MR Thermometry Accuracy and Prospective Imaging-Based Patient Selection in MR-Guided Hyperthermia Treatment for Locally Advanced Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Protocol
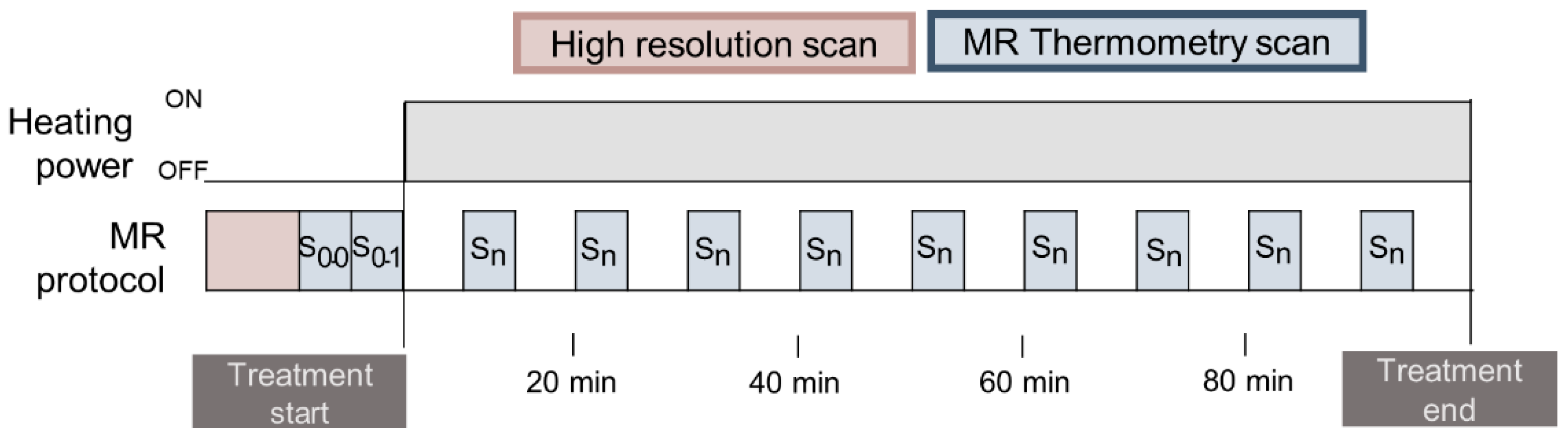
2.2. MR Thermometry Image Acquisition
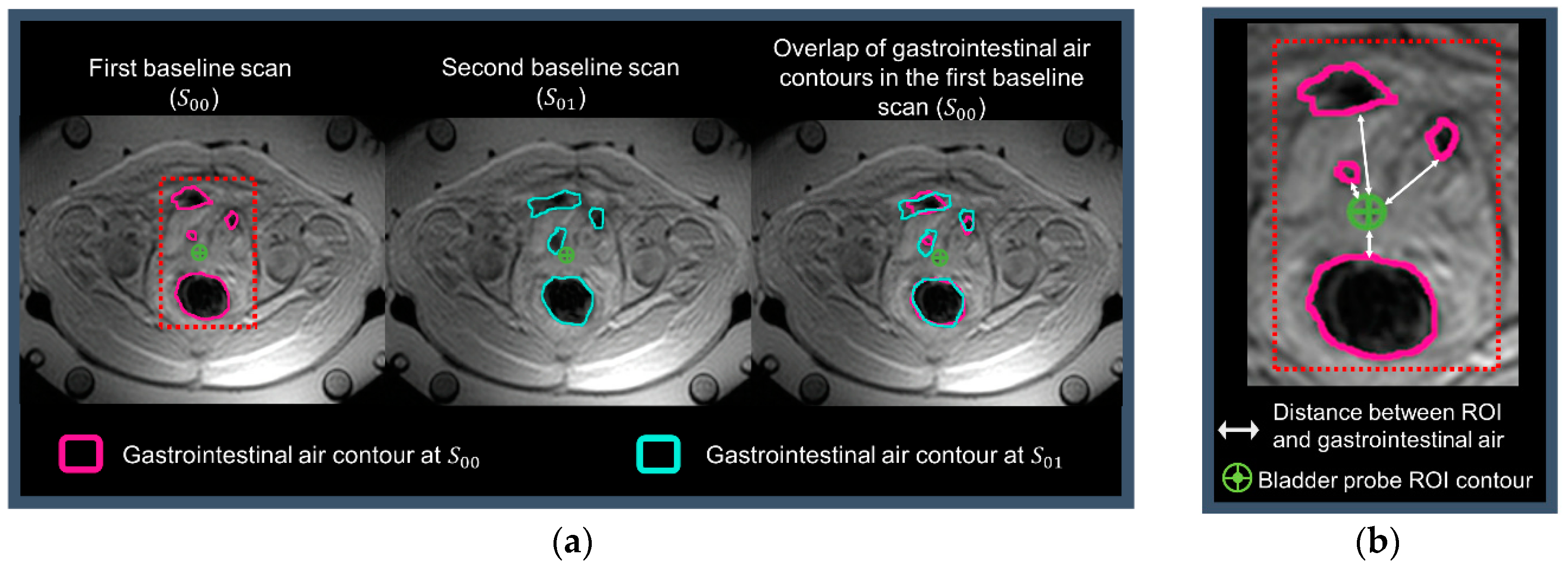
2.3. Contouring and Region Selection
2.4. MR Thermometry Processing
- Uncorrected MR thermometry maps: MR thermometry maps were calculated by taking the difference between the phase maps ( and ), which is formulated as:where is the gyromagnetic ratio and equal to 267.5 × 106 rad/T∙s; is the PRF change coefficient, which is equal to −0.001 ppm/°C; is the magnetic field strength equal to 1.5 T; TE is the echo time equal to 19.1 ms; and n is the scan time.
- Low SNR masking: For each MR thermometry map, voxels with low SNR corresponding to a temperature deviation > 3 °C with respect to three-by-three neighbors were masked to prevent the inclusion of noisy data in the voxels used for drift correction.
- B0 drift correction: In addition to the four fat-like tubes included in the hyperthermia device, body fat (Figure 2) was used to compensate for changes of the static magnetic field B0. A 2D polynomial spatial-temporal correction was applied across the MR temperature maps such that temperature changes are reversed to zero in the selected fat regions. Hence, denotes the final corrected MR thermometry.
- Inaccurate data exclusion: Unrealistic data was removed to avoid pollution by data points affected by confounders such as moving air or other motion. The absolute difference between intraluminal measurements and average MR thermometry measurement within ROIs was minimized. The absolute difference between the two measurements is given by Equation (2), and the minimization is given by Equation (3). The threshold for removal was found to be 7 °C, which was iteratively found between 0 °C and 20 °C using an optimization cycle.where is the MR thermometry temperature; n is the scanning time; is the average intraluminal temperature along the catheter at each location (bladder, rectum, and vagina), j is the index of filtered voxels, and card(J) is the number of voxels within the ROI that were taken into account after applying the threshold.
- Average MR thermometry measurement within ROI: For each probe location, at each scanning time, the average temperature was calculated within the delineated ROIs ). The formulation of the average temperature is given by:where is the MR thermometry temperature, n is the scanning time, j is the index of voxels, and card(J) is the number of voxels within the ROI. Hence, for each intraluminal location and at each scan, a was calculated (, , ).
2.5. Imaging-Based MRT Accuracy Prediction Parameters
3. Results
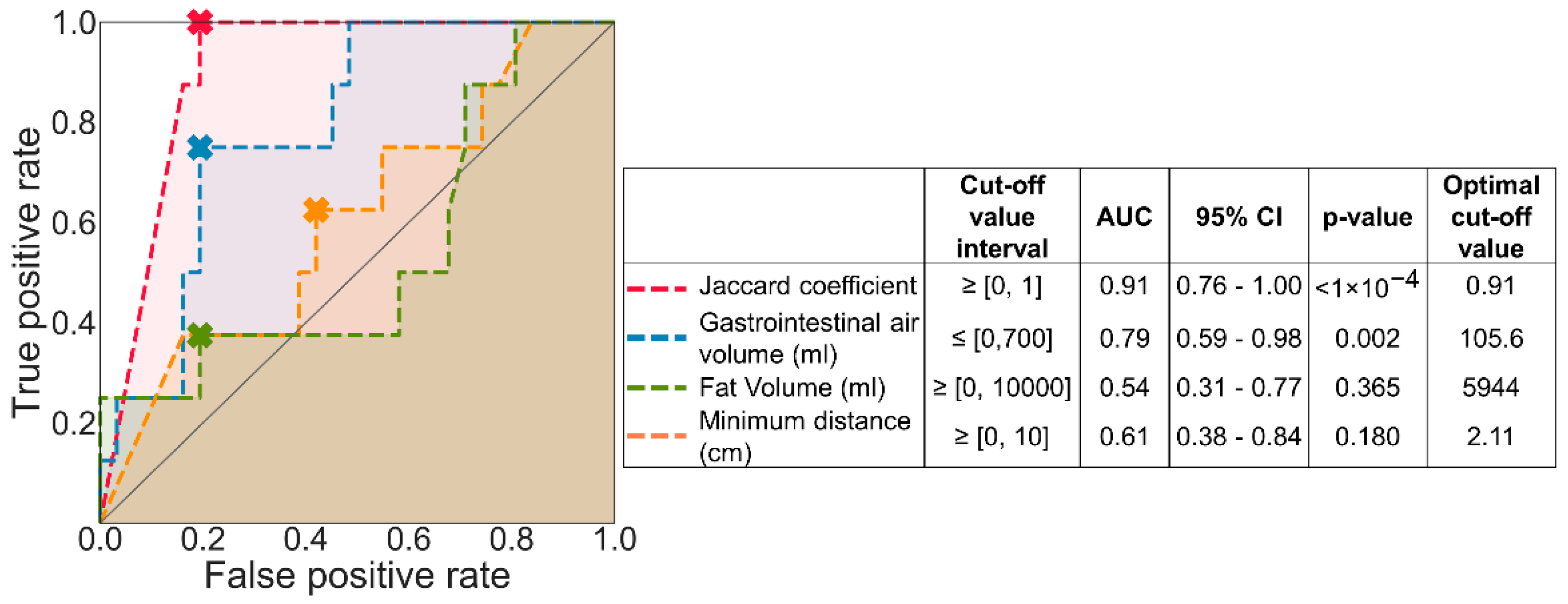
3.1. Predictive Value for MRT Accuracy of Imaging-Based Parameters
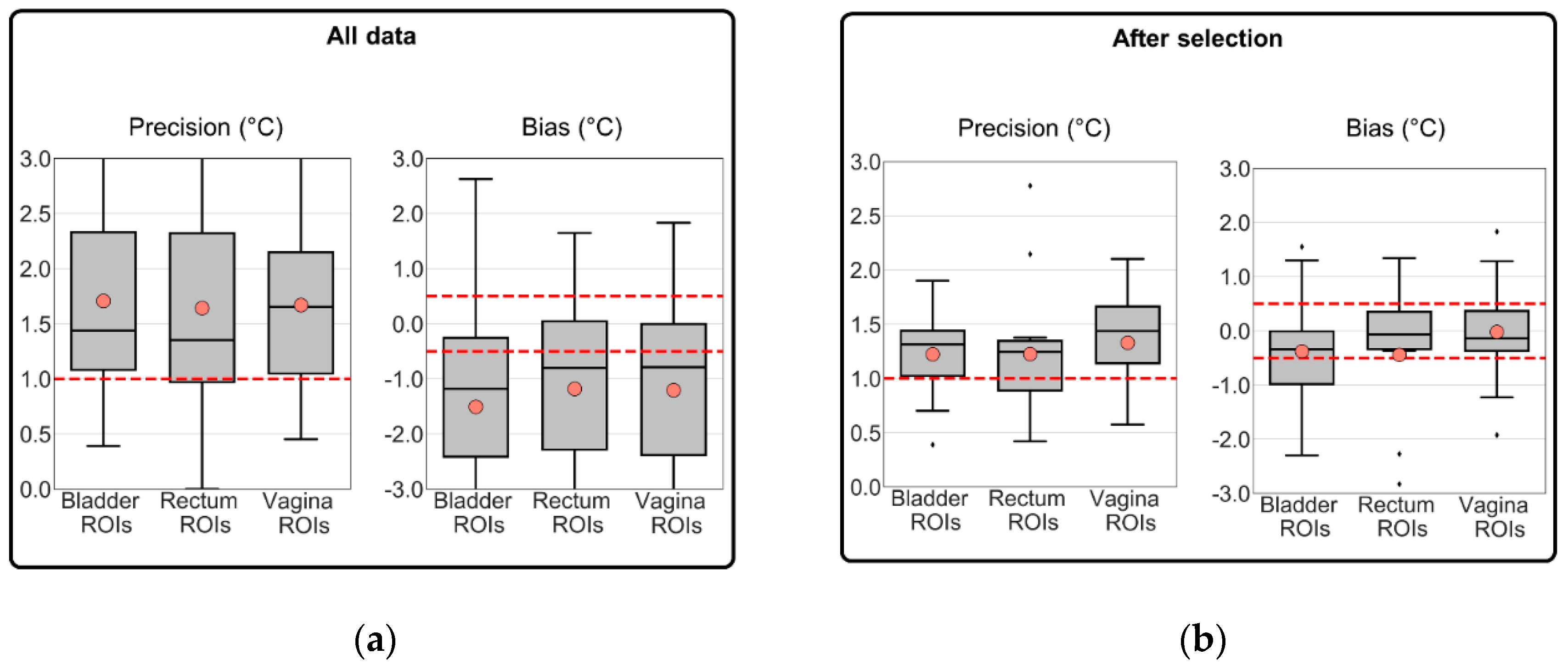
3.2. MRT Accuracy for All Data versus MRT Accuracy from Selected Sessions
4. Discussion
4.1. Image Parameters to Select Treatments with Robust MRT
4.2. Clinical Relevance
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Zee, J.; González, D.G.; Rhoon, G.C.; Van Dijk, J.D.P.; Van Putten, W.L.J. Van Comparison of Radiotherapy alone with Radiotherapy plus Hyperthermia in Locally Advanced Pelvic Tumors. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Datta, N.; Bose, A.; Kapoor, H.K. Thermoradiotherapy in the management of carcinoma cervix (stage IIIB): A controlled clinical study. Indian Med. Gaz. 1987, 121, 68–71. [Google Scholar]
- Sharma, S.; Patel, F.D.; Sandhu, A.P.S.; Gupta, B.D.; Yadav, N.S. A prospective randomized study of local hyperthermia as a supplement and radiosensitizer in the treatment of carcinoma of the cervix with radiotherapy. Endocuriether. Hyperth. Oncol. 1989, 5, 151–159. [Google Scholar]
- Chen, H.W.; Jun-Jie, F. Randomized trial of hyperthermo-radiochemotherapy for uterine cervix cancer. Chin. J. Clin. Oncol. 1997, 24, 249–251. [Google Scholar]
- Harima, Y.; Nagata, K.; Harima, K.; Ostapenko, V.V.; Tanaka, Y.S.S. “A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma” of Yoko Harima et al. (2001): Multiple biases and no advantage of hyperthermia. Int. J. Hyperth. 2018, 34, 1400. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Klingbiel, D.; Gómez, S.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: A systematic review with conventional and network meta-analyses. Int. J. Hyperth. 2016, 32, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Stutz, E.; Gomez, S.; Bodis, S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 411–437. [Google Scholar] [CrossRef]
- Lutgens, L.; van der Zee, J.; Pijls-Johannesma, M.; De Haas-Hock, D.F.M.; Buijsen, J.; Mastrigt, G.A.P.G.V.; Lammering, G.; De Ruysscher, D.K.M. Combined use of hyperthermia and radiation therapy for treating locally advanced cervical carcinoma. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Franckena, M.; Fatehi, D.; de Bruijne, M.; Canters, R.A.M.; van Norden, Y.; Mens, J.W.; van Rhoon, G.C.; van der Zee, J. Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur. J. Cancer 2009, 45, 1969–1978. [Google Scholar] [CrossRef]
- Perez, C.A.; Gillespie, B.; Pajak, T.; Hornback, N.B.; Emami, D.; Rubin, P. Quality assurance problems in clinical hyperthermia and their impact on therapeutic outcome: A report by the radiation therapy oncology group. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 551–558. [Google Scholar] [CrossRef]
- Van Der Zee, J.; van Rhoon, G.C.; Wust, P. In regard to Dr. Vasanthan et al. (Int J Radiat Oncol Biol Phys 2005;61:145-153). Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 940–945. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther. Onkol. 2011, 187, 605–610. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Ganter, H.; Nadobny, J.; Fähling, H.; Seebass, M.; Felix, R.; Wust, P. A practical approach to thermography in a hyperthermia/magnetic resonance hybrid system: Validation in a heterogeneous phantom. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 267–277. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Feussner, A.; Fähling, H.; Nadobny, J.; Hildebrandt, B.; Felix, R.; Wust, P. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int. J. Hyperth. 2005, 21, 497–513. [Google Scholar] [CrossRef]
- De Senneville, B.D.; Quesson, B.; Moonen, C.T.W. Magnetic resonance temperature imaging. Int. J. Hyperth. 2005, 21, 515–531. [Google Scholar] [CrossRef]
- Adibzadeh, F.; Sumser, K.; Curto, S.; Yeo, D.T.B.; Shishegar, A.A.; Paulides, M.M. Systematic review of pre-clinical and clinical devices for magnetic resonance-guided radiofrequency hyperthermia. Int. J. Hyperth. 2020, 37, 15–27. [Google Scholar] [CrossRef]
- Curto, S.; Aklan, B.; Mulder, T.; Mils, O.; Schmidt, M.; Lamprecht, U.; Peller, M.; Wessalowski, R.; Lindner, L.H.; Fietkau, R.; et al. Quantitative, Multi-institutional Evaluation of MR Thermometry Accuracy for Deep-Pelvic MR-Hyperthermia Systems Operating in Multi-vendor MR-systems Using a New Anthropomorphic Phantom. Cancers 2019, 11, 1709. [Google Scholar] [CrossRef]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 2012, 188, 198–211. [Google Scholar] [CrossRef]
- Fatehi, D.; Van Der Zee, J.; Notenboom, A.; Van Rhoon, G.C. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther. Onkol. 2007, 183, 479–486. [Google Scholar] [CrossRef]
- Wust, P.; Cho, C.H.; Hildebrandt, B.; Gellermann, J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int. J. Hyperth. 2006, 22, 255–262. [Google Scholar] [CrossRef]
- Jones, E.; Secord, A.A.; Prosnitz, L.R.; Samulski, T.V.; Oleson, J.R.; Berchuck, A.; Clarke-Pearson, D.; Soper, J.; Dewhirst, M.W.; Vujaskovic, Z. Intra-peritoneal cisplatin and whole abdomen hyperthermia for relapsed ovarian carcinoma. Int. J. Hyperth. 2006, 22, 161–172. [Google Scholar] [CrossRef]
- Van der Zee, J.; Peer-Valstar, J.N.; Rietveld, P.J.; de Graaf-Strukowska, L.; van Rhoon, G.C. Practical limitations of interstitial thermometry during deep hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1205–1212. [Google Scholar] [CrossRef]
- Kok, H.P.; Schooneveldt, G.; Bakker, A.; de Kroon-Oldenhof, R.; Korshuize-van Straten, L.; de Jong, C.E.; Steggerda-Carvalho, E.; Geijsen, E.D.; Stalpers, L.J.A.; Crezee, J. Predictive value of simulated SAR and temperature for changes in measured temperature after phase-amplitude steering during locoregional hyperthermia treatments. Int. J. Hyperth. 2018, 35, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, R.W.M.; Curto, S.; De Jager, B.; Maljaars, E.; Van Rhoon, G.C.; Paulides, M.M.; Heemels, W.P.M.H. POD-Based Recursive Temperature Estimation for MR-Guided RF Hyperthermia Cancer Treatment: A Pilot Study. In Proceedings of the 57th IEEE Conference on Decision and Control, Miami Beach, FL, USA, 17–19 December 2018; pp. 5201–5208. [Google Scholar]
- Hutchinson, E.; Dahleh, M.; Hynynen, K. The feasibility of MRI feedback control for intracavitary phased array hyperthermia treatments. Int. J. Hyperth. 1998, 14, 39–56. [Google Scholar] [CrossRef]
- Cheng, K.S.; Stakhursky, V.; Craciunescu, O.I.; Stauffer, P.; Dewhirst, M.; Das, S.K. Fast temperature optimization of multi-source hyperthermia applicators with reduced-order modeling of “virtual sources”. Phys. Med. Biol. 2008, 53, 1619–1635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pacheco, C.C.; Orlande, H.R.B.; Colaço, M.J.; Dulikravich, G.S.; Varón, L.A.B.; Lamien, B. Real-time temperature estimation with enhanced spatial resolution during MR-guided hyperthermia therapy. Numer. Heat Transf. Part A Appl. 2020, 77, 782–806. [Google Scholar] [CrossRef]
- Stakhursky, V.L.; Arabe, O.; Cheng, K.S.; MacFall, J.; MacCarini, P.; Craciunescu, O.; Dewhirst, M.; Stauffer, P.; Das, S.K. Real-time MRI-guided hyperthermia treatment using a fast adaptive algorithm. Phys. Med. Biol. 2009, 54, 2131–2145. [Google Scholar] [CrossRef]
- Potocki, J.K.; Tharp, H.S. Concurrent hyperthermia estimation schemes based on extended kalman filtering and reduced-order modelling. Int. J. Hyperth. 1993, 9, 849–865. [Google Scholar] [CrossRef]
- Kowalski, M.E.; Jin, J.M. A temperature-based feedback control system for electromagnetic phased-array hyperthermia: Theory and simulation. Phys. Med. Biol. 2003, 48, 633–651. [Google Scholar] [CrossRef]
- Gellermann, J.; Weihrauch, M.; Cho, C.H.; Wlodarczyk, W.; Fähling, H.; Felix, R.; Budach, V.; Weiser, M.; Nadobny, J.; Wust, P. Comparison of MR-thermography and planning calculations in phantoms. Med. Phys. 2006, 33, 3912–3920. [Google Scholar] [CrossRef]
- Cline, H.E.; Hynynen, K.; Schneider, E.; Hardy, C.J.; Maier, S.E.; Watkins, R.D.; Jolesz, F.A. Simultaneous magnetic resonance phase and magnitude temperature maps in muscle. Magn. Reson. Med. 1996, 35, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Unsoeld, M.; Lamprecht, U.; Traub, F.; Hermes, B.; Scharpf, M.; Potkrajcic, V.; Zips, D.; Paulsen, F.; Eckert, F. MR thermometry data correlate with pathological response for soft tissue sarcoma of the lower extremity in a single center analysis of prospectively registered patients. Cancers 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.I.; Raaymakers, B.W.; Kotte, A.N.T.J.; Das, S.K.; Samulski, T.V.; Lagendijk, J.J.W. Discretizing large traceable vessels and using DE-MRI perfusion maps yields numerical temperature contours that match the MR non-invasive measurements. Med. Phys. 2001, 28, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Poorman, M.E.; Braškutė, I.; Bartels, L.W.; Grissom, W.A. Multi-echo MR thermometry using iterative separation of baseline water and fat images. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- De Poorter, J.; De Wagter, C.; De Deene, Y.; Thomsen, C.; Ståhlberg, F.; Achten, E. Noninvasive MRI Thermometry with the Proton Resonance Frequency (PRF) Method: In Vivo Results in Human Muscle. Magn. Reson. Med. 1995, 33, 74–81. [Google Scholar] [CrossRef]
- Peters, R.D.; Hinks, R.S.; Henkelman, R.M. Ex vivo tissue-type independence in proton-resonance frequency shift MR thermometry. Magn. Reson. Med. 1998, 40, 454–459. [Google Scholar] [CrossRef]
- Quesson, B.; De Zwart, J.A.; Moonen, C.T.W. Magnetic resonance temperature imaging for guidance of thermotherapy. J. Magn. Reson. Imaging 2000, 12, 525–533. [Google Scholar] [CrossRef]
- Young, I.R.; Hajnal, J.V.; Roberts, I.G.; Ling, J.X.; Hill-cottingham, R.J.; Oatridge, A.; Wilson, J.A. An Evaluation of the Effects of Susceptibility Changes on the Water Chemical Shift Method of Temperature Measurement in Human Peripheral Muscle. Magn. Reson. Med. 1996, 36, 366–374. [Google Scholar] [CrossRef]
- Rieke, V.; Pauly, P.K.B. MR Thermometry Viola. North 2008, 29, 1883–1889. [Google Scholar]
- Numan, W.C.M.; Hofstetter, L.W.; Kotek, G.; Bakker, J.F.; Fiveland, E.W.; Houston, G.C.; Kudielka, G.; Yeo, D.T.B.; Paulides, M.M. Exploration of MR-guided head and neck hyperthermia by phantom testing of a modified prototype applicator for use with proton resonance frequency shift thermometry. Int. J. Hyperth. 2014, 30, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Oberacker, E.; Kuehne, A.; Nadobny, J.; Zschaeck, S.; Weihrauch, M.; Waiczies, H.; Ghadjar, P.; Wust, P.; Niendorf, T.; Winter, L. Radiofrequency applicator concepts for simultaneous MR imaging and hyperthermia treatment of glioblastoma multiforme. Curr. Dir. Biomed. Eng. 2017, 3, 473–477. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Hildebrandt, B.; Ganter, H.; Nicolau, A.; Rau, B.; Tilly, W.; Fähling, H.; Nadobny, J.; Felix, R.; et al. Non-invasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 tesla hybrid system. Cancer Res. 2005, 65, 5872–5880. [Google Scholar] [CrossRef]
- Gellermann, J.; Hildebrandt, B.; Issels, R.; Ganter, H.; Wlodarczyk, W.; Budach, V.; Felix, R.; Tunn, P.U.; Reichardt, P.; Wust, P. Non-invasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006, 107, 1373–1382. [Google Scholar] [CrossRef]
- Craciunescu, O.I.; Stauffer, P.R.; Soher, B.J.; Wyatt, C.R.; Arabe, O.; MacCarini, P.; Das, S.K.; Cheng, K.S.; Wong, T.Z.; Jones, E.L.; et al. Accuracy of real time non-invasive temperature measurements using magnetic resonance thermal imaging in patients treated for high grade extremity soft tissue sarcomas. Med. Phys. 2009, 36, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, P.R.; Craciunescu, O.I.; Maccarini, P.F.; Wyatt, C.; Arunachalam, K.; Arabe, O.; Stakhursky, V.; Soher, B.; MacFall, J.R.; Li, Z.; et al. Clinical utility of magnetic resonance thermal imaging (MRTI) for real-time guidance of deep hyperthermia. In Proceedings of the Energy-based Treatment of Tissue and Assessment V, San Jose, CA, USA, 24–29 January 2009; Volume 7181, p. 71810I. [Google Scholar]
- Dadakova, T.; Gellermann, J.; Voigt, O.; Korvink, J.G.; Pavlina, J.M.; Hennig, J.; Bock, M. Fast PRF-based MR thermometry using double-echo EPI: In vivo comparison in a clinical hyperthermia setting. Magn. Reson. Mater. Phys. Biol. Med. 2015, 28, 305–314. [Google Scholar] [CrossRef]
- Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical performance and future potential of magnetic resonance thermometry in hyperthermia. Cancers 2021, 13, 1–19. [Google Scholar]
- Rijnen, Z.; Bakker, J.F.; Canters, R.A.M.; Togni, P.; Verduijn, G.M.; Levendag, P.C.; Van Rhoon, G.C.; Paulides, M.M. Clinical integration of software tool VEDO for adaptive and quantitative application of phased array hyperthermia in the head and neck. Int. J. Hyperth. 2013, 29, 181–193. [Google Scholar] [CrossRef]
- Canters, R.A.M.; Paulides, M.M.; Franckena, M.F.; Van Der Zee, J.; Van Rhoon, G.C. Implementation of treatment planning in the routine clinical procedure of regional hyperthermia treatment of cervical cancer: An overview and the Rotterdam experience. Int. J. Hyperth. 2012, 28, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of the Flora in the Alpine Zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- McGuinness, K.; O’Connor, N.E. A comparative evaluation of interactive segmentation algorithms. Pattern Recognit. 2010, 43, 434–444. [Google Scholar] [CrossRef]
- Niwattanakul, S.; Singthongchai, J.; Naenudorn, E.; Wanapu, S. Using of jaccard coefficient for keywords similarity. Lect. Notes Eng. Comput. Sci. 2013, 2202, 380–384. [Google Scholar]
- Metz, C.E.; Kronman, H.B. Statistical significance tests for binormal ROC curves. J. Math. Psychol. 1980, 22, 218–243. [Google Scholar] [CrossRef]
- Fan, J.; Upadhye, S.; Worster, A. Understanding receiver operating characteristic (ROC) curves. Can. J. Emerg. Med. 2006, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, S.V.V.N.; Altman, M.B.; Zhu, L.; Partanen, A.; Cheng, G.; Gach, H.M.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Evaluation and selection of anatomic sites for magnetic resonance imaging-guided mild hyperthermia therapy: A healthy volunteer study. Int. J. Hyperth. 2018, 34, 1381–1389. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. Int. J. Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.A.; Moore, J.L. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Miller, R.L.; Acton, C.; Fullerton, D.A.; Maltby, J. Analysis of Variance (ANOVA). In SPSS for Social Scientists; Campling, J., Ed.; Palgrave: London, UK, 2002. [Google Scholar] [CrossRef]
- Wang, P. Evaluation of MR thermometry with proton resonance frequency method at 7T. Quant. Imaging Med. Surg. 2017, 7, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Canters, R.A.M.; Paulides, M.M.; Franckena, M.; Mens, J.W.; Van Rhoon, G.C. Benefit of replacing the Sigma-60 by the Sigma-Eye applicator: A Monte Carlo-based uncertainty analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 189, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, D.; van der Zee, J.; de Bruijne, M.; Franckena, M.; van Rhoon, G.C. RF-power and temperature data analysis of 444 patients with primary cervical cancer: Deep hyperthermia using the Sigma-60 applicator is reproducible. Int. J. Hyperth. 2007, 23, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Van Rhoon, G.C.; Van Der Heuvel, D.J.; Ameziane, A.; Rietveld, P.J.M.; Volenec, K.; Van Der Zee, J. Characterization of the SAR-distribution of the Sigma-60 applicator for regional hyperthermia using a Schottky diode sheet. Int. J. Hyperth. 2003, 19, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Mulder, H.T.; Baron, P.; Coello, E.; Menzel, M.I.; van Rhoon, G.C.; Haase, A. Correction of motion-induced susceptibility artifacts and B0 drift during proton resonance frequency shift-based MR thermometry in the pelvis with background field removal methods. Magn. Reson. Med. 2020, 84, 2495–2511. [Google Scholar] [CrossRef]
- De Denis Senneville, B.; Quesson, B.; Desbarats, P.; Salomir, R.; Palussière, J.; Moonen, C.T.W. Atlas-based motion correction for on-line MR temperature mapping. Proc. Int. Conf. Image Process. ICIP 2004, 4, 2571–2574. [Google Scholar]
- Yuan, J.; Mei, C.-S.; Panych, L.P.; McDannold, N.J.; Madore, B. Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry. Quant. Imaging Med. Surg. 2012, 2, 21–32. [Google Scholar]
- Tanner, C.; Zur, Y.; French, K.; Samei, G.; Strehlow, J.; Sat, G.; McLeod, H.; Houston, G.; Kozerke, S.; Székely, G.; et al. In vivo validation of spatio-temporal liver motion prediction from motion tracked on MR thermometry images. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1143–1152. [Google Scholar] [CrossRef]
- Celicanin, Z.; Auboiroux, V.; Bieri, O.; Petrusca, L.; Santini, F.; Viallon, M.; Scheffler, K.; Salomir, R. Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs. Magn. Reson. Med. 2014, 72, 1087–1095. [Google Scholar] [CrossRef]
- De Senneville, B.D.; Roujol, S.; Moonen, C.; Ries, M. Motion correction in MR thermometry of abdominal organs: A comparison of the referenceless vs. the multibaseline approach. Magn. Reson. Med. 2010, 64, 1373–1381. [Google Scholar] [CrossRef]
- De Senneville, B.D.; Desbarats, P.; Salomir, R.; Quesson, B.; Moonen, C.T.W. Correction of accidental patient motion for online Mr thermometry. In Proceedings of the 7th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI 2004), Saint-Malo, France, 26–29 September 2004; pp. 637–644. [Google Scholar]
- Todd, N.; Diakite, M.; Payne, A.; Parker, D.L. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magn. Reson. Med. 2014, 72, 793–799. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kokuryo, D.; Kaihara, T.; Fujii, N.; Kumamoto, E. Image reconstruction method with compressed sensing for high-speed MR temperature measurement of abdominal organs. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2731–2735. [Google Scholar]
- Poorter, J. De Noninvasive MRI thermometry with the proton resonance frequency method: Study of susceptibility effects. Magn. Reson. Med. 1995, 34, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Partanen, A.; Werner, B.; Ghanouni, P.; Bitton, R.; Butts Pauly, K.; Grissom, W.A. Correcting heat-induced chemical shift distortions in proton resonance frequency-shift thermometry. Magn. Reson. Med. 2016, 76, 172–182. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Categories | Value |
|---|---|---|
| Patient/Tumor Characteristics | ||
| Total number of patients | 14 | |
| Age (years) | 56.5 ± 16.7 | |
| Median age (years) | 60 | |
| Histology | Adenocarcinoma | 3 |
| Squamous cell carcinoma | 10 | |
| Carcinosarcoma | 1 | |
| FIGO stage | IA | 1 |
| IB | 2 | |
| IIB | 5 | |
| IIIB | 4 | |
| IVA | 2 | |
| Hyperthermia Treatment Session Characteristics | ||
| Total number of sessions | 39 | |
| Number of treatment sessions per patient | 2.8 ± 1.5 | |
| Duration of each treatment session (minutes) | 89.5 ± 1.6 | |
| MR thermometry scans per treatment session | 8.8 ± 1.5 | |
| The time between the start of the two baseline scans (seconds) | 97.0 ± 10.0 | |
| Number of MR thermometry slices with identified probes | 7.3 ± 2.4 | |
| Number of probe mapping measurements during treatment time | 15.2 ± 3.0 | |
| Maximum probe measurements range (cm) | Bladder | 9.9 ± 2.2 |
| Rectum | 6.9 ± 2.1 | |
| Vagina | 8.4 ± 2.5 | |
| Maximum net heating power (W) | 941.1 ± 118.7 | |
| All Treatment Sessions | Selected for Air Motion (Jaccard Coefficient ≥ 0.91) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bladder | Rectum | Vagina | Deviation from the Acceptable Threshold | Bladder | Rectum | Vagina | Deviation from the Acceptable Threshold | |
| Accuracy | 2.2 ± 1.6 | 1.9 ± 1.4 | 2.0 ± 1.5 | +1.0 °C | 1.1 ± 0.7 | 1.1 ± 1.1 | 0.9 ± 0.6 | −0.0 °C |
| Precision | 1.7 ± 0.9 | 1.6 ± 0.9 | 1.7 ± 0.8 | +0.7 °C | 1.2 ± 0.4 | 1.2 ± 0.6 | 1.3 ± 0.4 | +0.2 °C |
| Bias | −1.5 ± 2.1 | −1.2 ± 1.7 | −1.2 ± 1.8 | +0.8 °C | −0.4 ± 1.1 | −0.4 ± 1.4 | 0.0 ± 1.0 | −0.3 °C |
| Sessions | 39 sessions (100%) | 15 sessions (38%) | ||||||
| Patients | 14 patients (100%) | 9 patients (64%) | ||||||
| Measurements: Mean Temperature Increase (°C) | ||||||
|---|---|---|---|---|---|---|
| This Study | Other Studies | |||||
| Location | LACC | Gellermann et al. [43] Recurrent Rectal cancer | Gellermann et al. [44] Soft Tissue Sarcoma | |||
| MRT | Intraluminal | MRT | Intraluminal | MRT | Intraluminal | |
| Bladder | 2.4 °C ± 1.7 °C | 2.5 °C ± 1.2 °C | >7 °C | No data | ≤4 to 5 °C | 2.6 °C ± 1.3 °C |
| Vagina | 2.6 °C ± 1.6 °C | 2.4 °C ± 1.2 °C | No data | 2.2 °C ± 0.6 °C | ||
| Rectum | 2.1 °C ± 1.4 °C | 2.5 °C ± 1.3 °C | ~3 °C | 3.5 °C ± 1.0 °C | ||
| Sessions | 15 sessions (38%) | 15 sessions (20%) | 15 sessions (50%) | |||
| Patients | 9 patients (64%) | 15 patients (100%) | 9 patients (100%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
VilasBoas-Ribeiro, I.; Curto, S.; van Rhoon, G.C.; Franckena, M.; Paulides, M.M. MR Thermometry Accuracy and Prospective Imaging-Based Patient Selection in MR-Guided Hyperthermia Treatment for Locally Advanced Cervical Cancer. Cancers 2021, 13, 3503. https://doi.org/10.3390/cancers13143503
VilasBoas-Ribeiro I, Curto S, van Rhoon GC, Franckena M, Paulides MM. MR Thermometry Accuracy and Prospective Imaging-Based Patient Selection in MR-Guided Hyperthermia Treatment for Locally Advanced Cervical Cancer. Cancers. 2021; 13(14):3503. https://doi.org/10.3390/cancers13143503
Chicago/Turabian StyleVilasBoas-Ribeiro, Iva, Sergio Curto, Gerard C. van Rhoon, Martine Franckena, and Margarethus M. Paulides. 2021. "MR Thermometry Accuracy and Prospective Imaging-Based Patient Selection in MR-Guided Hyperthermia Treatment for Locally Advanced Cervical Cancer" Cancers 13, no. 14: 3503. https://doi.org/10.3390/cancers13143503
APA StyleVilasBoas-Ribeiro, I., Curto, S., van Rhoon, G. C., Franckena, M., & Paulides, M. M. (2021). MR Thermometry Accuracy and Prospective Imaging-Based Patient Selection in MR-Guided Hyperthermia Treatment for Locally Advanced Cervical Cancer. Cancers, 13(14), 3503. https://doi.org/10.3390/cancers13143503










