Development of Randomized Trials in Adults with Medulloblastoma—The Example of EORTC 1634-BTG/NOA-23
Abstract
Simple Summary
Abstract
1. Introduction
2. Classification of Medulloblastoma
3. Backbone of Therapeutic Strategy in EORTC 1634-BTG/NOA-23
3.1. Resection
3.2. Radiotherapy
3.3. Combined Radio-Chemotherapy
3.4. Targeted Therapy
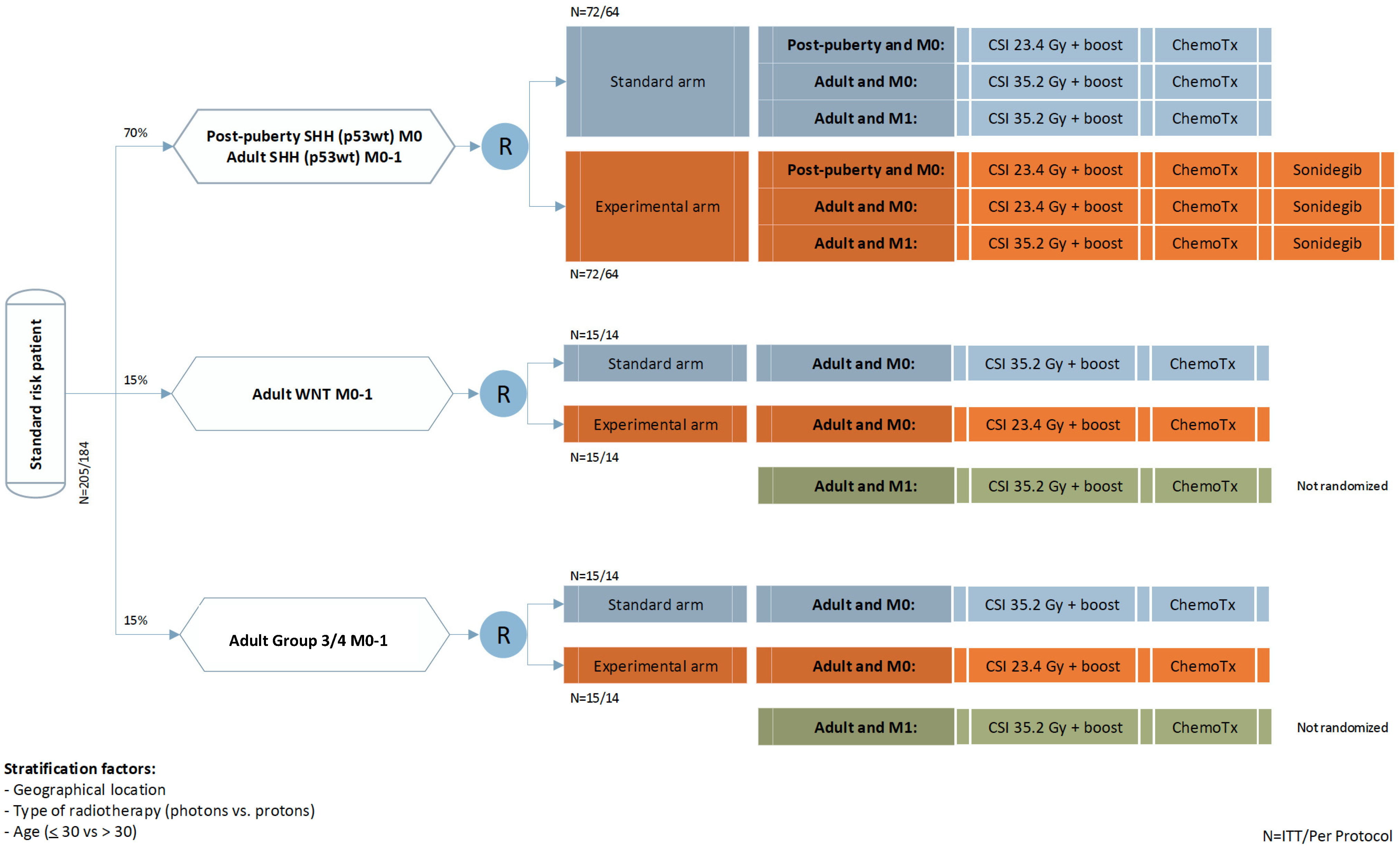
4. Trial Design
5. Objectives
6. Evaluation of Efficacy and Statistics
7. Translational Research
7.1. Neuropathology Reference and Subgrouping
7.2. Genotype-Based Subgrouping and Target Detection from Liquid Biopsies
7.3. Imaging and Radiotherapy-Related Biomarkers
7.4. Neurocognitive Function
7.5. Health-Related Quality of Life
7.6. Fertility and Endocrine Function
7.7. Comparison of Data with Data from Pediatric Trials
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smoll, N.R. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer 2012, 118, 1313–1322. [Google Scholar] [CrossRef]
- Peris-Bonet, R.; Martinez-Garcia, C.; Lacour, B.; Petrovich, S.; Giner-Ripoll, B.; Navajas, A.; Steliarova-Foucher, E. Childhood central nervous system tumours–incidence and survival in Europe (1978–1997): Report from Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 2064–2080. [Google Scholar] [CrossRef] [PubMed]
- Smoll, N.R.; Drummond, K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J. Clin. Neurosci. 2012, 19, 1541–1544. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Remke, M.; Jones, D.T.; Schlanstein, M.; Northcott, P.A.; Cho, Y.J.; Koster, J.; Schouten-van Meeteren, A.; van Vuurden, D.; et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012, 123, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Housepian, E.M.; Herbert, C., Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 1969, 93, 1351–1359. [Google Scholar] [CrossRef]
- Padovani, L.; Sunyach, M.P.; Perol, D.; Mercier, C.; Alapetite, C.; Haie-Meder, C.; Hoffstetter, S.; Muracciole, X.; Kerr, C.; Wagner, J.P.; et al. Common strategy for adult and pediatric medulloblastoma: A multicenter series of 253 adults. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 433–440. [Google Scholar] [CrossRef]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Ermani, M. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 2007, 110, 2035–2041. [Google Scholar] [CrossRef]
- Miralbell, R.; Bieri, S.; Huguenin, P.; Feldges, A.; Morin, A.M.; Garcia, E.; Wagner, H.P.; Wacker, P.; von der Weid, N. Prognostic value of cerebrospinal fluid cytology in pediatric medulloblastoma. Swiss Pediatric Oncology Group. Ann. Oncol. 1999, 10, 239–241. [Google Scholar] [CrossRef]
- Zeltzer, P.M.; Boyett, J.M.; Finlay, J.L.; Albright, A.L.; Rorke, L.B.; Milstein, J.M.; Allen, J.C.; Stevens, K.R.; Stanley, P.; Li, H.; et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children’s Cancer Group 921 randomized phase III study. J. Clin. Oncol. 1999, 17, 832–845. [Google Scholar] [CrossRef]
- Kool, M.; Korshunov, A.; Pfister, S.M. Update on molecular and genetic alterations in adult medulloblastoma. Memo 2012, 5, 228–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Remke, M.; Hielscher, T.; Northcott, P.A.; Witt, H.; Ryzhova, M.; Wittmann, A.; Benner, A.; von Deimling, A.; Scheurlen, W.; Perry, A.; et al. Adult medulloblastoma comprises three major molecular variants. J. Clin. Oncol. 2011, 29, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.H.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell 2017, 31, 737–754.e6. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.J.; Laperriere, N.J.; Wong, C.S.; Milosevic, M.F.; Simpson, W.J.; Pintilie, M. Medulloblastoma in adults. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 951–957. [Google Scholar] [CrossRef]
- Becker, R.L.; Becker, A.D.; Sobel, D.F. Adult medulloblastoma: Review of 13 cases with emphasis on MRI. Neuroradiology 1995, 37, 104–108. [Google Scholar] [CrossRef]
- Kool, M.; Jones, D.T.; Jager, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef]
- Franceschi, E.; Hofer, S.; Brandes, A.A.; Frappaz, D.; Kortmann, R.D.; Bromberg, J.; Dangouloff-Ros, V.; Boddaert, N.; Hattingen, E.; Wiestler, B.; et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019, 20, e715–e728. [Google Scholar] [CrossRef]
- Packer, R.J.; Goldwein, J.; Nicholson, H.S.; Vezina, L.G.; Allen, J.C.; Ris, M.D.; Muraszko, K.; Rorke, L.B.; Wara, W.M.; Cohen, B.H.; et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J. Clin. Oncol. 1999, 17, 2127–2136. [Google Scholar] [CrossRef]
- Taylor, R.E.; Bailey, C.C.; Robinson, K.; Weston, C.L.; Ellison, D.; Ironside, J.; Lucraft, H.; Gilbertson, R.; Tait, D.M.; Walker, D.A.; et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J. Clin. Oncol. 2003, 21, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Hauerstock, D.; Guiot, M.C.; Kasymjanova, G.; Roberge, D.; Kavan, P.; Muanza, T. Characteristics and outcomes of medulloblastoma in adults. Pediatr. Blood Cancer 2008, 51, 603–607. [Google Scholar] [CrossRef]
- Frappaz, D.; Faure-Conter, C.; Bonneville Levard, A.; Barritault, M.; Meyronet, D.; Sunyach, M.P. Medulloblastomas in adolescents and adults—Can the pediatric experience be extrapolated? Neurochirurgie 2018, 67, 76–82. [Google Scholar] [CrossRef]
- Beier, D.; Proescholdt, M.; Reinert, C.; Pietsch, T.; Jones, D.T.W.; Pfister, S.M.; Hattingen, E.; Seidel, C.; Dirven, L.; Luerding, R.; et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro Oncol. 2018, 20, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.M.; Rotondo, R.L.; Bradley, J.A.; Pincus, D.W.; Wynn, T.T.; Fort, J.A.; Morris, C.G.; Mendenhall, N.P.; Marcus, R.B., Jr.; Indelicato, D.J. Late toxicity following craniospinal radiation for early-stage medulloblastoma. Acta Oncol. 2014, 53, 471–480. [Google Scholar] [CrossRef]
- Friedrich, C.; von Bueren, A.O.; von Hoff, K.; Kwiecien, R.; Pietsch, T.; Warmuth-Metz, M.; Hau, P.; Deinlein, F.; Kuehl, J.; Kortmann, R.D.; et al. Treatment of adult nonmetastatic medulloblastoma patients according to the paediatric HIT 2000 protocol: A prospective observational multicentre study. Eur. J. Cancer 2013, 49, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Sutton, L.N.; Elterman, R.; Lange, B.; Goldwein, J.; Nicholson, H.S.; Mulne, L.; Boyett, J.; D’Angio, G.; Wechsler-Jentzsch, K.; et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J. Neurosurg. 1994, 81, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, R.D.; Kuhl, J.; Timmermann, B.; Mittler, U.; Urban, C.; Budach, V.; Richter, E.; Willich, N.; Flentje, M.; Berthold, F.; et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT ’91. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 269–279. [Google Scholar] [CrossRef]
- Lannering, B.; Rutkowski, S.; Doz, F.; Pizer, B.; Gustafsson, G.; Navajas, A.; Massimino, M.; Reddingius, R.; Benesch, M.; Carrie, C.; et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J. Clin. Oncol. 2012, 30, 3187–3193. [Google Scholar] [CrossRef]
- Franceschi, E.; Bartolotti, M.; Paccapelo, A.; Marucci, G.; Agati, R.; Volpin, L.; Danieli, D.; Ghimenton, C.; Gardiman, M.P.; Sturiale, C.; et al. Adjuvant chemotherapy in adult medulloblastoma: Is it an option for average-risk patients? J. Neurooncol. 2016, 128, 235–240. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Zhukova, N.; Ramaswamy, V.; Remke, M.; Pfaff, E.; Shih, D.J.; Martin, D.C.; Castelo-Branco, P.; Baskin, B.; Ray, P.N.; Bouffet, E.; et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J. Clin. Oncol. 2013, 31, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.J.; Northcott, P.A.; Remke, M.; Korshunov, A.; Ramaswamy, V.; Kool, M.; Luu, B.; Yao, Y.; Wang, X.; Dubuc, A.M.; et al. Cytogenetic prognostication within medulloblastoma subgroups. J. Clin. Oncol. 2014, 32, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Liu, L.; Raffel, C.; Hui, C.C.; Mainprize, T.G.; Zhang, X.; Agatep, R.; Chiappa, S.; Gao, L.; Lowrance, A.; et al. Mutations in SUFU predispose to medulloblastoma. Nat. Genet. 2002, 31, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Hielscher, T.; Bouffet, E.; Remke, M.; Luu, B.; Gururangan, S.; McLendon, R.E.; Bigner, D.D.; Lipp, E.S.; Perreault, S.; et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016, 17, 484–495. [Google Scholar] [CrossRef]
- Albright, A.L.; Wisoff, J.H.; Zeltzer, P.M.; Boyett, J.M.; Rorke, L.B.; Stanley, P. Effects of medulloblastoma resections on outcome in children: A report from the Children’s Cancer Group. Neurosurgery 1996, 38, 265–271. [Google Scholar] [CrossRef]
- Abacioglu, U.; Uzel, O.; Sengoz, M.; Turkan, S.; Ober, A. Medulloblastoma in adults: Treatment results and prognostic factors. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 855–860. [Google Scholar] [CrossRef]
- Taylor, R.E.; Bailey, C.C.; Robinson, K.J.; Weston, C.L.; Ellison, D.; Ironside, J.; Lucraft, H.; Gilbertson, R.; Tait, D.M.; Saran, F.; et al. Impact of radiotherapy parameters on outcome in the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1184–1193. [Google Scholar] [CrossRef]
- Carrie, C.; Lasset, C.; Alapetite, C.; Haie-Meder, C.; Hoffstetter, S.; Demaille, M.C.; Kerr, C.; Wagner, J.P.; Lagrange, J.L.; Maire, J.P.; et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer 1994, 74, 2352–2360. [Google Scholar] [CrossRef]
- Dietzsch, S.; Braesigk, A.; Seidel, C.; Remmele, J.; Kitzing, R.; Schlender, T.; Mynarek, M.; Geismar, D.; Jablonska, K.; Schwarz, R.; et al. Pretreatment central quality control for craniospinal irradiation in non-metastatic medulloblastoma: First experiences of the German radiotherapy quality control panel in the SIOP PNET5 MB trial. Strahlenther. Onkol. 2020. [Google Scholar] [CrossRef]
- Kortmann, R.D.; Timmermann, B.; Kuhl, J.; Willich, N.; Flentje, M.; Meisner, C.; Bamberg, M. HIT ’91 (prospective, co-operative study for the treatment of malignant brain tumors in childhood): Accuracy and acute toxicity of the irradiation of the craniospinal axis. Results of the quality assurance program. Strahlenther. Onkol. 1999, 175, 162–169. [Google Scholar] [CrossRef]
- Ibrahim, N.Y.; Abdel Aal, H.H.; Abdel Kader, M.S.; Makaar, W.S.; Shaaban, A.H. Reducing late effects of radiotherapy in average risk medulloblastoma. Chin. Clin. Oncol. 2014, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Sunyach, M.P.; Barretta, F.; Gandola, L.; Garegnani, A.; Pecori, E.; Spreafico, F.; Bonneville-Levard, A.; Meyronet, D.; Mottolese, C.; et al. Reduced-dose craniospinal irradiation is feasible for standard-risk adult medulloblastoma patients. J. Neurooncol. 2020, 148, 619–628. [Google Scholar] [CrossRef]
- Packer, R.J.; Gajjar, A.; Vezina, G.; Rorke-Adams, L.; Burger, P.C.; Robertson, P.L.; Bayer, L.; LaFond, D.; Donahue, B.R.; Marymont, M.H.; et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J. Clin. Oncol. 2006, 24, 4202–4208. [Google Scholar] [CrossRef]
- Silber, J.H.; Radcliffe, J.; Peckham, V.; Perilongo, G.; Kishnani, P.; Fridman, M.; Goldwein, J.W.; Meadows, A.T. Whole-brain irradiation and decline in intelligence: The influence of dose and age on IQ score. J. Clin. Oncol. 1992, 10, 1390–1396. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Spiegler, B.J.; Greenberg, M.L.; Rutka, J.T.; Hyder, D.J.; Bouffet, E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J. Clin. Oncol. 2005, 23, 2256–2263. [Google Scholar] [CrossRef]
- Moxon-Emre, I.; Bouffet, E.; Taylor, M.D.; Laperriere, N.; Scantlebury, N.; Law, N.; Spiegler, B.J.; Malkin, D.; Janzen, L.; Mabbott, D. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J. Clin. Oncol. 2014, 32, 1760–1768. [Google Scholar] [CrossRef]
- Ris, M.D.; Packer, R.; Goldwein, J.; Jones-Wallace, D.; Boyett, J.M. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J. Clin. Oncol. 2001, 19, 3470–3476. [Google Scholar] [CrossRef]
- Palmer, S.L.; Armstrong, C.; Onar-Thomas, A.; Wu, S.; Wallace, D.; Bonner, M.J.; Schreiber, J.; Swain, M.; Chapieski, L.; Mabbott, D.; et al. Processing speed, attention, and working memory after treatment for medulloblastoma: An international, prospective, and longitudinal study. J. Clin. Oncol. 2013, 31, 3494–3500. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Seidel, K.; Di, C.; Leisenring, W.M.; Perkins, S.M.; Krull, K.R.; Sklar, C.A.; Green, D.M.; Armstrong, G.T.; Zeltzer, L.K.; et al. Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: A report from the Childhood Cancer Survivor Study. Neuro Oncol. 2017, 19, 689–698. [Google Scholar] [CrossRef]
- Kumar, N.; Miriyala, R.; Thakur, P.; Madan, R.; Salunke, P.; Yadav, B.; Gupta, A. Impact of acute hematological toxicity on treatment interruptions during cranio-spinal irradiation in medulloblastoma: A tertiary care institute experience. J. Neurooncol. 2017, 134, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.C.; Goldberg, S.I.; Kuhl, D.; Brainau, K.A.; Lawell, M.P.; Weyman, E.A.; Gallotto, S.L.; Hess, C.B.; Huang, M.S.; Friedmann, A.M.; et al. Quality of life in patients with proton-treated pediatric medulloblastoma: Results of a prospective assessment with 5-year follow-up. Cancer 2018, 124, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.P.; Barney, C.L.; Grosshans, D.R.; McAleer, M.F.; de Groot, J.F.; Puduvalli, V.K.; Tucker, S.L.; Crawford, C.N.; Khan, M.; Khatua, S.; et al. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; MacDonald, T.; Ebb, D.; MacDonald, S.M.; Tarbell, N.J.; et al. Clinical Outcomes Among Children With Standard-Risk Medulloblastoma Treated With Proton and Photon Radiation Therapy: A Comparison of Disease Control and Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kocakaya, S.; Beier, C.P.; Beier, D. Chemotherapy increases long-term survival in patients with adult medulloblastoma—A literature-based meta-analysis. Neuro Oncol. 2016, 18, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Kann, B.H.; Lester-Coll, N.H.; Park, H.S.; Yeboa, D.N.; Kelly, J.R.; Baehring, J.M.; Becker, K.P.; Yu, J.B.; Bindra, R.S.; Roberts, K.B. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017, 19, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Ermani, M.; Amista, P.; Basso, U.; Vastola, F.; Gardiman, M.; Iuzzolino, P.; Turazzi, S.; Rotilio, A.; Volpin, L.; et al. The treatment of adults with medulloblastoma: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 755–761. [Google Scholar] [CrossRef]
- Herrlinger, U.; Steinbrecher, A.; Rieger, J.; Hau, P.; Kortmann, R.D.; Meyermann, R.; Schabet, M.; Bamberg, M.; Dichgans, J.; Bogdahn, U.; et al. Adult medulloblastoma: Prognostic factors and response to therapy at diagnosis and at relapse. J. Neurol. 2005, 252, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Minichillo, S.; Mura, A.; Tosoni, A.; Mascarin, M.; Tomasello, C.; Bartolini, S.; Brandes, A.A. Adjuvant chemotherapy in average-risk adult medulloblastoma patients improves survival: A long term study. BMC Cancer 2020, 20, 755. [Google Scholar] [CrossRef]
- Von Bueren, A.O.; Friedrich, C.; von Hoff, K.; Kwiecien, R.; Muller, K.; Pietsch, T.; Warmuth-Metz, M.; Hau, P.; Benesch, M.; Kuehl, J.; et al. Metastatic medulloblastoma in adults: Outcome of patients treated according to the HIT2000 protocol. Eur. J. Cancer 2015, 51, 2434–2443. [Google Scholar] [CrossRef]
- Pak, E.; MacKenzie, E.L.; Zhao, X.; Pazyra-Murphy, M.F.; Park, P.M.C.; Wu, L.; Shaw, D.L.; Addleson, E.C.; Cayer, S.S.; Lopez, B.G.; et al. A large-scale drug screen identifies selective inhibitors of class I HDACs as a potential therapeutic option for SHH medulloblastoma. Neuro Oncol. 2019, 21, 1150–1163. [Google Scholar] [CrossRef]
- Northcott, P.A.; Robinson, G.W.; Kratz, C.P.; Mabbott, D.J.; Pomeroy, S.L.; Clifford, S.C.; Rutkowski, S.; Ellison, D.W.; Malkin, D.; Taylor, M.D.; et al. Medulloblastoma. Nat. Rev. Dis. Prim. 2019, 5, 11. [Google Scholar] [CrossRef]
- Thompson, E.M.; Ashley, D.; Landi, D. Current medulloblastoma subgroup specific clinical trials. Transl. Pediatr. 2020, 9, 157–162. [Google Scholar] [CrossRef]
- Frappaz, D.; Barritault, M.; Montane, L.; Laigle-Donadey, F.; Chinot, O.; Le Rhun, E.; Bonneville-Levard, A.; Hottinger, A.F.; Meyronnet, D.; Bidaux, A.S.; et al. MEVITEM—A Phase I/II of vismodegib + temozolomide vs temozolomide in patients with recurrent/refractory medulloblastoma with Sonic Hedgehog pathway activation. Neuro Oncol. 2021. [Google Scholar] [CrossRef]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef]
- Samkari, A.; White, J.; Packer, R. SHH inhibitors for the treatment of medulloblastoma. Expert Rev. Neurother. 2015, 15, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gajjar, A.; Stewart, C.F.; Ellison, D.W.; Kaste, S.; Kun, L.E.; Packer, R.J.; Goldman, S.; Chintagumpala, M.; Wallace, D.; Takebe, N.; et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: A pediatric brain tumor consortium study. Clin. Cancer Res. 2013, 19, 6305–6312. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.W.; Orr, B.A.; Wu, G.; Gururangan, S.; Lin, T.; Qaddoumi, I.; Packer, R.J.; Goldman, S.; Prados, M.D.; Desjardins, A.; et al. Vismodegib Exerts Targeted Efficacy Against Recurrent Sonic Hedgehog-Subgroup Medulloblastoma: Results From Phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 2015, 33, 2646–2654. [Google Scholar] [CrossRef]
- Shou, Y.; Robinson, D.M.; Amakye, D.D.; Rose, K.L.; Cho, Y.J.; Ligon, K.L.; Sharp, T.; Haider, A.S.; Bandaru, R.; Ando, Y.; et al. A five-gene hedgehog signature developed as a patient preselection tool for hedgehog inhibitor therapy in medulloblastoma. Clin. Cancer Res. 2015, 21, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Tawbi, H.A.; Thomas, A.L.; Stoller, R.G.; Turtschi, C.P.; Baselga, J.; Sarantopoulos, J.; Mahalingam, D.; Shou, Y.; Moles, M.A.; et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 2014, 20, 1900–1909. [Google Scholar] [CrossRef]
- Kieran, M.W.; Chisholm, J.; Casanova, M.; Brandes, A.A.; Aerts, I.; Bouffet, E.; Bailey, S.; Leary, S.; MacDonald, T.J.; Mechinaud, F.; et al. Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro Oncol. 2017, 19, 1542–1552. [Google Scholar] [CrossRef]
- Robinson, G.W.; Kaste, S.C.; Chemaitilly, W.; Bowers, D.C.; Laughton, S.; Smith, A.; Gottardo, N.G.; Partap, S.; Bendel, A.; Wright, K.D.; et al. Irreversible growth plate fusions in children with medulloblastoma treated with a targeted hedgehog pathway inhibitor. Oncotarget 2017, 8, 69295–69302. [Google Scholar] [CrossRef]
- Morrissy, A.S.; Garzia, L.; Shih, D.J.; Zuyderduyn, S.; Huang, X.; Skowron, P.; Remke, M.; Cavalli, F.M.; Ramaswamy, V.; Lindsay, P.E.; et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 2016, 529, 351–357. [Google Scholar] [CrossRef]
- Li, Y.; Song, Q.; Day, B.W. Phase I and phase II sonidegib and vismodegib clinical trials for the treatment of paediatric and adult MB patients: A systemic review and meta-analysis. Acta Neuropathol. Commun. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Song, R.; Xie, J. Sonidegib: Mechanism of action, pharmacology, and clinical utility for advanced basal cell carcinomas. Onco Targets Ther. 2017, 10, 1645–1653. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef]
- Peng, J.; Zhou, H.; Tang, O.; Chang, K.; Wang, P.; Zeng, X.; Shen, Q.; Wu, J.; Xiao, Y.; Patel, S.H.; et al. Evaluation of RAPNO criteria in medulloblastoma and other leptomeningeal seeding tumors using MRI and clinical data. Neuro Oncol. 2020, 22, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, H.; Yakisich, J.S. Overcoming the blood-brain barrier for chemotherapy: Limitations, challenges and rising problems. Anticancer Agents Med. Chem. 2014, 14, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.; Demko, S.; Shord, S.; Zhao, H.; Chen, H.; He, K.; Putman, A.; Helms, W.; Keegan, P.; Pazdur, R. FDA Approval Summary: Sonidegib for Locally Advanced Basal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2377–2381. [Google Scholar] [CrossRef]
- Atalar, B.; Ozsahin, M.; Call, J.; Napieralska, A.; Kamer, S.; Villa, S.; Erpolat, P.; Negretti, L.; Lassen-Ramshad, Y.; Onal, C.; et al. Treatment outcome and prognostic factors for adult patients with medulloblastoma: The Rare Cancer Network (RCN) experience. Radiother. Oncol. 2018, 127, 96–102. [Google Scholar] [CrossRef]
- Oyharcabal-Bourden, V.; Kalifa, C.; Gentet, J.C.; Frappaz, D.; Edan, C.; Chastagner, P.; Sariban, E.; Pagnier, A.; Babin, A.; Pichon, F.; et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: A French Society of Pediatric Oncology Study. J. Clin. Oncol. 2005, 23, 4726–4734. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Jones, D.T.; Meyer, J.; Kratz, A.; Reuss, D.; Capper, D.; Koelsche, C.; Korshunov, A.; Wiestler, B.; et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016, 131, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Escudero, L.; Llort, A.; Arias, A.; Diaz-Navarro, A.; Martinez-Ricarte, F.; Rubio-Perez, C.; Mayor, R.; Caratu, G.; Martinez-Saez, E.; Vazquez-Mendez, E.; et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat. Commun. 2020, 11, 5376. [Google Scholar] [CrossRef]
- Keil, V.C.; Warmuth-Metz, M.; Reh, C.; Enkirch, S.J.; Reinert, C.; Beier, D.; Jones, D.T.W.; Pietsch, T.; Schild, H.H.; Hattingen, E.; et al. Imaging Biomarkers for Adult Medulloblastomas: Genetic Entities May Be Identified by Their MR Imaging Radiophenotype. AJNR Am. J. Neuroradiol. 2017, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.W.; Ghamdi, M.A.A.; Hussain, M.; Khan, M.A.; Khan, K.M.; Almotiri, S.H.; Butt, S.A. Brain Tumor Analysis Empowered with Deep Learning: A Review, Taxonomy, and Future Challenges. Brain Sci. 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Grinband, J.; Weinberg, B.D.; Bardis, M.; Khy, M.; Cadena, G.; Su, M.Y.; Cha, S.; Filippi, C.G.; Bota, D.; et al. Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, P.; Alberts, E.; Delbridge, C.; Trebeschi, S.; Valentinitsch, A.; Bette, S.; Huber, T.; Gempt, J.; Meyer, B.; Schlegel, J.; et al. Diffusion tensor image features predict IDH genotype in newly diagnosed WHO grade II/III gliomas. Sci. Rep. 2017, 7, 13396. [Google Scholar] [CrossRef]
- Metz, M.C.; Molina-Romero, M.; Lipkova, J.; Gempt, J.; Liesche-Starnecker, F.; Eichinger, P.; Grundl, L.; Menze, B.; Combs, S.E.; Zimmer, C.; et al. Predicting Glioblastoma Recurrence from Preoperative MR Scans Using Fractional-Anisotropy Maps with Free-Water Suppression. Cancers 2020, 12, 728. [Google Scholar] [CrossRef]
- Lipkova, J.; Angelikopoulos, P.; Wu, S.; Alberts, E.; Wiestler, B.; Diehl, C.; Preibisch, C.; Pyka, T.; Combs, S.E.; Hadjidoukas, P.; et al. Personalized Radiotherapy Design for Glioblastoma: Integrating Mathematical Tumor Models, Multimodal Scans, and Bayesian Inference. IEEE Trans. Med. Imaging 2019, 38, 1875–1884. [Google Scholar] [CrossRef]
- De, B.; Beal, K.; De Braganca, K.C.; Souweidane, M.M.; Dunkel, I.J.; Khakoo, Y.; Gilheeney, S.W.; DeAngelis, L.M.; Menzel, P.; Patel, S.H.; et al. Long-term outcomes of adult medulloblastoma patients treated with radiotherapy. J. Neurooncol. 2018, 136, 95–104. [Google Scholar] [CrossRef]
- Taphoorn, M.J.; Klein, M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004, 3, 159–168. [Google Scholar] [CrossRef]
- Klein, M.; Postma, T.J.; Taphoorn, M.J.; Aaronson, N.K.; Vandertop, W.P.; Muller, M.; van der Ploeg, H.M.; Heimans, J.J. The prognostic value of cognitive functioning in the survival of patients with high-grade glioma. Neurology 2003, 61, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.L.; Goldstein, B.; Shera, D.; Ledakis, G.E.; Tallent, E.M. The predictive value of longitudinal neuropsychologic assessment in the early detection of brain tumor recurrence. Cancer 2003, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Benedict, R.H.; Schretlen, D.; Brandt, J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin. Neuropsychol. 1999, 13, 348–358. [Google Scholar] [CrossRef]
- Ehrenstein, W.H.; Heister, G.; Cohen, R. Trail Making Test and visual search. Arch. Psychiatr. Nervenkr. 1982, 231, 333–338. [Google Scholar] [CrossRef]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef]
- Monteiro, S.; Torres, A.; Morgadinho, R.; Pereira, A. Psychosocial outcomes in young adults with cancer: Emotional distress, quality of life and personal growth. Arch. Psychiatr. Nurs. 2013, 27, 299–305. [Google Scholar] [CrossRef]
- Sodergren, S.C.; Husson, O.; Robinson, J.; Rohde, G.E.; Tomaszewska, I.M.; Vivat, B.; Dyar, R.; Darlington, A.S.; Group, E.Q.o.L. Systematic review of the health-related quality of life issues facing adolescents and young adults with cancer. Qual. Life Res. 2017, 26, 1659–1672. [Google Scholar] [CrossRef]
- Dirven, L.; Luerding, R.; Beier, D.; Bumes, E.; Reinert, C.; Seidel, C.; Bonsanto, M.M.; Bremer, M.; Rieken, S.; Combs, S.E.; et al. Neurocognitive functioning and health-related quality of life in adult medulloblastoma patients: Long-term outcomes of the NOA-07 study. J. Neurooncol. 2020, 148, 117–130. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Van Leeuwen, M.; Husson, O.; Alberti, P.; Arraras, J.I.; Chinot, O.L.; Costantini, A.; Darlington, A.S.; Dirven, L.; Eichler, M.; Hammerlid, E.B.; et al. Understanding the quality of life (QOL) issues in survivors of cancer: Towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual. Life Outcomes 2018, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, S.; Dunkel, I.J.; Khakoo, Y.; Wolden, S.; Allen, J.; Sklar, C.A. Ovarian function in survivors of childhood medulloblastoma: Impact of reduced dose craniospinal irradiation and high-dose chemotherapy with autologous stem cell rescue. Pediatr. Blood Cancer 2015, 62, 317–321. [Google Scholar] [CrossRef]
- Cuny, A.; Trivin, C.; Brailly-Tabard, S.; Adan, L.; Zerah, M.; Sainte-Rose, C.; Alapetite, C.; Brugieres, L.; Habrand, J.L.; Doz, F.; et al. Inhibin B and anti-Mullerian hormone as markers of gonadal function after treatment for medulloblastoma or posterior fossa ependymoma during childhood. J. Pediatr. 2011, 158, 1016–1022.e1. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Perz, J.; Ussher, J.M.; Peate, M.; Anazodo, A. Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology 2019, 28, 22–30. [Google Scholar] [CrossRef]
- Anazodo, A.; Ataman-Millhouse, L.; Jayasinghe, Y.; Woodruff, T.K. Oncofertility-An emerging discipline rather than a special consideration. Pediatr. Blood Cancer 2018, 65, e27297. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.; Perz, J.; Ussher, J.; Peate, M.; Anazodo, A. Clinician provision of oncofertility support in cancer patients of a reproductive age: A systematic review. Psychooncology 2018, 27, 748–756. [Google Scholar] [CrossRef]
- Dauti, A.; Gerstl, B.; Chong, S.; Chisholm, O.; Anazodo, A. Improvements in Clinical Trials Information Will Improve the Reproductive Health and Fertility of Cancer Patients. J. Adolesc. Young Adult Oncol. 2017, 6, 235–269. [Google Scholar] [CrossRef]
- Volckmar, X.; Vallejo, M.; Bertoldo, M.J.; Nguyen, Q.N.; Handelsman, D.J.; Chisholm, O.; Anazodo, A. Oncofertility Information Available for Recently Approved Novel Non Cytotoxic and Immunotherapy Oncology Drugs. Clin. Pharmacol. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hau, P.; Frappaz, D.; Hovey, E.; McCabe, M.G.; Pajtler, K.W.; Wiestler, B.; Seidel, C.; Combs, S.E.; Dirven, L.; Klein, M.; et al. Development of Randomized Trials in Adults with Medulloblastoma—The Example of EORTC 1634-BTG/NOA-23. Cancers 2021, 13, 3451. https://doi.org/10.3390/cancers13143451
Hau P, Frappaz D, Hovey E, McCabe MG, Pajtler KW, Wiestler B, Seidel C, Combs SE, Dirven L, Klein M, et al. Development of Randomized Trials in Adults with Medulloblastoma—The Example of EORTC 1634-BTG/NOA-23. Cancers. 2021; 13(14):3451. https://doi.org/10.3390/cancers13143451
Chicago/Turabian StyleHau, Peter, Didier Frappaz, Elizabeth Hovey, Martin G. McCabe, Kristian W. Pajtler, Benedikt Wiestler, Clemens Seidel, Stephanie E. Combs, Linda Dirven, Martin Klein, and et al. 2021. "Development of Randomized Trials in Adults with Medulloblastoma—The Example of EORTC 1634-BTG/NOA-23" Cancers 13, no. 14: 3451. https://doi.org/10.3390/cancers13143451
APA StyleHau, P., Frappaz, D., Hovey, E., McCabe, M. G., Pajtler, K. W., Wiestler, B., Seidel, C., Combs, S. E., Dirven, L., Klein, M., Anazodo, A., Hattingen, E., Hofer, S., Pfister, S. M., Zimmer, C., Kortmann, R.-D., Sunyach, M.-P., Tanguy, R., Effeney, R., ... Weller, M. (2021). Development of Randomized Trials in Adults with Medulloblastoma—The Example of EORTC 1634-BTG/NOA-23. Cancers, 13(14), 3451. https://doi.org/10.3390/cancers13143451










