The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
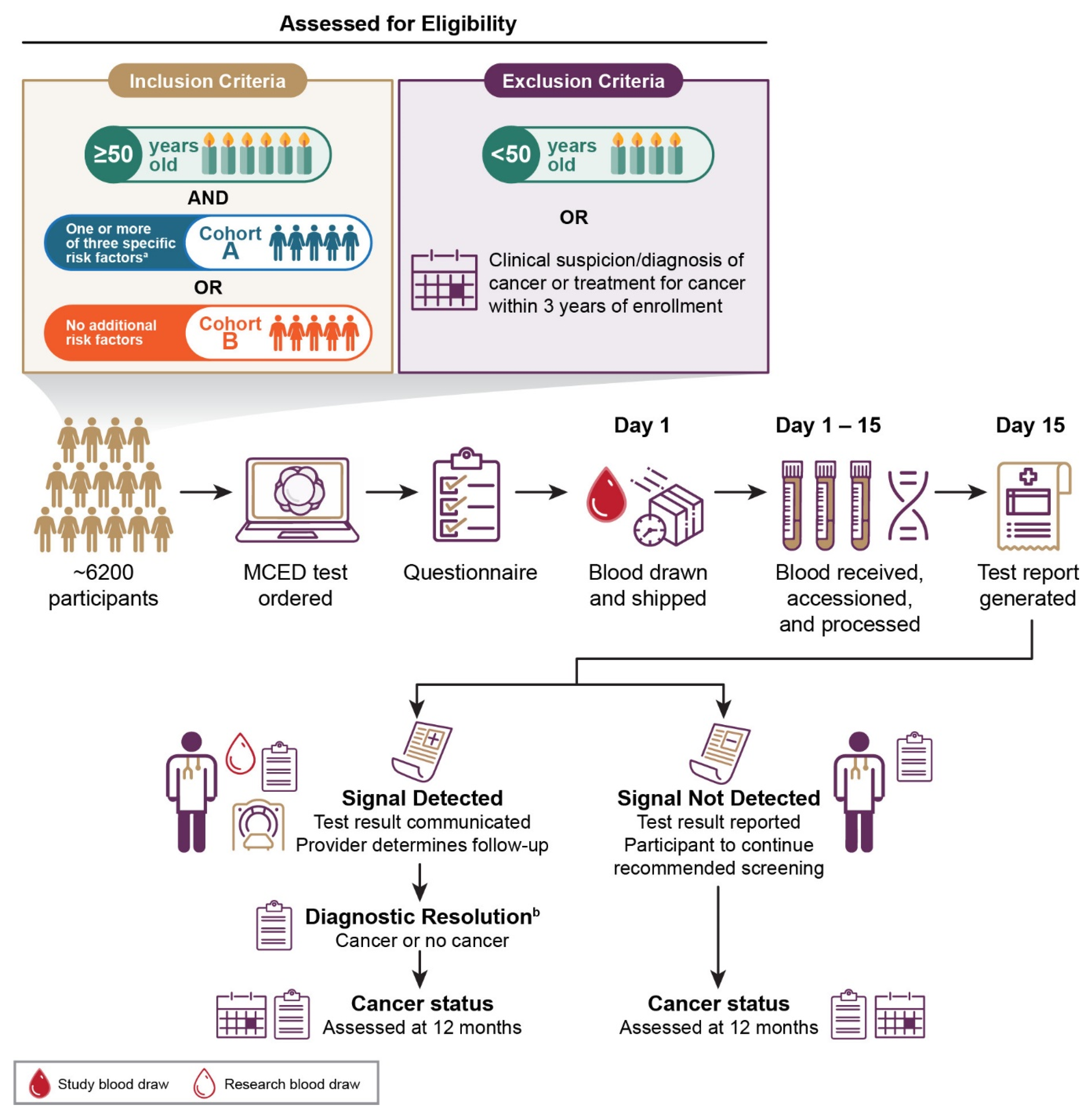
2.1. Study Design
2.2. Study Objectives and Endpoints
- Assess participants’ attitudes towards adherence to screening and subsequent MCED testing. Endpoints will include (a) change in attitude towards adherence to guideline-recommended screening [1,2,3,4,5] after an MCED test for participants with “signal not detected” result, and (b) attitude towards subsequent MCED testing.
- Assess turnaround time for test results from blood draw to when the patient receives results.
- Evaluate the impact of the COVID-19 pandemic on study endpoints, such as time to diagnostic resolution and participant anxiety levels.
- Assess the performance of follow-up MCED tests (number and proportion of participants who undergo 1 or more follow-up tests, number and proportion of “signal detected” results, and cancer incidence), health resource utilization, and time needed to achieve diagnostic resolution (e.g., the number of clinic or lab visits, imaging or invasive tests) after a follow-up “signal detected” result.
- Assess participants’ comprehension of study communications and educational materials about the MCED test.
- Evaluate changes from baseline in blood biomarkers from multiple blood draws in participants with “signal detected” results.
2.3. Eligibility
2.4. Statistical Analyses
3. Simulated Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siu, A.L.; U.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 279–296. [Google Scholar] [CrossRef] [Green Version]
- U.S. Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef] [Green Version]
- U.S. Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; García, F.A.R.; Gillman, M.W.; Harper, D.M.; Kemper, A.R.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 2564–2575. [Google Scholar] [PubMed]
- Moyer, V.A.; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [PubMed]
- Armaroli, P.; Villain, P.; Suonio, E.; Almonte, M.; Anttila, A.; Atkin, W.S.; Dean, P.B.; de Koning, H.J.; Dillner, L.; Herrero, R.; et al. European Code against Cancer, 4th Edition: Cancer screening. Cancer Epidemiol. 2015, 39 (Suppl. 1), S139–S152. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef]
- Hall, I.J.; Tangka, F.K.L.; Sabatino, S.A.; Thompson, T.D.; Graubard, B.I.; Breen, N. Patterns and trends in cancer screening in the United States. Prev. Chronic Dis. 2018, 15, E97. [Google Scholar] [CrossRef] [Green Version]
- Munoz, D.; Near, A.M.; van Ravesteyn, N.T.; Lee, S.J.; Schechter, C.B.; Alagoz, O.; Berry, D.A.; Burnside, E.S.; Chang, Y.; Chisholm, G.; et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J. Natl. Cancer Inst. 2014, 106, dju289. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Cancer-Preventive Strategies. IARC Handbooks of Cancer Prevention: Cervix Cancer Screening; IARC Press: Lyon, France, 2005; Volume 10. [Google Scholar]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Banegas, M.P.; Yabroff, K.R.; O’Keeffe-Rosetti, M.C.; Ritzwoller, D.P.; Fishman, P.A.; Salloum, R.G.; Lafata, J.E.; Hornbrook, M.C. Medical care costs associated with cancer in integrated delivery systems. J. Natl. Compr. Cancer Netw. 2018, 16, 402–410. [Google Scholar] [CrossRef] [Green Version]
- DaCosta Byfield, S.; Nash Smyth, E.; Mytelka, D.; Bowman, L.; Teitelbaum, A. Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population. J. Med. Econ. 2013, 16, 1379–1386. [Google Scholar] [CrossRef]
- Croswell, J.M.; Kramer, B.S.; Kreimer, A.R.; Prorok, P.C.; Xu, J.-L.; Baker, S.G.; Fagerstrom, R.; Riley, T.L.; Clapp, J.D.; Berg, C.D.; et al. Cumulative incidence of false-positive results in repeated, multimodal cancer screening. Ann. Fam. Med. 2009, 7, 212–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlquist, D.A. Universal cancer screening: Revolutionary, rational, and realizable. NPJ Precis. Oncol. 2018, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- Pepe, M.S.; Etzioni, R.; Feng, Z.; Potter, J.D.; Thompson, M.L.; Thornquist, M.; Winget, M.; Yasui, Y. Phases of biomarker development for early detection of cancer. J. Natl. Cancer Inst. 2001, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.; Lin, W.; Ramaiah, M.; Jung, B.; Ji, L.; Revenkova, E.; Shah, P.; Croisetiere, C.; Berman, J.; Eubank, L.; et al. Analytical Validation of a Multi-Cancer Early Detection Test with Tissue Localization Using a Cell-Free DNA-Based Targeted Methylation assay. In Proceedings of the American Association for Cancer Research Annual Meeting, Virtual Meeting, 22 June 2020; Available online: https://www.aacr.org/meeting/aacr-annual-meeting-2020/ (accessed on 22 June 2020).
- Liu, M.C.; Cummings, S.; Vachon, C.; Kerlikowske, K.; Couch, F.J.; Morris, E.A.; Olson, J.E.; Polley, E.C.; Conners, A.L.; Ellis, R.E.; et al. Development of cell-free nucleic acid-based tests for detection of invasive breast cancer: The STRIVE study. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 5–9 December 2017. [Google Scholar]
- Janes, S.M.; Dickson, J.L.; Devaraj, A.; Horst, C.; Quaife, S.; Levermore, C.; Gyertson, K.; Mullin, A.; Farrelly, L.; Allen, B.; et al. Trial in progress: Cancer screening study with or without low dose lung CT to validate a multi-cancer early detection blood test: SUMMIT. In Proceedings of the International Association for the Study of Lung Cancer, Barcelona, Spain, 7–10 September 2019. [Google Scholar]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Bevers, T.B.; Helvie, M.; Bonaccio, E.; Calhoun, K.E.; Daly, M.B.; Farrar, W.B.; Garber, J.E.; Gray, R.; Greenberg, C.C.; Greenup, R.; et al. Breast Cancer Screening and Diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 1362–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.-J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Maruish, M.E. User’s Manual for the SF-12v2 Health Survey, 3rd ed.; Quality Metric Incorporated: Lincoln, RI, USA, 2012. [Google Scholar]
- Cella, D.; Hughes, C.; Peterman, A.; Chang, C.-H.; Peshkin, B.N.; Schwartz, M.D.; Wenzel, L.; Lemke, A.; Marcus, A.C.; Lerman, C. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002, 21, 564–572. [Google Scholar] [CrossRef]
- Dewitt, B.; Feeny, D.; Fischhoff, B.; Cella, D.; Hays, R.D.; Hess, R.; Pilkonis, P.A.; Revicki, D.A.; Roberts, M.S.; Tsevat, J.; et al. Estimation of a Preference-Based Summary Score for the Patient-Reported Outcomes Measurement Information System: The PROMIS((R))-Preference (PROPr) Scoring System. Med. Decis. Mak. 2018, 38, 683–698. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.A.; Hubbell, E.; Ofman, J.J. Multi-cancer early detection: A new paradigm for reducing cancer-specific and all-cause mortality. Cancer Cell. 2021, 39, 447–448. [Google Scholar] [CrossRef]
- Freedman, N.D.; Abnet, C.C.; Caporaso, N.E.; Fraumeni, J.F., Jr.; Murphy, G.; Hartge, P.; Hollenbeck, A.R.; Park, Y.; Shiels, M.S.; Silverman, D.T. Impact of changing US cigarette smoking patterns on incident cancer: Risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int. J. Epidemiol. 2016, 45, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Center for Health Statistics. National Health Interview Survey. Available online: https://www.cdc.gov/nchs/nhis/index.htm (accessed on 30 July 2020).
- Hubbell, E.; Clarke, C.A.; Aravanis, A.M.; Berg, C.D. Modeled reductions in last-stage cancer with a multi-cancer early detection test. Cancer Epidemiol. Biomark. Prev. 2021, 30, 460–468. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Klein, E.A.; Seiden, M.V.; Hubbell, E.; Venn, O.; Jamshidi, A.; Zhang, N.; Beausang, J.F.; Gross, S.; Kurtzman, K.N.; et al. Simultaneous multi-cancer detection and tissue of origin (TOO) localization using targeted bisulfite sequencing of plasma cell-free DNA (cfDNA). Ann. Oncol. 2019, 30 (Suppl. 5), v912. [Google Scholar] [CrossRef]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Østrup Jensen, S.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019, 570, 385–389. [Google Scholar] [CrossRef]
- Shen, S.Y.; Singhania, R.; Fehringer, G.; Chakravarthy, A.; Roehrl, M.H.A.; Chadwick, D.; Zuzarte, P.C.; Borgida, A.; Wang, T.T.; Li, T.; et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018, 563, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.; Yu, J.X.; Oliva, C.; Hooton, M.; Hodge, J.; Hubbard-Lucey, V.M. Impact of COVID-19 on oncology clinical trials. Nat. Rev. Drug Discov. 2020, 19, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.A.; Myers, L.; Fields, B.K.K.; Demirjian, N.L.; Patel, D.; Roberge, E.; Gholamrezanezhad, A.; Reddy, S. Coronavirus disease 2019 (COVID-19) pandemic: Review of guidelines for resuming non-urgent imaging and procedures in radiology during Phase II. Clin. Imaging 2020, 67, 30–36. [Google Scholar] [CrossRef] [PubMed]
| Cancer Signal Origin Prediction | Proposed First-Line Procedures | |
|---|---|---|
| Multiple myeloma | Blood workup including peripheral blood smear, complete blood count (CBC) with differential; chemistry tests including creatinine clearance, protein electrophoresis of blood/urine | |
| Upper GI (esophagus, stomach) | Blood work | Endoscopy |
| Colorectal | Colonoscopy | |
| Head and neck | Physical exam, fiber optic exam, ultrasound | |
| Pancreas, gallbladder | CT abdomen with IV contrast, MRCP | |
| Ovary | Abdominal/pelvic exam, ultrasound (preferred) | |
| Lung | CT chest with or without IV contrast | |
| Liver, bile duct | Ultrasound | |
| Breast | Diagnostic mammography with ultrasound (MRI if mammography screening within last 3 months) | |
| Lymphoid neoplasm | CT (neck, chest, abdomen, pelvis) with IV contrast, PET-CT | |
| Indeterminate | CT (neck, chest, abdomen, pelvis) with IV contrast, PET-CT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadauld, L.D.; McDonnell, C.H., III; Beer, T.M.; Liu, M.C.; Klein, E.A.; Hudnut, A.; Whittington, R.A.; Taylor, B.; Oxnard, G.R.; Lipson, J.; et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers 2021, 13, 3501. https://doi.org/10.3390/cancers13143501
Nadauld LD, McDonnell CH III, Beer TM, Liu MC, Klein EA, Hudnut A, Whittington RA, Taylor B, Oxnard GR, Lipson J, et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers. 2021; 13(14):3501. https://doi.org/10.3390/cancers13143501
Chicago/Turabian StyleNadauld, Lincoln D., Charles H. McDonnell, III, Tomasz M. Beer, Minetta C. Liu, Eric A. Klein, Andrew Hudnut, Richard A. Whittington, Bruce Taylor, Geoffrey R. Oxnard, Jafi Lipson, and et al. 2021. "The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice" Cancers 13, no. 14: 3501. https://doi.org/10.3390/cancers13143501
APA StyleNadauld, L. D., McDonnell, C. H., III, Beer, T. M., Liu, M. C., Klein, E. A., Hudnut, A., Whittington, R. A., Taylor, B., Oxnard, G. R., Lipson, J., Lopatin, M., Shaknovich, R., Chung, K. C., Fung, E. T., Schrag, D., & Marinac, C. R. (2021). The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers, 13(14), 3501. https://doi.org/10.3390/cancers13143501






