Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
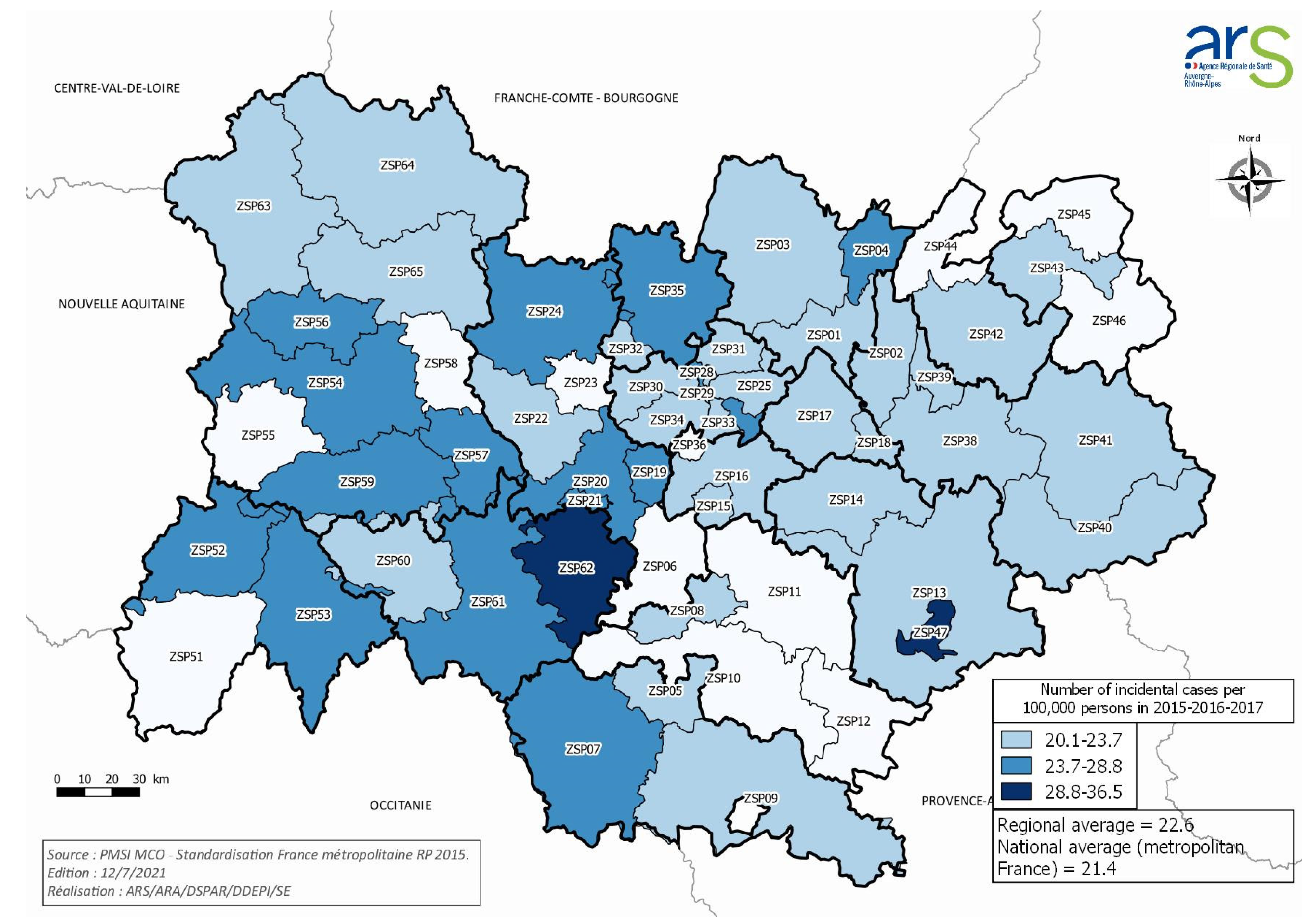
3.1. Population, Structures, and Incidence
3.2. Patient and Treatment Characteristics
3.3. Management within the 30 Days before the First Hospital Stay for PDAC
3.4. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.-L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995–2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [Green Version]
- Romain, G.; Boussari, O.; Bossard, N.; Remontet, L.; Bouvier, A.-M.; Mounier, M.; Iwaz, J.; Colonna, M.; Jooste, V. French Network of Cancer Registries (FRANCIM) Time-to-Cure and Cure Proportion in Solid Cancers in France. A Population Based Study. Cancer Epidemiol. 2019, 60, 93–101. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Partensky, C.; Bray, F. More Deaths from Pancreatic Cancer than Breast Cancer in the EU by 2017. Acta Oncol. Stockh. Swed. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, X.; Yang, L.; Wang, Q.; Bian, X.; Ye, J.; Li, Y.; Li, L. Rising Trends in Pancreatic Cancer Incidence and Mortality in 2000–2014. Clin. Epidemiol. 2018, 10, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowska, M.K.; Weeks, J.C.; Earle, C.C. Treatment of Locally Advanced Pancreatic Cancer in the Real World: Population-Based Practices and Effectiveness. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3409–3414. [Google Scholar] [CrossRef]
- Abraham, A.; Al-Refaie, W.B.; Parsons, H.M.; Dudeja, V.; Vickers, S.M.; Habermann, E.B. Disparities in Pancreas Cancer Care. Ann. Surg. Oncol. 2013, 20, 2078–2087. [Google Scholar] [CrossRef]
- Nipp, R.; Tramontano, A.C.; Kong, C.Y.; Pandharipande, P.; Dowling, E.C.; Schrag, D.; Hur, C. Disparities in Cancer Outcomes across Age, Sex, and Race/Ethnicity among Patients with Pancreatic Cancer. Cancer Med. 2018, 7, 525–535. [Google Scholar] [CrossRef]
- Powers, B.D.; Fulp, W.; Dhahri, A.; DePeralta, D.K.; Ogami, T.; Rothermel, L.; Permuth, J.B.; Vadaparampil, S.T.; Kim, J.-K.; Pimiento, J.; et al. The Impact of Socioeconomic Deprivation on Clinical Outcomes for Pancreatic Adenocarcinoma at a High-Volume Cancer Center: A Retrospective Cohort Analysis. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Maire, F.; Cibot, J.-O.; Compagne, C.; Hentic, O.; Hammel, P.; Muller, N.; Ponsot, P.; Levy, P.; Ruszniewski, P. Epidemiology of Pancreatic Cancer in France: Descriptive Study from the French National Hospital Database. Eur. J. Gastroenterol. Hepatol. 2017, 29, 904–908. [Google Scholar] [CrossRef]
- Barreto, S.G. Pancreatic Cancer in Australia: Is Not It Time We Address the Inequitable Resource Problem? Future Oncol. Lond. Engl. 2020, 16, 1385–1392. [Google Scholar] [CrossRef]
- Diaz, A.; Paredes, A.Z.; Hyer, J.M.; Pawlik, T.M. Variation in Value among Hospitals Performing Complex Cancer Operations. Surgery 2020, 168, 106–112. [Google Scholar] [CrossRef]
- Mehta, V.V.; Friedmann, P.; McAuliffe, J.C.; Muscarella, P.; In, H. Pancreatic Cancer Surgery Following Emergency Department Admission: Understanding Poor Outcomes and Disparities in Care. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020. [Google Scholar] [CrossRef]
- Bezin, J.; Duong, M.; Lassalle, R.; Droz, C.; Pariente, A.; Blin, P.; Moore, N. The National Healthcare System Claims Databases in France, SNIIRAM and EGB: Powerful Tools for Pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. 2017, 26, 954–962. [Google Scholar] [CrossRef]
- Doat, S.; Samson, S.; Fagot-Campagna, A.; Tuppin, P.; Menegaux, F. Estimation of Breast, Prostate, and Colorectal Cancer Incidence Using a French Administrative Database (General Sample of Health Insurance Beneficiaries). Rev. Epidemiol. Sante Publique 2016, 64, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Maroun, R.; Maunoury, F.; Benjamin, L.; Nachbaur, G.; Durand-Zaleski, I. In-Hospital Economic Burden of Metastatic Renal Cell Carcinoma in France in the Era of Targeted Therapies: Analysis of the French National Hospital Database from 2008 to 2013. PLoS ONE 2016, 11, e0162864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farges, O.; Bendersky, N.; Truant, S.; Delpero, J.R.; Pruvot, F.R.; Sauvanet, A. The Theory and Practice of Pancreatic Surgery in France. Ann. Surg. 2017, 266, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Luft, H.S.; Bunker, J.P.; Enthoven, A.C. Should Operations Be Regionalized? The Empirical Relation between Surgical Volume and Mortality. N. Engl. J. Med. 1979, 301, 1364–1369. [Google Scholar] [CrossRef]
- Schneider, E.B.; Ejaz, A.; Spolverato, G.; Hirose, K.; Makary, M.A.; Wolfgang, C.L.; Ahuja, N.; Weiss, M.; Pawlik, T.M. Hospital Volume and Patient Outcomes in Hepato-Pancreatico-Biliary Surgery: Is Assessing Differences in Mortality Enough? J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2014, 18, 2105–2115. [Google Scholar] [CrossRef]
- Amini, N.; Spolverato, G.; Kim, Y.; Pawlik, T.M. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2015, 19, 1581–1592. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.A.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital Volume and Surgical Mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef]
- Urbach, D.R. Pledging to Eliminate Low-Volume Surgery. N. Engl. J. Med. 2015, 373, 1388–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooiker, G.A.; van Gijn, W.; Wouters, M.W.J.M.; Post, P.N.; van de Velde, C.J.H.; Tollenaar, R.a.E.M. Signalling Committee Cancer of the Dutch Cancer Society Systematic Review and Meta-Analysis of the Volume-Outcome Relationship in Pancreatic Surgery. Br. J. Surg. 2011, 98, 485–494. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Clement, G.; Lenne, X.; Farges, O.; Delpero, J.-R.; Theis, D.; Pruvot, F.-R.; Truant, S. Failure-to-Rescue in Patients Undergoing Pancreatectomy: Is Hospital Volume a Standard for Quality Improvement Programs? Nationwide Analysis of 12,333 Patients. Ann. Surg. 2018, 268, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Onete, V.G.; Besselink, M.G.; Salsbach, C.M.; Van Eijck, C.H.; Busch, O.R.; Gouma, D.J.; de Hingh, I.H.; Sieders, E.; Dejong, C.H.; Offerhaus, J.G.; et al. Impact of Centralization of Pancreatoduodenectomy on Reported Radical Resections Rates in a Nationwide Pathology Database. HPB 2015, 17, 736–742. [Google Scholar] [CrossRef] [Green Version]
- Ahola, R.; Siiki, A.; Vasama, K.; Vornanen, M.; Sand, J.; Laukkarinen, J. Effect of Centralization on Long-Term Survival after Resection of Pancreatic Ductal Adenocarcinoma. Br. J. Surg. 2017, 104, 1532–1538. [Google Scholar] [CrossRef]
- Ahola, R.; Sand, J.; Laukkarinen, J. Centralization of Pancreatic Surgery Improves Results: Review. Scand. J. Surg. SJS Off. Organ Finn. Surg. Soc. Scand. Surg. Soc. 2020, 109, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Pasquer, A.; Renaud, F.; Hec, F.; Gandon, A.; Vanderbeken, M.; Drubay, V.; Caranhac, G.; Piessen, G.; Mariette, C. FREGAT Working GroupFRENCH Is Centralization Needed for Esophageal and Gastric Cancer Patients with Low Operative Risk? A Nationwide Study. Ann. Surg. 2016, 264, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Polonski, A.; Izbicki, J.R.; Uzunoglu, F.G. Centralization of Pancreatic Surgery in Europe. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2019, 23, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Sella, T.; Margalit, O.; Amit, U.; Halpern, N.; Aderka, D.; Shacham-Shmueli, E.; Urban, D.; Lawrence, Y.R. Short- and Long-Term Survival in Metastatic Pancreatic Adenocarcinoma, 1993–2013. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
| Institution Type | N Hospital Sites | Level 1 | Level 2 | Level 3 | N patients (%) |
|---|---|---|---|---|---|
| General hospital | 59 | 13 | 42 | 4 | 640 (34%) |
| Private clinic | 36 | 2 | 30 | 4 | 637 (34%) |
| University hospital | 12 | 1 | 7 | 4 | 435 (23%) |
| Private hospital committed to public service | 9 | 3 | 6 | 0 | 107 (5.7%) |
| Cancer care center | 2 | 0 | 2 | 0 | 53 (2.8%) |
| Total | 118 | 19 | 87 | 12 | 1872 (100%) |
| Hospital Type | N Hospital Sites | N Patients | (%) |
|---|---|---|---|
| General hospital | 14 | 42 | −12% |
| Private clinic | 20 | 123 | −35% |
| University hospital | 4 | 142 | −40% |
| Private hospital committed to public service | 3 | 19 | −5% |
| Cancer care center | 1 | 20 | −6% |
| Out of region hospital sites | 7 | 7 | −2% |
| Total | 42 | 353 | |
| Hospital Expertise Levels | N Hospital Sites | N Patients |
|---|---|---|
| 1 | 0 | 21 |
| 2 | 31 | 117 |
| 3 | 11 | 229 |
| Total | 42 | 346 * |
| Number of PS/Year/Hospital Site | N hospital Sites | N Patients | (%) |
|---|---|---|---|
| 1 | 14 | 14 | (4.0%) |
| 2–5 | 15 | 49 | (14.2%) |
| 6–10 | 4 | 31 | (9.0%) |
| 11–20 | 6 | 98 | (28.3%) |
| >20 | 3 | 154 | (44.5%) |
| Total | 42 | 346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fouchardière, C.; Adham, M.; Marion-Audibert, A.-M.; Duclos, A.; Darcha, C.; Berthelet, O.; Hervieu, V.; Artru, P.; Labrosse, H.; Fayet, Y.; et al. Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database. Cancers 2021, 13, 3515. https://doi.org/10.3390/cancers13143515
de la Fouchardière C, Adham M, Marion-Audibert A-M, Duclos A, Darcha C, Berthelet O, Hervieu V, Artru P, Labrosse H, Fayet Y, et al. Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database. Cancers. 2021; 13(14):3515. https://doi.org/10.3390/cancers13143515
Chicago/Turabian Stylede la Fouchardière, Christelle, Mustapha Adham, Anne-Marie Marion-Audibert, Antoine Duclos, Claude Darcha, Olivier Berthelet, Valérie Hervieu, Pascal Artru, Hélène Labrosse, Yohan Fayet, and et al. 2021. "Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database" Cancers 13, no. 14: 3515. https://doi.org/10.3390/cancers13143515
APA Stylede la Fouchardière, C., Adham, M., Marion-Audibert, A.-M., Duclos, A., Darcha, C., Berthelet, O., Hervieu, V., Artru, P., Labrosse, H., Fayet, Y., Ferroud-Plattet, B., Aublet-Cuvellier, B., Chambon, G., Baconnier, M., Rebischung, C., Farsi, F., Ray-Coquard, I., Mastier, C., Ternamian, P.-J., ... Herr, A.-L. (2021). Management of Patients with Pancreatic Ductal Adenocarcinoma in the Real-Life Setting: Lessons from the French National Hospital Database. Cancers, 13(14), 3515. https://doi.org/10.3390/cancers13143515







