Impaired Set-Shifting from Dorsal Stream Disconnection: Insights from a European Series of Right Parietal Lower-Grade Glioma Resection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Participant Data
2.3. Cognitive Data
2.3.1. Neuropsychological Assessment
2.3.2. Analyses of the Neuropsychological Data
2.4. Imaging Data
2.5. Image Analyses
3. Results
3.1. Demographic Data
3.2. Neuropsychological Outcome
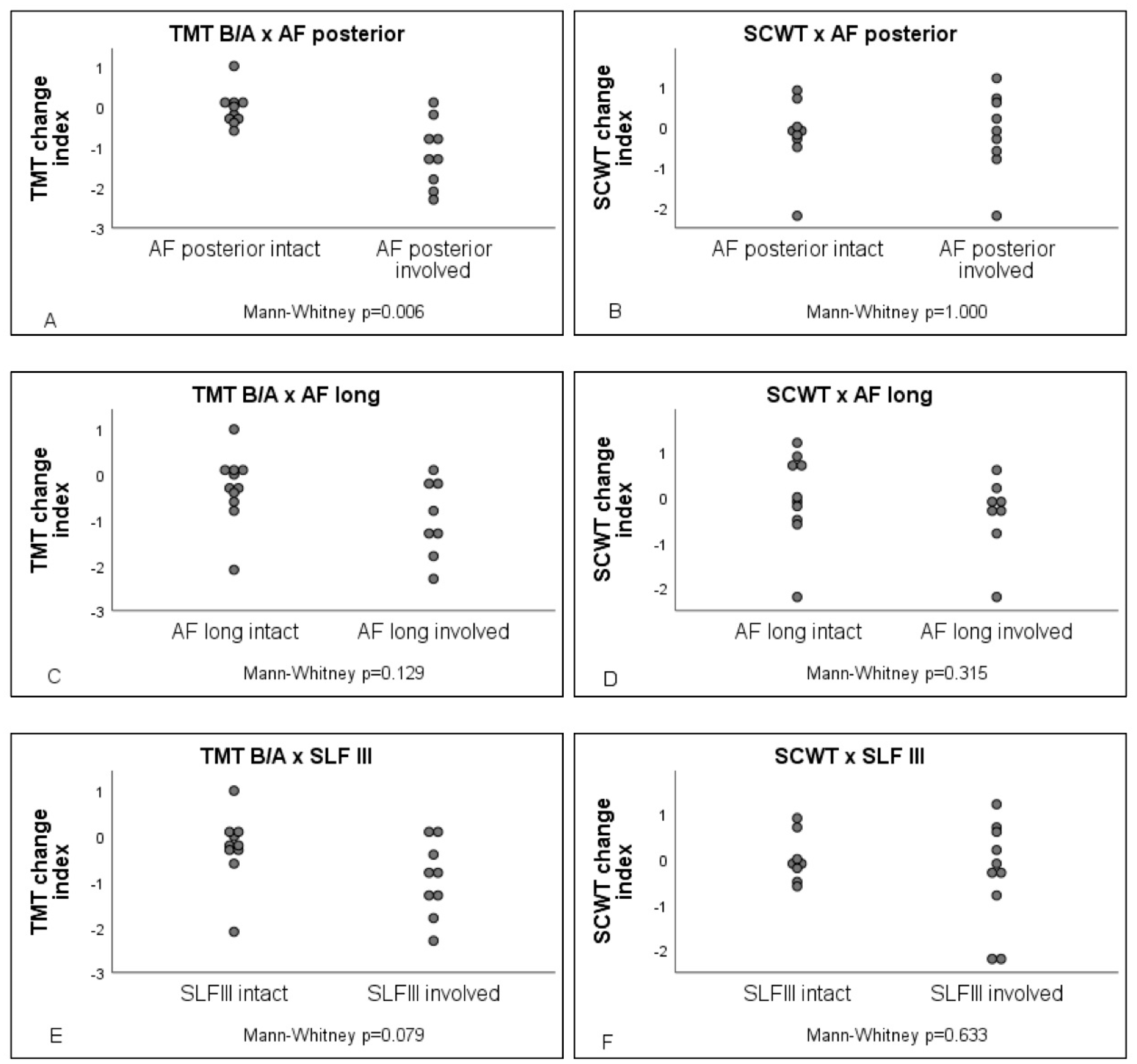
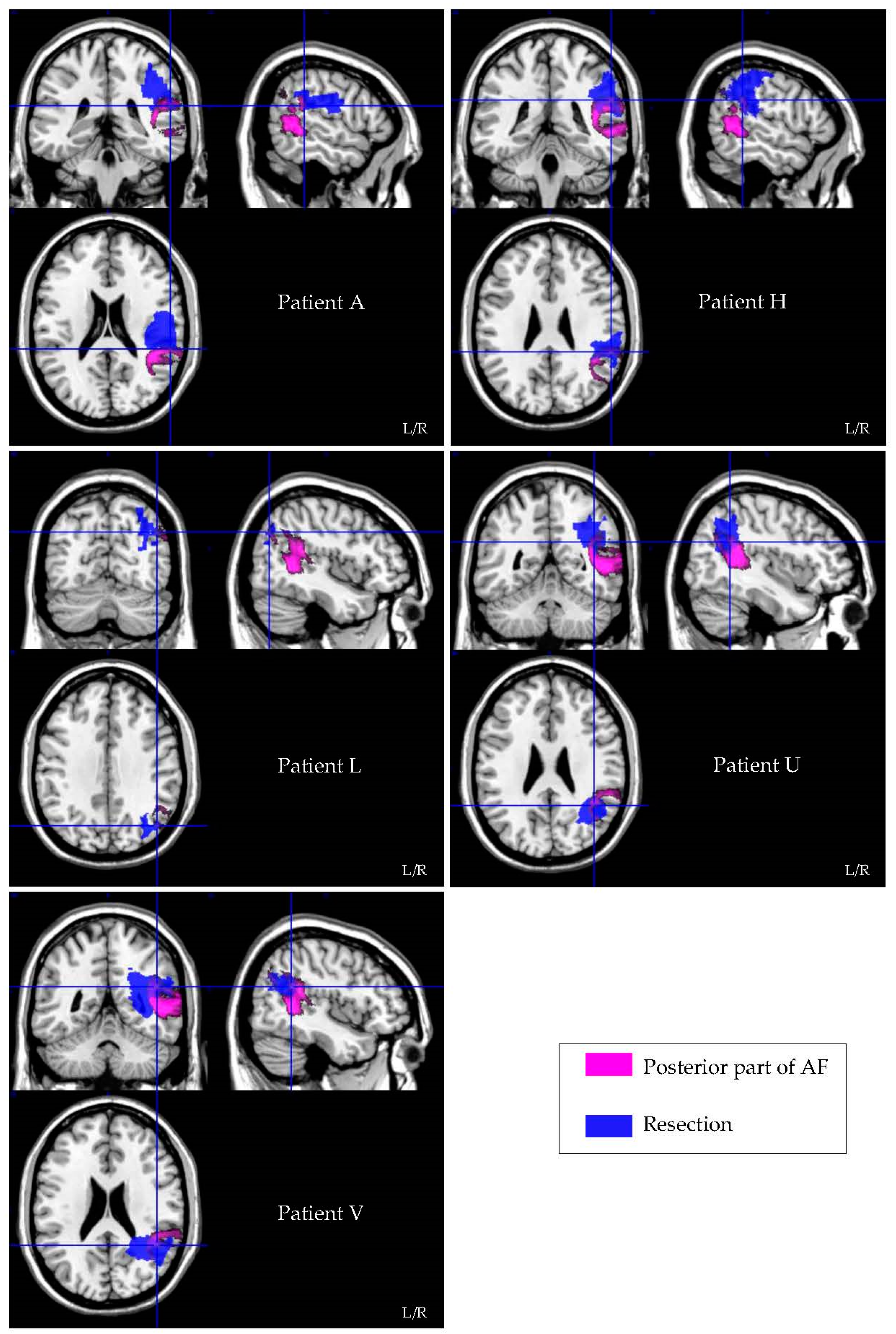
3.3. Lesion Symptom Mapping Analysis
3.4. Resection Volume Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandonnet, E.; Vincent, M.; Valero-Cabré, A.; Facque, V.; Barberis, M.; Bonnetblanc, F.; Rheault, F.; Volle, E.; Descoteaux, M.; Margulies, D.S. Network-level causal analysis of set-shifting during trail making test part B: A multimodal analysis of a glioma surgery case. Cortex 2020, 132, 238–249. [Google Scholar] [CrossRef]
- Altieri, R.; Raimondo, S.; Tiddia, C.; Sammarco, D.; Cofano, F.; Zeppa, P.; Monticelli, M.; Melcarne, A.; Junemann, C.; Zenga, F.; et al. Glioma surgery: From preservation of motor skills to conservation of cognitive functions. J. Clin. Neurosci. 2019, 70, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgeman, B.; Young, A.W. Functions of the Right Cerebral Hemisphere. Am. J. Psychol. 1986, 99, 564. [Google Scholar] [CrossRef]
- Gogos, A.J.; Young, J.S.; Morshed, R.A.; Hervey-Jumper, S.L.; Berger, M.S. Awake glioma surgery: Technical evolution and nuances. J. Neuro Oncol. 2020, 147, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Barbey, A.K.; Colom, R.; Grafman, J. Architecture of cognitive flexibility revealed by lesion mapping. NeuroImage 2013, 82, 547–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandonnet, E.; Cerliani, L.; Siuda-Krzywicka, K.; Poisson, I.; Zhi, N.; Volle, E.; De Schotten, M.T. A net-work-level approach of cognitive flexibility impairment after surgery of a right temporo-parietal glioma. Neurochirurgie 2017, 63, 308–313. [Google Scholar] [CrossRef]
- Mandonnet, E.; Sarubbo, S.; Petit, L. The Nomenclature of Human White Matter Association Pathways: Proposal for a Systematic Taxonomic Anatomical Classification. Front. Neuroanat. 2018, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Dajani, D.R.; Uddin, L.Q. Demystifying cognitive flexibility: Implications for clinical and developmental neu-roscience. Trends Neurosci. 2015, 38, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Danckert, J.; Stöttinger, E.; Quehl, N.; Anderson, B. Right Hemisphere Brain Damage Impairs Strategy Updating. Cereb. Cortex 2011, 22, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Stroop, J.R. Stroop color word test. J. Exp. Physiol. 1935, 18, 643–662. [Google Scholar]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.M.; Vogels, R.L.C.; Van Harten, B.; Gouw, A.A.; Poggesi, A.; Scheltens, P.; Kessels, R.P.C.; Scherder, E.J.A. Assessing mental flexibility: Neuroanatomical and neuropsychological correlates of the trail making test in elderly people. Clin. Neuropsychol. 2010, 24, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cubillo, I.; Perianez, J.A.; Adrover-Roig, D.; Rodriguez-Sanchez, J.M.; Rios-Lago, M.; Tirapu, J.E.E.A.; Barcelo, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibi-tion/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbuthnott, K.; Frank, J. Trail Making Test, Part B as a Measure of Executive Control: Validation Using a Set-Switching Paradigm. J. Clin. Exp. Neuropsychol. 2000, 22, 518–528. [Google Scholar] [CrossRef]
- Christidi, F.; Kararizou, E.; Triantafyllou, N.; Anagnostouli, M.; Zalonis, I. Derived Trail Making Test indices: Demographics and cognitive background variables across the adult life span. Aging Neuropsychol. Cogn. 2015, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Periáñez, A.J.; Lubrini, G.; García-Gutiérrez, A.; Rios-Lago, M. Construct Validity of the Stroop Color-Word Test: Influence of Speed of Visual Search, Verbal Fluency, Working Memory, Cognitive Flexibility, and Conflict Monitoring. Arch. Clin. Neuropsychol. 2021, 36, 99–111. [Google Scholar] [CrossRef]
- Comalli, P.E., Jr.; Wapner, S.; Werner, H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J. Genet. Psychol. 1962, 100, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Stuss, D.T.; Floden, D.; Alexander, M.P.; Levine, B.; Katz, D. Stroop performance in focal lesion patients: Dis-sociation of processes and frontal lobe lesion location. Neuropsychologia 2001, 39, 771–786. [Google Scholar] [CrossRef]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. Detecting the significance of changes in per-formance on the Stroop Color-Word Test, Rey’s Verbal Learning Test, and the Letter Digit Substitution Test: The regres-sion-based change approach. J. Int. Neuropsychol. Soc. 2008, 14, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Van der Elst, W.; Van Boxtel, M.P.; Van Breukelen, G.J.; Jolles, J. The Stroop color-word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment 2006, 13, 62–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar] [PubMed]
- Rorden, C.; Bonilha, L.; Fridriksson, J.; Bender, B.; Karnath, H.-O. Age-specific CT and MRI templates for spatial normalization. NeuroImage 2012, 61, 957–965. [Google Scholar] [CrossRef] [Green Version]
- The Math Works, Inc. MATLAB, Version R2018b; The Math Works, Inc.: Natick, MA, USA, 2018. [Google Scholar]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Brett, M.; Leff, A.; Rorden, C.; Ashburner, J. Spatial Normalization of Brain Images with Focal Lesions Using Cost Function Masking. NeuroImage 2001, 14, 486–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorden, C.; Karnath, H.-O.; Bonilha, L. Improving Lesion-Symptom Mapping. J. Cogn. Neurosci. 2007, 19, 1081–1088. [Google Scholar] [CrossRef]
- Rojkova, K.; Volle, E.; Urbanski, M.; Humbert, F.; Dell’Acqua, F.; De Schotten, M.T. Atlasing the frontal lobe connections and their variability due to age and education: A spherical deconvolution tractography study. Brain Struct. Funct. 2016, 221, 1751–1766. [Google Scholar] [CrossRef]
- Chaytor, N.; Schmitter-Edgecombe, M.; Burr, R. Improving the ecological validity of executive functioning as-sessment. Arch. Clin. Neuropsychol. 2006, 21, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Buttelmann, F.; Karbach, J. Development and Plasticity of Cognitive Flexibility in Early and Middle Childhood. Front. Psychol. 2017, 8, 1040. [Google Scholar] [CrossRef] [Green Version]
- Colé, P.; Duncan, L.G.; Blaye, A. Cognitive flexibility predicts early reading skills. Front. Psychol. 2014, 5, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, T.U.; Iannaccone, R.; Walitza, S.; Brandeis, D.; Brem, S. Cognitive flexibility in adolescence: Neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage 2015, 104, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Genet, J.J.; Siemer, M. Flexible control in processing affective and non-affective material predicts individual differences in trait resilience. Cogn. Emot. 2011, 25, 380–388. [Google Scholar] [CrossRef]
- Mandonnet, E.; Herbet, G.; Duffau, H. Letter: Introducing New Tasks for Intraoperative Mapping in Awake Glioma Surgery: Clearing the Line Between Patient Care and Scientific Research. Neurosurgery 2019, 86, E256–E257. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Awake Surgery for Nonlanguage Mapping. Neurosurgery 2010, 66, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Parisot, S.; Darlix, A.; Baumann, C.; Zouaoui, S.; Yordanova, Y.N.; Blonski, M.; Rigau, V.; Chemouny, S.; Taillandier, L.; Bauchet, L.; et al. A Probabilistic Atlas of Diffuse WHO Grade II Glioma Locations in the Brain. PLoS ONE 2016, 11, e0144200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pa, J.; Possin, K.L.; Wilson, S.M.; Quitania, L.C.; Kramer, J.H.; Boxer, A.L.; Weiner, M.W.; Johnson, J.K. Gray matter correlates of set-shifting among neurodegenerative disease, mild cognitive impairment, and healthy older adults. J. Int. Neuropsychol. Soc. 2010, 16, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Klein, M. Neurocognitive functioning in adult WHO grade II gliomas: Impact of old and new treatment modalities. Neuro-Oncology 2012, 14, iv17–iv24. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.G.; Reid, R.I.; Gunter, J.L.; Senjem, M.L.; Przybelski, S.A.; Zuk, S.M.; Whitwell, J.L.; Vemuri, P.; Josephs, K.A.; Kantarci, K.; et al. Improved DTI registration allows voxel-based analysis that outperforms Tract-Based Spatial Statistics. NeuroImage 2014, 94, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient | Age | Sex | Months * | Radiotherapy * | Chemotherapy * | Rehabilitation * | Seizures * | Start AED * |
|---|---|---|---|---|---|---|---|---|
| A | 47 | M | 6 | N | N | N | N | N |
| B | 36 | F | 4 | Y | N | N | Y | Y |
| C | 31 | M | 9 | N | N | N | N | N |
| D | 34 | M | 6 | Y | N | N | N | N |
| E | 55 | M | 6 | N | N | Y | N | Y |
| F | 33 | F | 3 | Y | N | N | N | N |
| G | 21 | M | 3 | N | N | N | N | N |
| H | 40 | F | 4 | N | N | Y | N | N |
| I | 53 | M | 4 | N | N | Y | N | N |
| J | 36 | M | 4 | N | N | Y | N | N |
| K | 31 | F | 3 | N | N | Y | N | N |
| L | 67 | F | 3 | N | N | Y | N | N |
| M | 49 | F | 3 | N | N | Y | N | N |
| N | 39 | F | 12 | Y | N | N | Y | Y |
| O | 34 | M | 15 | Y | N | Y | N | N |
| P | 23 | F | 11 | Y | N | Y | N | N |
| Q | 39 | F | 17 | Y | N | N | N | N |
| R | 48 | F | 18 | Y | Y | Y | N | N |
| S | 30 | M | 3 | N | N | N | N | N |
| T | 48 | M | 4 | N | N | N | N | N |
| U | 59 | F | 5 | N | N | N | Y | Y |
| V | 49 | M | 3 | N | N | N | N | N |
| TMT | SCWT | |||||
|---|---|---|---|---|---|---|
| Preoperative * | Postoperative * | Change Index | Preoperative * | Postoperative * | Change Index | |
| A | +1.0 | −0.3 | −1.3 | +1.3 | +1.0 | −0.3 |
| B | +0.3 | +0.4 | +0.1 | +1.0 | +0.7 | −0.3 |
| C | +1.1 | +0.9 | −0.2 | +1.8 | +1.7 | −0.1 |
| D | +0.5 | +0.2 | −0.3 | +0.6 | +0.1 | −0.5 |
| E | −0.6 | +0.4 | +1.0 | - | +0.2 | - |
| F | +0.3 | +0.3 | 0.0 | +0.4 | +0.3 | −0.1 |
| G | +0.1 | −0.8 | −0.8 | −0.6 | +0.1 | +0.7 |
| H | −0.1 | −1.9 | −1.8 | −0.2 | −2.4 | −2.2 |
| I | +0.3 | +0.4 | +0.1 | −1.8 | −0.7 | +1.2 |
| J | +1.0 | +0.7 | −0.3 | −1.0 | −0.1 | +0.9 |
| K | −0.1 | −0.8 | −0.8 | −0.3 | −1.2 | −0.8 |
| L | −1.2 | −3.3 | −2.1 | +0.7 | +0.1 | −0.6 |
| M | +0.2 | +0.3 | +0.1 | +0.8 | +0.6 | −0.2 |
| N | −0.1 | −0.5 | −0.4 | +0.2 | −1.9 | −2.2 |
| O | +0.9 | +0.3 | −0.6 | +0.5 | +1.2 | +0.7 |
| P | +0.4 | +0.5 | +0.1 | +0.8 | +0.8 | +0.0 |
| Q | - | - | - | +1.1 | +1.0 | −0.1 |
| R | - | - | - | +2.2 | +2.4 | +0.2 |
| S | - | - | - | +0.1 | +0.7 | +0.6 |
| T | +0.5 | +0.3 | −0.2 | - | - | - |
| U | −2.6 | −3.9 | −1.3 | - | - | - |
| V | +1 | −1.3 | −2.3 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartung, S.L.; Mandonnet, E.; de Witt Hamer, P.; Klein, M.; Wager, M.; Rech, F.; Pallud, J.; Pessanha Viegas, C.; Ille, S.; Krieg, S.M.; et al. Impaired Set-Shifting from Dorsal Stream Disconnection: Insights from a European Series of Right Parietal Lower-Grade Glioma Resection. Cancers 2021, 13, 3337. https://doi.org/10.3390/cancers13133337
Hartung SL, Mandonnet E, de Witt Hamer P, Klein M, Wager M, Rech F, Pallud J, Pessanha Viegas C, Ille S, Krieg SM, et al. Impaired Set-Shifting from Dorsal Stream Disconnection: Insights from a European Series of Right Parietal Lower-Grade Glioma Resection. Cancers. 2021; 13(13):3337. https://doi.org/10.3390/cancers13133337
Chicago/Turabian StyleHartung, Suzanne L., Emmanuel Mandonnet, Philip de Witt Hamer, Martin Klein, Michel Wager, Fabien Rech, Johan Pallud, Catarina Pessanha Viegas, Sebastian Ille, Sandro M. Krieg, and et al. 2021. "Impaired Set-Shifting from Dorsal Stream Disconnection: Insights from a European Series of Right Parietal Lower-Grade Glioma Resection" Cancers 13, no. 13: 3337. https://doi.org/10.3390/cancers13133337
APA StyleHartung, S. L., Mandonnet, E., de Witt Hamer, P., Klein, M., Wager, M., Rech, F., Pallud, J., Pessanha Viegas, C., Ille, S., Krieg, S. M., Robe, P. A., & van Zandvoort, M. J. E. (2021). Impaired Set-Shifting from Dorsal Stream Disconnection: Insights from a European Series of Right Parietal Lower-Grade Glioma Resection. Cancers, 13(13), 3337. https://doi.org/10.3390/cancers13133337







