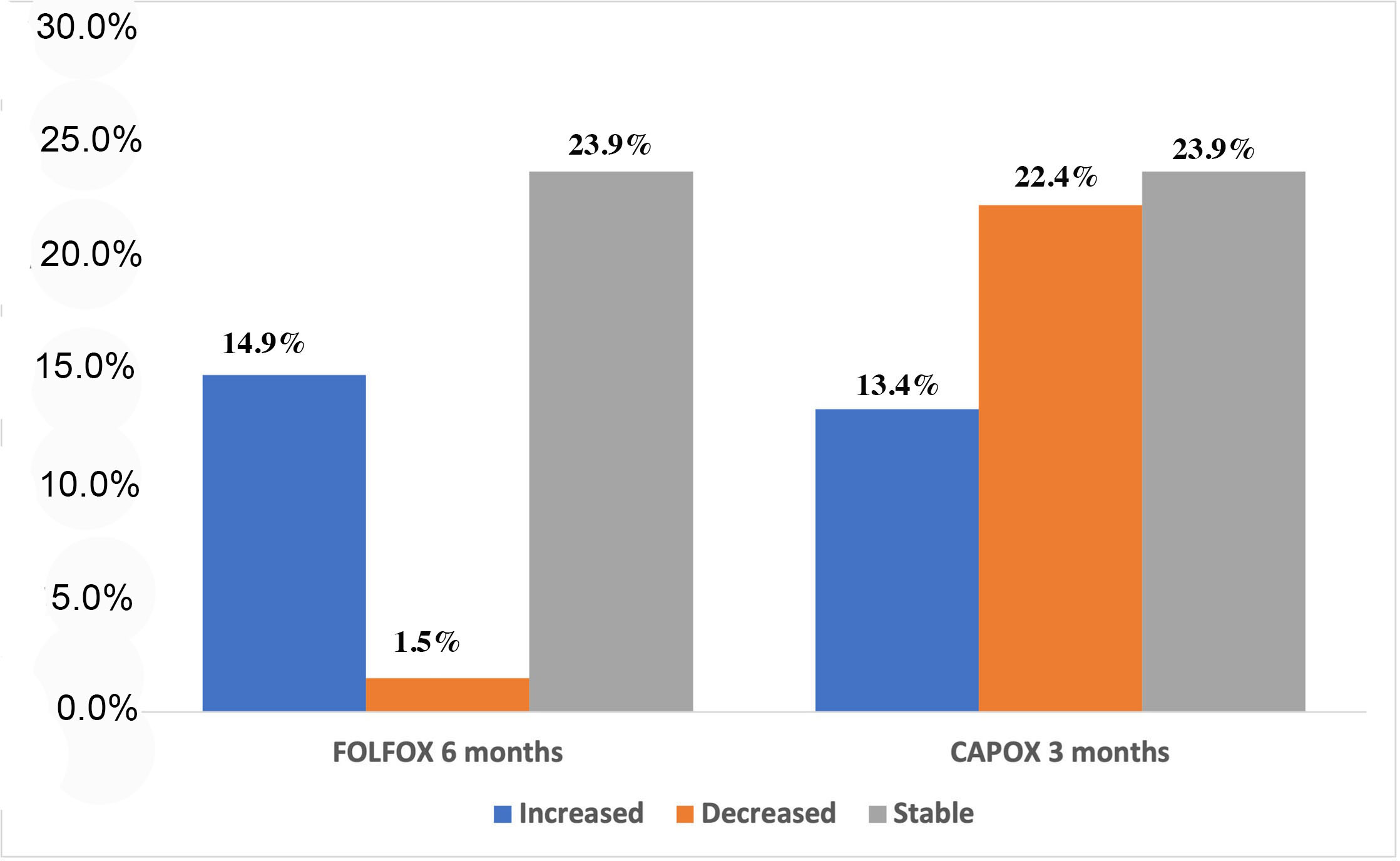
3.2.3. Association of Chemotherapy Regimen with Changes in the Absolute Number and Copy Number of CTCs during Treatment
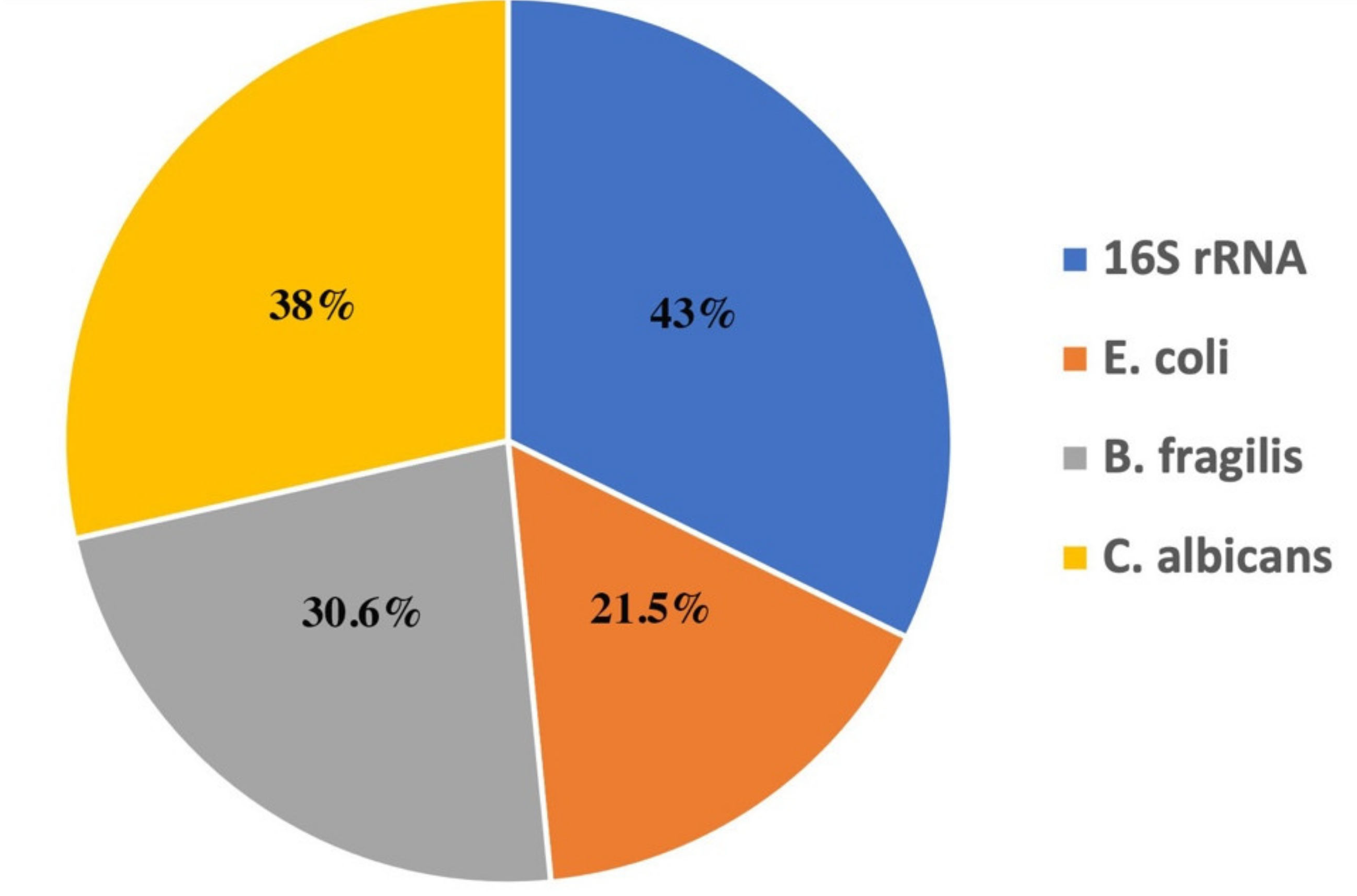
3.3. Detection of Microbial DNA Fragments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Andre, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, D.G.; Tabernero, J.; Maroun, J.; de Braud, F.; Price, T.; Van Cutsem, E.; Hill, M.; Gilberg, F.; Rittweger, K.; Schmoll, H.J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J. Clin. Oncol. 2011, 29, 1465–1471. [Google Scholar] [CrossRef]
- Sobrero, A.; Douillard, J.Y. Reply to the letter to the editor: ‘The hard road to patient-centered care: 3 or 6 months of adjuvant chemotherapy for patients with stage III colon cancer?’ By P. Trendsz et al. Ann. Oncol. 2018, 29, 1883–1884. [Google Scholar] [CrossRef]
- Sobrero, A.; Grothey, A.; Iveson, T.; Labianca, R.; Yoshino, T.; Taieb, J.; Maughan, T.; Buyse, M.; Andre, T.; Meyerhardt, J.; et al. The hard road to data interpretation: 3 or 6 months of adjuvant chemotherapy for patients with stage III colon cancer? Ann. Oncol. 2018, 29, 1099–1107. [Google Scholar] [CrossRef]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Souglakos, J.; Boukovinas, I.; Kakolyris, S.; Xynogalos, S.; Ziras, N.; Athanasiadis, A.; Androulakis, N.; Christopoulou, A.; Vaslamatzis, M.; Ardavanis, A.; et al. Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: The efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann. Oncol. 2019, 30, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Meyerhardt, J.; Iveson, T.; Sobrero, A.; Yoshino, T.; Souglakos, I.; Grothey, A.; Niedzwiecki, D.; Saunders, M.; Labianca, R.; et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): Final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020, 21, 1620–1629. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, R.; Li, Z.; Li, J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017, 8, 55632–55645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef]
- Vardakis, N.; Messaritakis, I.; Papadaki, C.; Agoglossakis, G.; Sfakianaki, M.; Saridaki, Z.; Apostolaki, S.; Koutroubakis, I.; Perraki, M.; Hatzidaki, D.; et al. Prognostic significance of the detection of peripheral blood CEACAM5mRNA-positive cells by real-time polymerase chain reaction in operable colorectal cancer. Clin. Cancer Res. 2011, 17, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Messaritakis, I.; Sfakianaki, M.; Papadaki, C.; Koulouridi, A.; Vardakis, N.; Koinis, F.; Hatzidaki, D.; Georgoulia, N.; Kladi, A.; Kotsakis, A.; et al. Prognostic significance of CEACAM5mRNA-positive circulating tumor cells in patients with metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2018, 82, 767–775. [Google Scholar] [CrossRef]
- Huang, X.; Gao, P.; Song, Y.; Sun, J.; Chen, X.; Zhao, J.; Liu, J.; Xu, H.; Wang, Z. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: A meta-analysis. BMC Cancer 2014, 14, 976. [Google Scholar] [CrossRef] [PubMed]
- Bleday, R.; Song, J.; Walker, E.S.; Salcedo, B.F.; Thomas, P.; Wilson, R.E.; Chen, L.B.; Steele, G., Jr. Characterization of a new monoclonal antibody to a cell surface antigen on colorectal cancer and fetal gut tissues. Cancer 1986, 57, 433–440. [Google Scholar] [CrossRef]
- Tiernan, J.P.; Perry, S.L.; Verghese, E.T.; West, N.P.; Yeluri, S.; Jayne, D.G.; Hughes, T.A. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer 2013, 108, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Milaki, G.; Messaritakis, I.; Koinis, F.; Kotsakis, A.; Apostolaki, S.; Dermitzaki, E.K.; Perraki, M.; Hatzidaki, D.; Georgoulias, V. Prognostic value of chemotherapy-resistant CK19mRNA-positive circulating tumor cells in patients with advanced/metastatic non-small cell lung cancer. Cancer Chemother. Pharmacol. 2017, 80, 101–108. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, V.; Moreau, R.; Kamath, P.S.; Jalan, R.; Gines, P.; Nevens, F.; Fernandez, J.; To, U.; Garcia-Tsao, G.; Schnabl, B. Acute-on-chronic liver failure in cirrhosis. Nat. Rev. Dis. Primers 2016, 2, 16041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, T.D.; Alexander, J.W.; Johannigman, J.A. The detection of microbial DNA in the blood: A sensitive method for diagnosing bacteremia and/or bacterial translocation in surgical patients. Ann. Surg. 1998, 227, 1–9. [Google Scholar] [CrossRef]
- Barriere, S.L.; Lowry, S.F. An overview of mortality risk prediction in sepsis. Crit. Care Med. 1995, 23, 376–393. [Google Scholar] [CrossRef]
- Bruns, T.; Reuken, P.A.; Stengel, S.; Gerber, L.; Appenrodt, B.; Schade, J.H.; Lammert, F.; Zeuzem, S.; Stallmach, A. The prognostic significance of bacterial DNA in patients with decompensated cirrhosis and suspected infection. Liver Int. 2016, 36, 1133–1142. [Google Scholar] [CrossRef]
- Merlini, E.; Bellistri, G.M.; Tincati, C.; d’Arminio Monforte, A.; Marchetti, G. Sequencing of bacterial microflora in peripheral blood: Our experience with HIV-infected patients. J. Vis. Exp. 2011, e2830. [Google Scholar] [CrossRef] [Green Version]
- Messaritakis, I.; Vogiatzoglou, K.; Tsantaki, K.; Ntretaki, A.; Sfakianaki, M.; Koulouridi, A.; Tsiaoussis, J.; Mavroudis, D.; Souglakos, J. The Prognostic Value of the Detection of Microbial Translocation in the Blood of Colorectal Cancer Patients. Cancers 2020, 12, 1058. [Google Scholar] [CrossRef]
- Kallergi, G.; Papadaki, M.A.; Politaki, E.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, R59. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, M.A.; Kallergi, G.; Zafeiriou, Z.; Manouras, L.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Co-expression of putative stemness and epithelial-to-mesenchymal transition markers on single circulating tumour cells from patients with early and metastatic breast cancer. BMC Cancer 2014, 14, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messaritakis, I.; Stogiannitsi, M.; Koulouridi, A.; Sfakianaki, M.; Voutsina, A.; Sotiriou, A.; Athanasakis, E.; Xynos, E.; Mavroudis, D.; Tzardi, M.; et al. Evaluation of the detection of Toll-like receptors (TLRs) in cancer development and progression in patients with colorectal cancer. PLoS ONE 2018, 13, e0197327. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuebler, J.P.; Wieand, H.S.; O’Connell, M.J.; Smith, R.E.; Colangelo, L.H.; Yothers, G.; Petrelli, N.J.; Findlay, M.P.; Seay, T.E.; Atkins, J.N.; et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J. Clin. Oncol 2007, 25, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Grothey, A. Oxaliplatin-safety profile: Neurotoxicity. Semin. Oncol. 2003, 30, 5–13. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Wang, K.; Xu, C.; Hao, M.; Ding, L. Perspectives of the Application of Liquid Biopsy in Colorectal Cancer. Biomed. Res. Int 2020, 2020, 6843180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Zou, K.; Zheng, L.; Xiong, B. Prognostic and clinicopathological significance of circulating tumor cells detected by RT-PCR in non-metastatic colorectal cancer: A meta-analysis and systematic review. BMC Cancer 2017, 17, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, L.K.; Hissa, B.; Weitz, J.; Scholch, S.; Bork, U. The prognostic role of circulating tumor cells in colorectal cancer. Expert Rev. Anticancer Ther 2019, 19, 1077–1088. [Google Scholar] [CrossRef]
- Sotelo, M.J.; Sastre, J.; Maestro, M.L.; Veganzones, S.; Vieitez, J.M.; Alonso, V.; Gravalos, C.; Escudero, P.; Vera, R.; Aranda, E.; et al. Role of circulating tumor cells as prognostic marker in resected stage III colorectal cancer. Ann. Oncol. 2015, 26, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.H.; Kim, G.H.; Song, G.A.; Han, I.S.; Park, E.Y.; Kim, H.S.; Jo, H.J.; Ko, S.H.; Park, D.Y.; Cho, Y.K. Clinical Potential of Circulating Tumor Cells in Colorectal Cancer: A Prospective Study. Clin. Transl. Gastroenterol. 2019, 10, e00055. [Google Scholar] [CrossRef]
- Dizdar, L.; Fluegen, G.; van Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of circulating tumor cells in colorectal cancer patients using the GILUPI CellCollector: Results from a prospective, single-center study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Hendricks, A.; Brandt, B.; Geisen, R.; Dall, K.; Roder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Isolation and Enumeration of CTC in Colorectal Cancer Patients: Introduction of a Novel Cell Imaging Approach and Comparison to Cellular and Molecular Detection Techniques. Cancers 2020, 12, 2643. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kiavue, N.; Ychou, M.; Cabel, L.; Stern, M.H.; Madic, J.; Saliou, A.; Rampanou, A.; Decraene, C.; Bouche, O.; et al. Circulating Tumor Cells and Circulating Tumor DNA Detection in Potentially Resectable Metastatic Colorectal Cancer: A Prospective Ancillary Study to the Unicancer Prodige-14 Trial. Cells 2019, 8, 516. [Google Scholar] [CrossRef] [Green Version]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of Gut Microbiota in the Development and Treatment of Colorectal Cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Frequency (n = 121) | % |
|---|---|---|
| Age (range) | 62 (37–83) | |
| <70 | 83 | 68.9 |
| ≥70 | 38 | 31.4 |
| Gender | ||
| Male | 73 | 60.3 |
| Female | 48 | 39.7 |
| PS (ECOG) | ||
| 0–1 | 120 | 99.2 |
| ≥2 | 1 | 0.8 |
| Surgery | ||
| Yes | 121 | 100 |
| No | 0 | 0.0 |
| Location | ||
| Colon/Sigmoid | 112 | 92.6 |
| Rectum | 9 | 7.4 |
| Right/Left site | ||
| Right colon | 28 | 23.1 |
| Left colon | 93 | 76.9 |
| Histology | ||
| Adenocarcinoma | 99 | 85.3 |
| Mucinous | 17 | 14.7 |
| Unknown | 5 | |
| Regimen | ||
| FOLFOX | 50 | 41.3 |
| CAPOX | 71 | 58.7 |
| Treatment Duration | ||
| 3 months | 37 | 30.6 |
| 6 months | 84 | 69.4 |
| Regimen and Duration | ||
| FOLFOX—3 months | 9 | 7.4 |
| FOLFOX—6 months | 41 | 33.9 |
| CAPOX—3 months | 28 | 23.1 |
| CAPOX—6 months | 43 | 35.6 |
| Timepoint | Immunofluorescence (IFAT) | RT-qPCR | |||
|---|---|---|---|---|---|
| Pos/Neg | No | Median CTC No (Range) | No (%) | Median Copy Number (Range) | |
| Baseline | Pos | 55 (45%) | 3 (1–119) | 46 (38.3%) * | 1.1 (0.71–6.7) |
| Neg | 66 (55%) | 74 (61.7%) | |||
| 3 months | Pos | 31 (44.9%) | 1 (1–6) * | 24 (34.8%) * | 1.1 (0.75–2.4) |
| Neg | 38 (55.1%) | 45 (65.2%) | |||
| 6 months | Pos | 24 (44.4%) | 2 (1–134) * | 23 (42.6%) * | 1.2 (0.71–12.33) |
| Neg | 30 (55.6%) | 31 (57.4%) | |||
| Detection of CTCs | Baseline—3 Months | Baseline—6 Months | 3 months—6 Months |
| +/+ | 27.5% | 22.2% | 57.1% |
| +/- | 20.3% * | 5.6% | 14.3% |
| -/- | 33.3% | 50% | 14.3% |
| -/+ | 18.8% | 22.2% | 14.3% |
| Absolute No of CTCs | Baseline—3 months | Baseline—6 months | 3 months—6 months |
| Increased | 23.2% | 29.6% | 42.9% ** |
| Decreased | 37.7% | 14.8% | 23.8% |
| Stable | 39.1% | 55.6% | 33.3% |
| Baseline vs. 3 Months | Baseline vs. 6 Months | 3 Months vs. 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Increase | Decrease | p | Increase | Decrease | p | Increase | Decrease | p | |
| FOLFOX | 7 (16.7%) | 9 (21.4%) | 0.554 | 13 (54.2%) | 1 (4.2%) | 0.001 | 8 (57.1%) | 2 (14.3%) | 0.05 |
| CAPOX | 9 (21.4%) | 17 (40.5%) | 3 (12.5%) | 7 (29.2%) | 1 (7.1%) | 2(21.4%) | |||
| CTCs Detection (All Treatments) | |||||||
| Baseline | 3 Months | 6 Months | Increase between Baseline—3 Months | Increase between Baseline—6 Months | Increase between 3–6 Months | ||
| 16S rRNA | p = 0.026 | p = 0.108 | p = 0.799 | p = 0.02 | p = 0.108 | p = 1 | |
| E. coli | p = 0.02 | p = 0.921 | p = 0.45 | p = 0.462 | p = 0.268 | p = 0.789 | |
| B. fragilis | p < 0.001 | p = 0.160 | p = 0.887 | p = 0.036 | p = 0.163 | p = 0.574 | |
| Microbial DNA | C. albicans | p = 0.029 | p = 0.392 | p = 0.257 | p = 0.201 | p = 0.11 | p = 0.5 |
| CTCs Detection (FOLFOX only) | |||||||
| Baseline | 3 months | 6 months | Increase between Baseline—3 months | Increase between Baseline—6 months | Increase between 3–6 months | ||
| 16S rRNA | p = 0.18 | p = 0.279 | p = 0.341 | p = 0.034 | p = 0.409 | p = 1 | |
| E. coli | p = 0.036 | p = 0.545 | p = 0.295 | p = 0.538 | p = 0.469 | p = 1 | |
| B. fragilis | p = 0.01 | p = 0.643 | p = 0.326 | p = 0508 | p = 0.404 | p = 0.667 | |
| Microbial DNA | C. albicans | p = 0.392 | p = 0.383 | p = 0.659 | p = 0.404 | p = 0.509 | p = 0.255 |
| CTCs Detection (CAPOX only) | |||||||
| Baseline | 3 months | 6 months | Increase between Baseline—3 months | Increase between Baseline—6 months | Increase between 3–6 months | ||
| 16S rRNA | p = 0.137 | p = 0.043 | p = 0.392 | p = 0.009 | p = 0.306 | p = 0.405 | |
| E. coli | p = 0.355 | p = 0.384 | p = 1 | p = 0.293 | p = 0.236 | p = 0.405 | |
| B. fragilis | p = 0.013 | p = 0.013 | p = 1 | p = 0.015 | p = 0.464 | p = 0.167 | |
| Microbial DNA | C. albicans | p = 0.029 | p = 0.082 | p = 0.76 | p = 0.322 | p = 0.094 | p = 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koulouridi, A.; Messaritakis, I.; Theodorakis, E.; Chondrozoumaki, M.; Sfakianaki, M.; Gouvas, N.; Tsiaoussis, J.; Mavroudis, D.; Tzardi, M.; Souglakos, J. Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment. Cancers 2021, 13, 3552. https://doi.org/10.3390/cancers13143552
Koulouridi A, Messaritakis I, Theodorakis E, Chondrozoumaki M, Sfakianaki M, Gouvas N, Tsiaoussis J, Mavroudis D, Tzardi M, Souglakos J. Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment. Cancers. 2021; 13(14):3552. https://doi.org/10.3390/cancers13143552
Chicago/Turabian StyleKoulouridi, Asimina, Ippokratis Messaritakis, Emmanouil Theodorakis, Maria Chondrozoumaki, Maria Sfakianaki, Nikolaos Gouvas, John Tsiaoussis, Dimitrios Mavroudis, Maria Tzardi, and John Souglakos. 2021. "Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment" Cancers 13, no. 14: 3552. https://doi.org/10.3390/cancers13143552
APA StyleKoulouridi, A., Messaritakis, I., Theodorakis, E., Chondrozoumaki, M., Sfakianaki, M., Gouvas, N., Tsiaoussis, J., Mavroudis, D., Tzardi, M., & Souglakos, J. (2021). Detection of Circulating Tumor Cells and Microbial DNA Fragments in Stage III Colorectal Cancer Patients under Three versus Six Months of Adjuvant Treatment. Cancers, 13(14), 3552. https://doi.org/10.3390/cancers13143552







