Crosstalk between Macrophages and Myxoid Liposarcoma Cells Increases Spreading and Invasiveness of Tumor Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Immunohistochemistry
2.3. Image Analysis
2.4. Primary MLPS Cell Culture
2.5. Isolation of Blood Monocytes
2.6. Non-Contact Co-Cultures and Collection of Conditioned Media
2.7. Cytofluorimetric Analysis
2.8. Dot Blot Array
2.9. Invasion Kinetic of MLPS Cells Monitored in Real Time
2.10. 3D Organotypic Co-Cultures
2.11. MLPS Cell Proliferation
2.12. Trans-Endothelial Migration
2.13. Statistical Analysis
2.14. Ethics Statement
3. Results
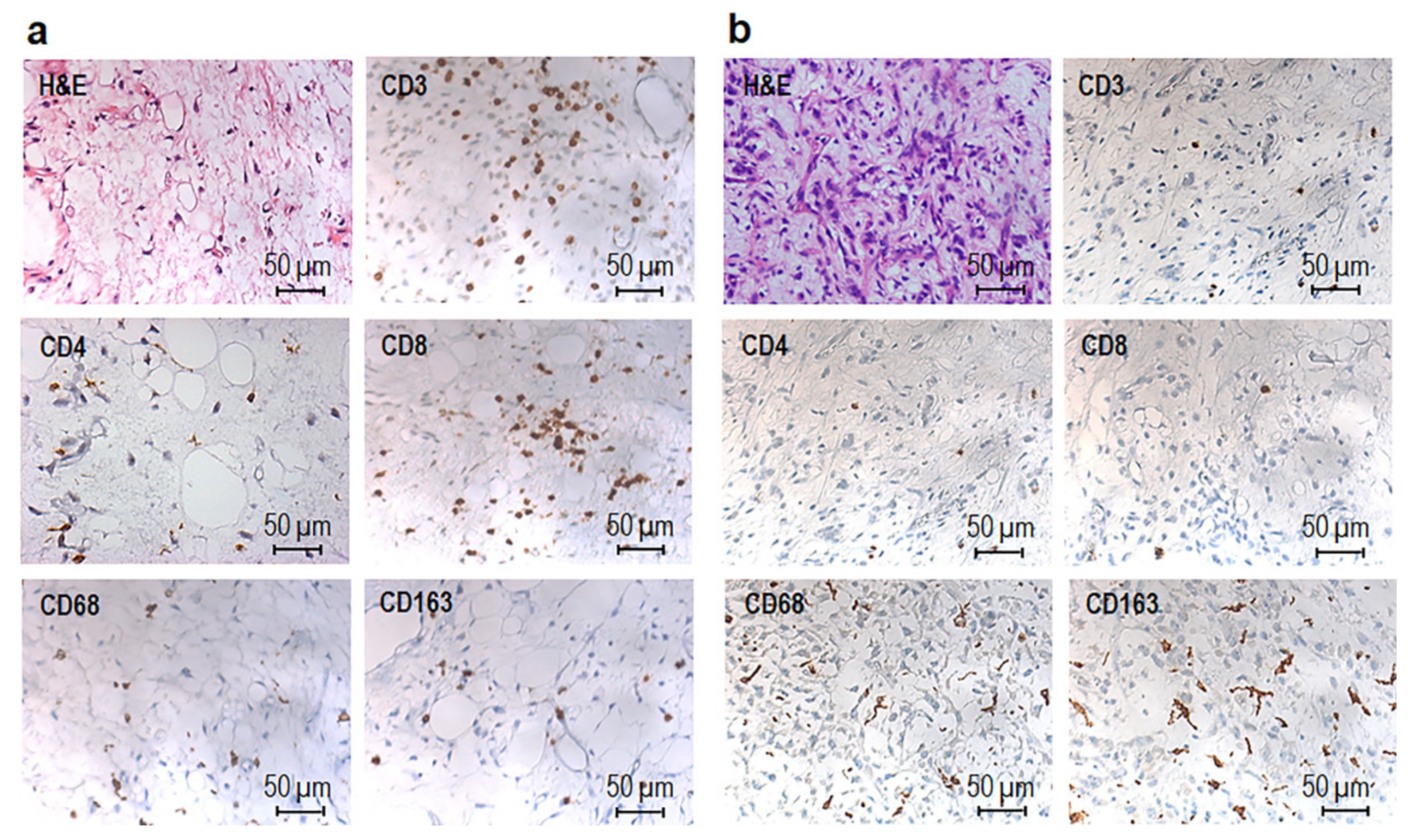
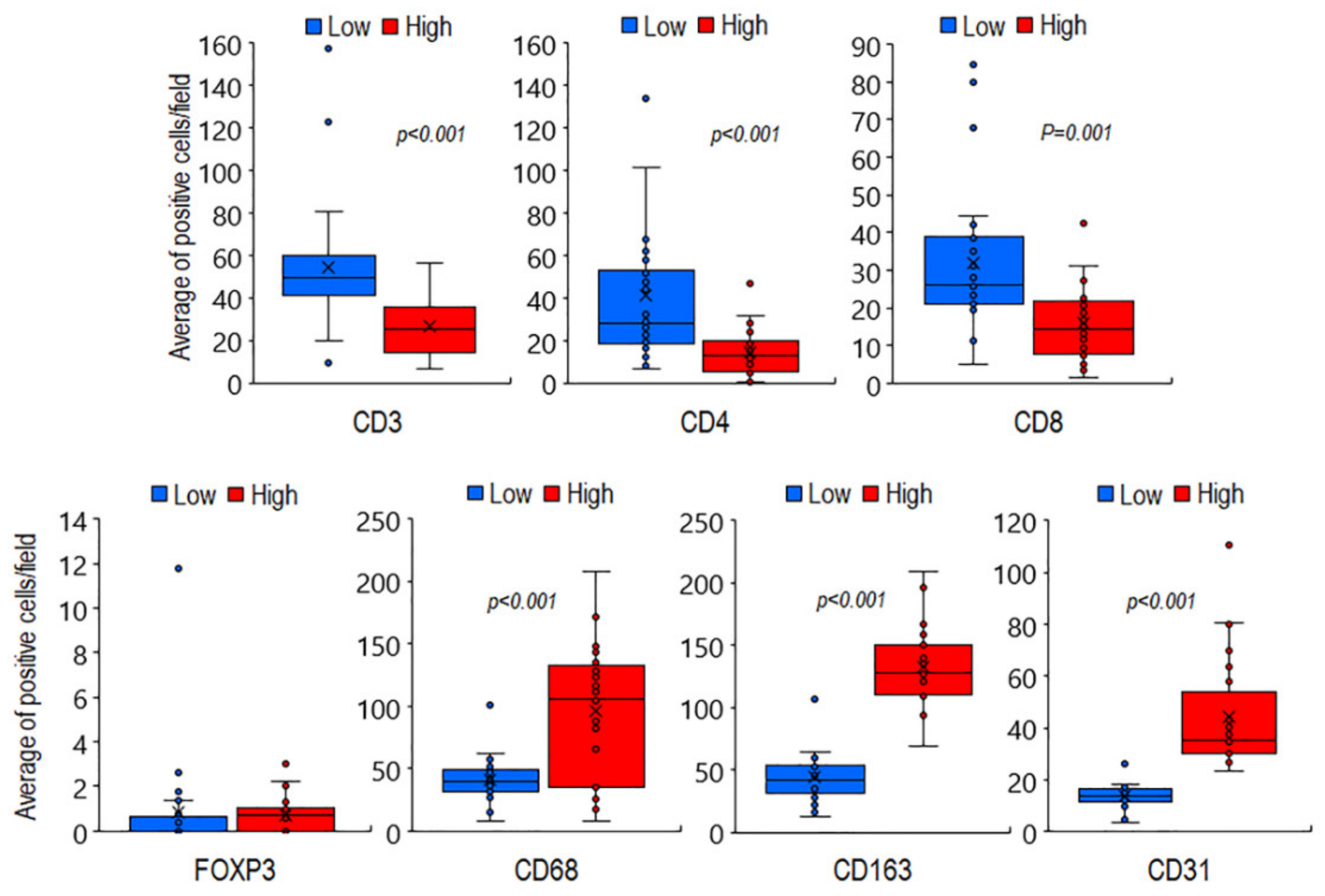
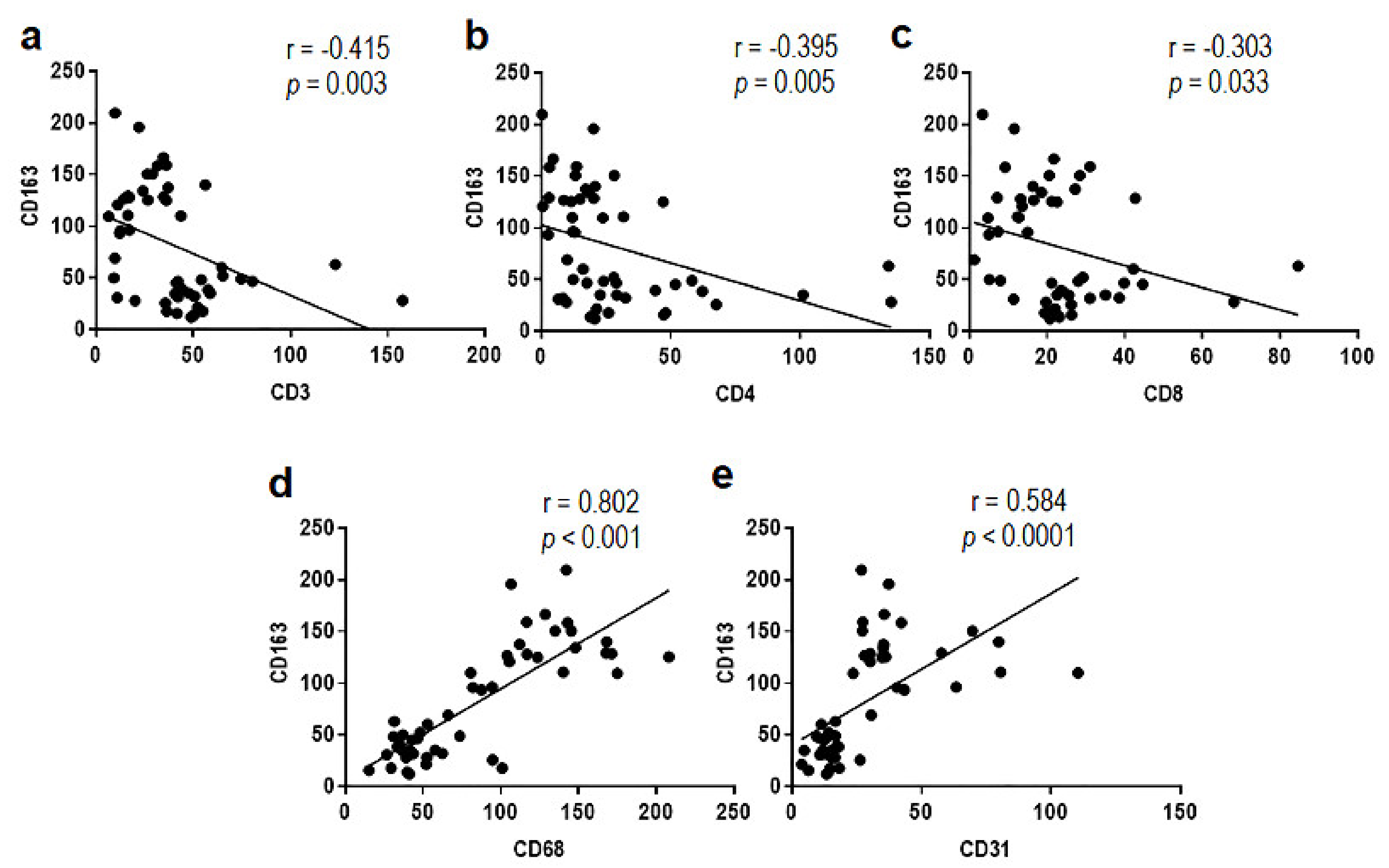
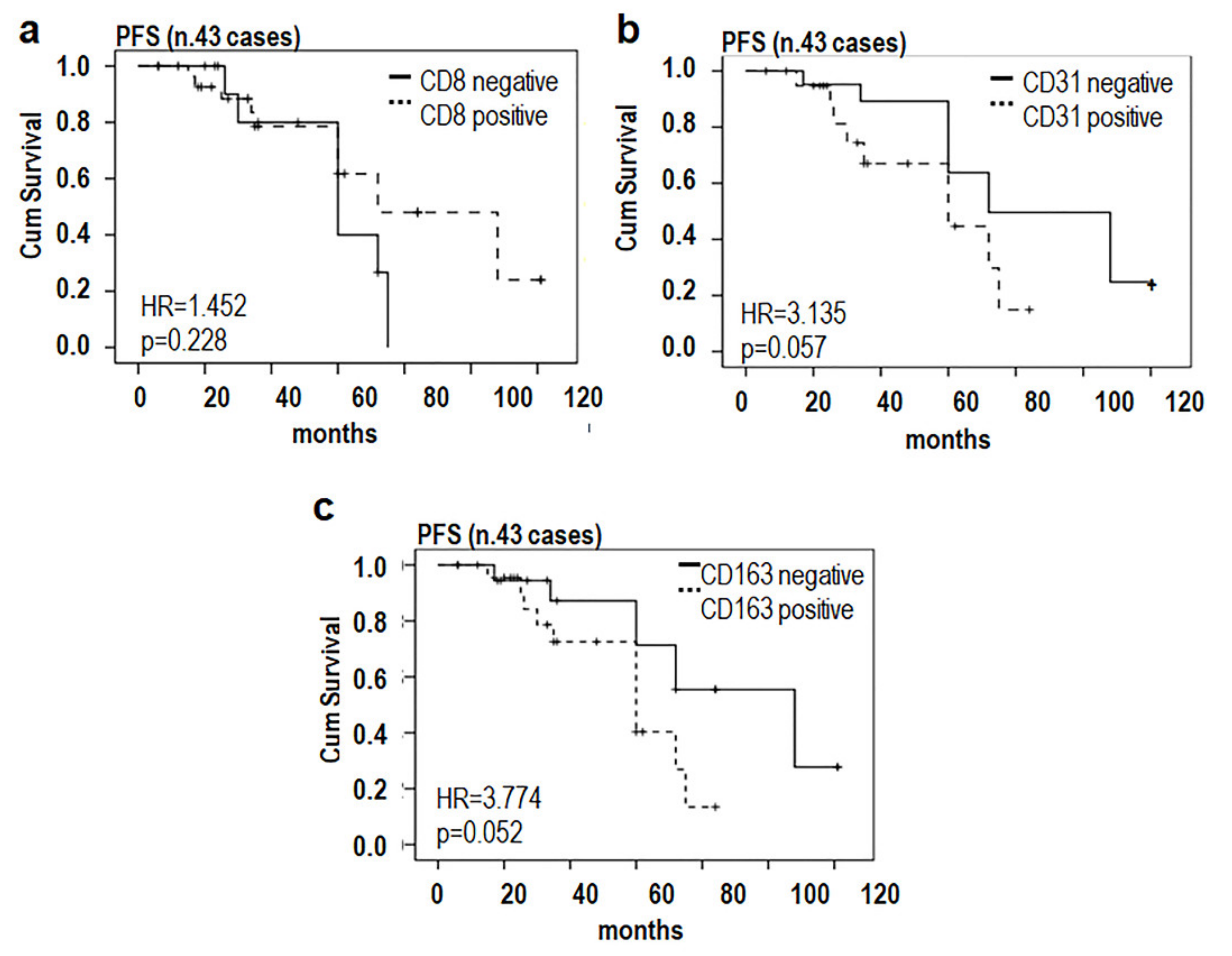
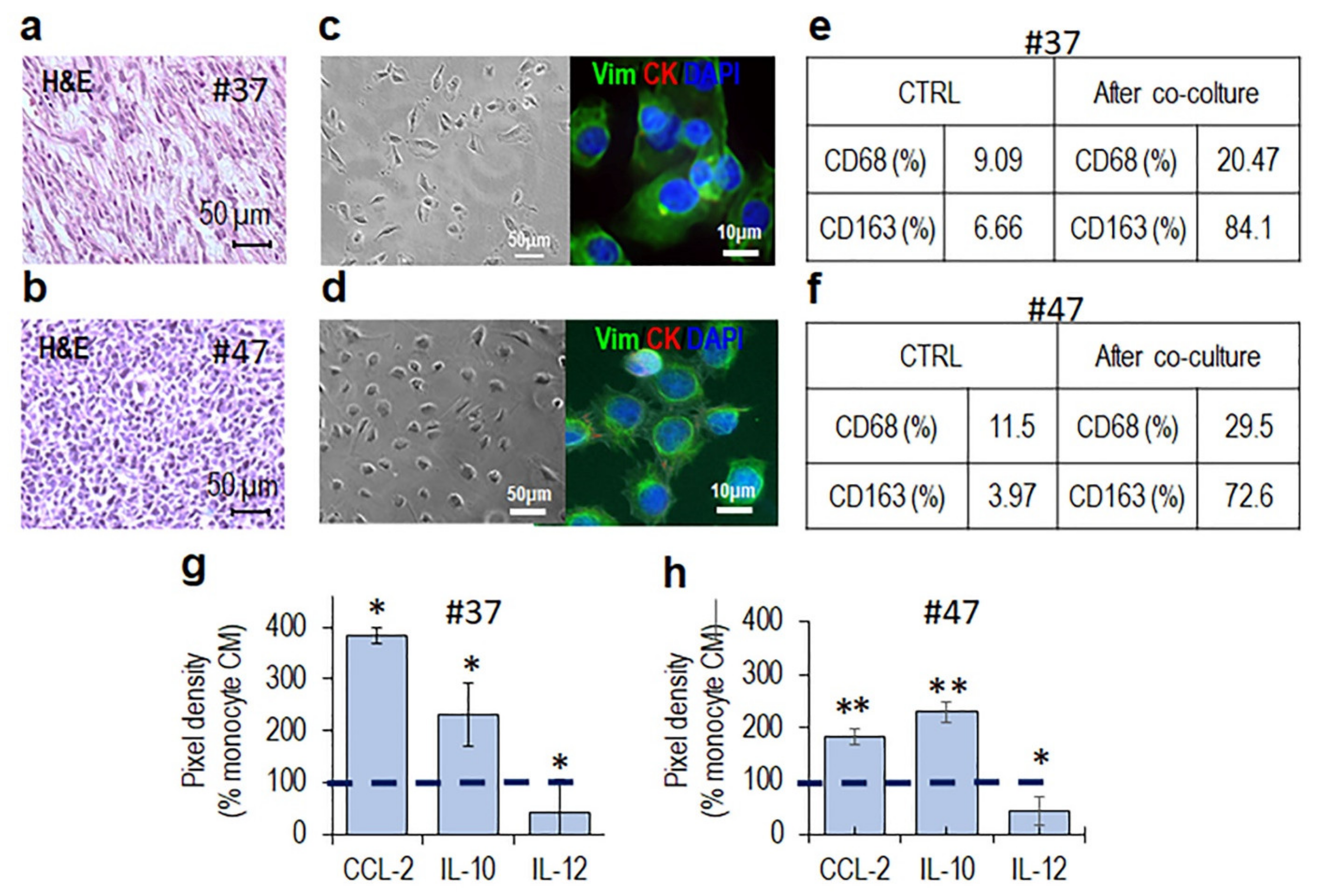
3.1. Phenotypic Characterization of Immune Cells Infiltrating MLPS Tissues
3.2. MLPS Cells Polarize Macrophages toward an M2-Like Pro-Tumor Phenotype
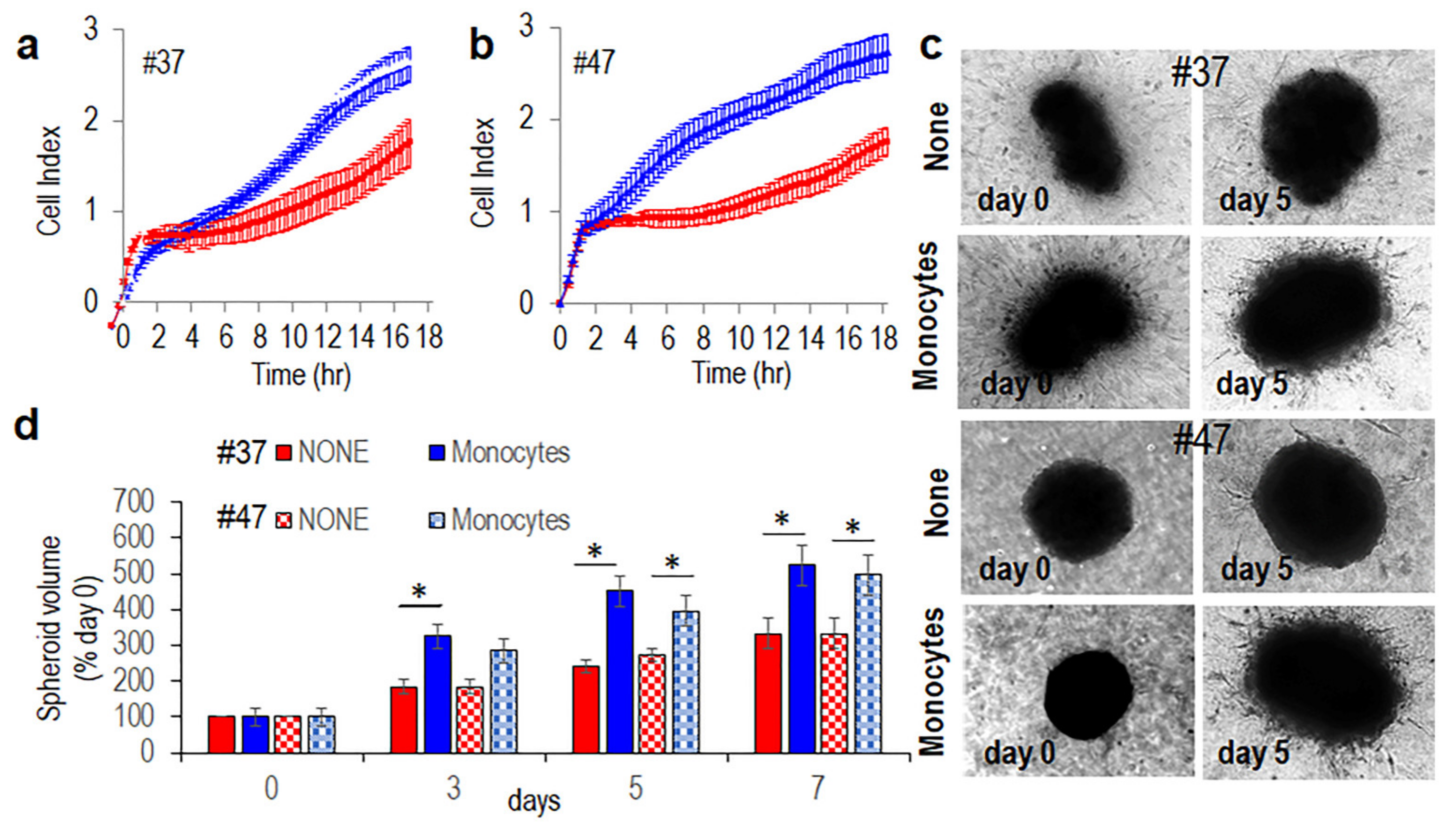
3.3. Monocytes Increase Spreading and Invasive Ability of MLPS Cells
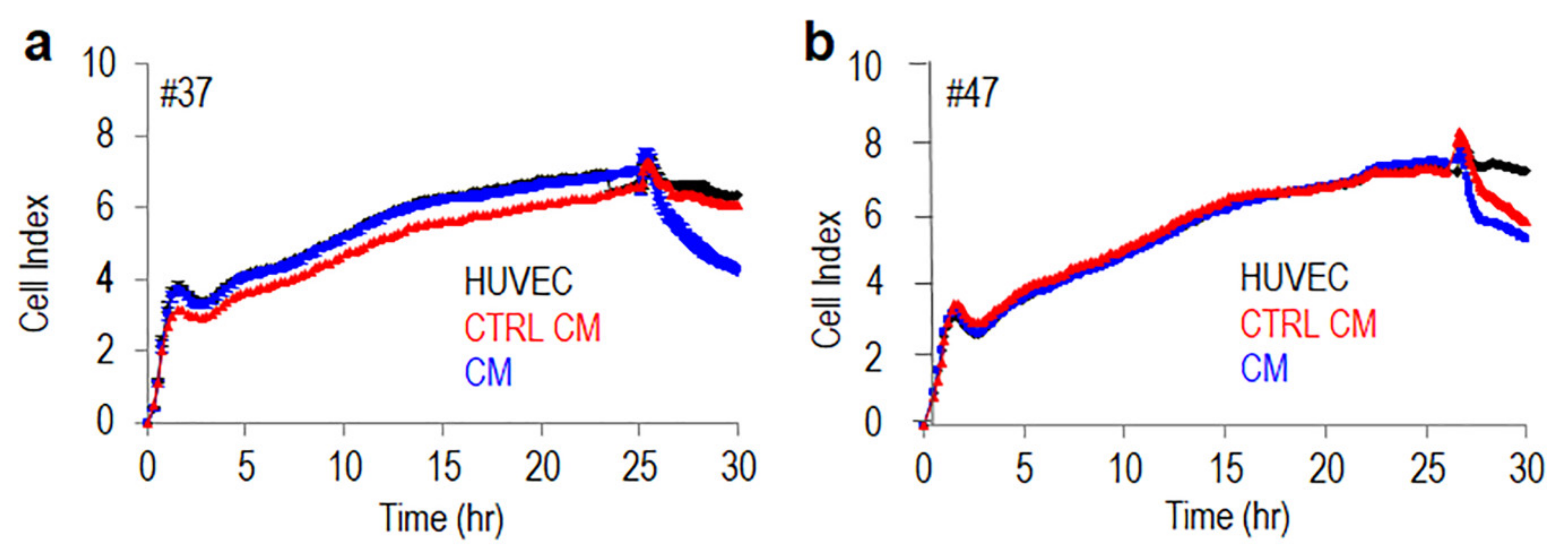
3.4. Monocytes Increase Transendothelial Migration of MLPS Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fletcher, C.; Bridge, J.; Hogendoorn, P.; Mertens, F. WHO Classification of Tumours of Soft Tissue and Bone; WHO: Geneva, Switzerland, 2013; ISBN 978-92-832-2434-1. [Google Scholar]
- Thway, K.; Nielsen, T. WHO Classification of Tumours of Soft Tissue and Bone, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2020; ISBN 978-92-832-4502-5. [Google Scholar]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO Classification: What’s New in Soft Tissue Tumor Pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Tschernyavsky, S.J.; Decuseara, R.; Leung, D.H.; Woodruff, J.M.; Brennan, M.F.; Bridge, J.A.; Neff, J.R.; Goldblum, J.R.; Ladanyi, M. Prognostic Impact of P53 Status, TLS-CHOP Fusion Transcript Structure, and Histological Grade in Myxoid Liposarcoma: A Molecular and Clinicopathologic Study of 82 Cases. Clin. Cancer Res. 2001, 7, 3977–3987. [Google Scholar]
- Fiore, M.; Grosso, F.; Lo Vullo, S.; Pennacchioli, E.; Stacchiotti, S.; Ferrari, A.; Collini, P.; Lozza, L.; Mariani, L.; Casali, P.G.; et al. Myxoid/Round Cell and Pleomorphic Liposarcomas: Prognostic Factors and Survival in a Series of Patients Treated at a Single Institution. Cancer 2007, 109, 2522–2531. [Google Scholar] [CrossRef]
- Lemeur, M.; Mattei, J.-C.; Souteyrand, P.; Chagnaud, C.; Curvale, G.; Rochwerger, A. Prognostic Factors for the Recurrence of Myxoid Liposarcoma: 20 Cases with up to 8 Years Follow-Up. Orthop. Traumatol. Surg. Res. 2015, 101, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Losada, J.; Sánchez-Martín, M.; Rodríguez-García, M.A.; Pérez-Mancera, P.A.; Pintado, B.; Flores, T.; Battaner, E.; Sánchez-Garćia, I. Liposarcoma Initiated by FUS/TLS-CHOP: The FUS/TLS Domain Plays a Critical Role in the Pathogenesis of Liposarcoma. Oncogene 2000, 19, 6015–6022. [Google Scholar] [CrossRef] [Green Version]
- Racanelli, D.; Brenca, M.; Baldazzi, D.; Goeman, F.; Casini, B.; De Angelis, B.; Guercio, M.; Milano, G.M.; Tamborini, E.; Busico, A.; et al. Next-Generation Sequencing Approaches for the Identification of Pathognomonic Fusion Transcripts in Sarcomas: The Experience of the Italian ACC Sarcoma Working Group. Front. Oncol. 2020, 10, 489. [Google Scholar] [CrossRef] [Green Version]
- Koelsche, C.; Renner, M.; Hartmann, W.; Brandt, R.; Lehner, B.; Waldburger, N.; Alldinger, I.; Schmitt, T.; Egerer, G.; Penzel, R.; et al. TERT Promoter Hotspot Mutations Are Recurrent in Myxoid Liposarcomas but Rare in Other Soft Tissue Sarcoma Entities. J. Exp. Clin. Cancer Res. 2014, 33, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, R.; Dei Tos, A.P.; Casali, P.G. Myxoid Liposarcoma and the Mammalian Target of Rapamycin Pathway. Curr. Opin. Oncol. 2013, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, M.; Cyra, M.; Isfort, I.; Jeiler, B.; Krüger, A.; Grünewald, I.; Steinestel, K.; Altvater, B.; Rossig, C.; Hafner, S.; et al. Phosphatidylinositol-3-Kinase (PI3K)/Akt Signaling Is Functionally Essential in Myxoid Liposarcoma. Mol. Cancer Ther. 2019, 18, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, T.; Virdis, E.; Brich, S.; Bozzi, F.; Tamborini, E.; Tarantino, E.; Jocollè, G.; Cassinelli, G.; Grosso, F.; Sanfilippo, R.; et al. Functional Mapping of Receptor Tyrosine Kinases in Myxoid Liposarcoma. Clin. Cancer Res. 2010, 16, 3581–3593. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Dodge, J.; Mehl, E.; Liu, S.; Poulin, N.; van de Rijn, M.; Nielsen, T.O. Validation of Immature Adipogenic Status and Identification of Prognostic Biomarkers in Myxoid Liposarcoma Using Tissue Microarrays. Hum. Pathol. 2009, 40, 1244–1251. [Google Scholar] [CrossRef]
- Haniball, J.; Sumathi, V.P.; Kindblom, L.-G.; Abudu, A.; Carter, S.R.; Tillman, R.M.; Jeys, L.; Spooner, D.; Peake, D.; Grimer, R.J. Prognostic Factors and Metastatic Patterns in Primary Myxoid/Round-Cell Liposarcoma. Sarcoma 2011, 2011, 538085. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.H.; Boland, P.; Guo, T.; Brennan, M.F.; Singer, S.; Healey, J.H.; Antonescu, C.R. Skeletal Metastases in Myxoid Liposarcoma: An Unusual Pattern of Distant Spread. Ann. Surg. Oncol. 2007, 14, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Susa, M.; Hosaka, S.; Nakayama, R.; Kobayashi, E.; Takeuchi, K.; Horiuchi, K.; Suzuki, Y.; Anazawa, U.; Mukai, M.; et al. Metastatic Patterns of Myxoid/Round Cell Liposarcoma: A Review of a 25-Year Experience. Sarcoma 2012, 2012, 345161. [Google Scholar] [CrossRef]
- Manji, G.A.; Schwartz, G.K. Managing Liposarcomas: Cutting Through the Fat. J. Oncol. Pract. 2016, 12, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Martín-Broto, J.; Moura, D.S.; Van Tine, B.A. Facts and Hopes in Immunotherapy of Soft-Tissue Sarcomas. Clin. Cancer Res. 2020, 26, 5801–5808. [Google Scholar] [CrossRef]
- Kirsanov, K.I.; Lesovaya, E.A.; Fetisov, T.I.; Bokhyan, B.Y.; Belitsky, G.A.; Yakubovskaya, M.G. Current Approaches for Personalized Therapy of Soft Tissue Sarcomas. Sarcoma 2020, 2020, 6716742. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Simone, M.; Tavecchio, M.; Erba, E.; Pesce, S.; Pasqualini, F.; Grosso, F.; Sanfilippo, R.; Casali, P.G.; et al. Antitumor and Anti-Inflammatory Effects of Trabectedin on Human Myxoid Liposarcoma Cells. Cancer Res. 2010, 70, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Greaves, M.; Maley, C.C. Clonal Evolution in Cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.-Z.; Pollard, J.W. Immune Cell Promotion of Metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Heymann, M.-F.; Schiavone, K.; Heymann, D. Bone Sarcomas in the Immunotherapy Era. Br. J. Pharmacol. 2020, 178, 1955–1972. [Google Scholar] [CrossRef]
- Simard, F.A.; Richert, I.; Vandermoeten, A.; Decouvelaere, A.-V.; Michot, J.-P.; Caux, C.; Blay, J.-Y.; Dutour, A. Description of the Immune Microenvironment of Chondrosarcoma and Contribution to Progression. Oncoimmunology 2017, 6, e1265716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minopoli, M.; Sarno, S.; Di Carluccio, G.; Azzaro, R.; Costantini, S.; Fazioli, F.; Gallo, M.; Apice, G.; Cannella, L.; Rea, D.; et al. Inhibiting Monocyte Recruitment to Prevent the Pro-Tumoral Activity of Tumor-Associated Macrophages in Chondrosarcoma. Cells 2020, 9, 1062. [Google Scholar] [CrossRef]
- Pollack, S.M.; He, Q.; Yearley, J.H.; Emerson, R.; Vignali, M.; Zhang, Y.; Redman, M.W.; Baker, K.K.; Cooper, S.; Donahue, B.; et al. T-Cell Infiltration and Clonality Correlate with Programmed Cell Death Protein 1 and Programmed Death-Ligand 1 Expression in Patients with Soft Tissue Sarcomas. Cancer 2017, 123, 3291–3304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.-W.; Sun, C.-M.; Calderaro, J.; Jeng, Y.-M.; Hsiao, L.-P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Ayodele, O.; Razak, A.R.A. Immunotherapy in Soft-Tissue Sarcoma. Curr. Oncol. 2020, 27, 17–23. [Google Scholar] [CrossRef]
- Pinto, M.L.; Rios, E.; Durães, C.; Ribeiro, R.; Machado, J.C.; Mantovani, A.; Barbosa, M.A.; Carneiro, F.; Oliveira, M.J. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front. Immunol. 2019, 10, 1875. [Google Scholar] [CrossRef] [Green Version]
- Bifulco, K.; Longanesi-Cattani, I.; Masucci, M.T.; De Chiara, A.; Fazioli, F.; Di Carluccio, G.; Pirozzi, G.; Gallo, M.; La Rocca, A.; Apice, G.; et al. Involvement of the Soluble Urokinase Receptor in Chondrosarcoma Cell Mobilization. Sarcoma 2011, 2011, 842842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carriero, M.V.; Bifulco, K.; Ingangi, V.; Costantini, S.; Botti, G.; Ragone, C.; Minopoli, M.; Motti, M.L.; Rea, D.; Scognamiglio, G.; et al. Retro-Inverso Urokinase Receptor Antagonists for the Treatment of Metastatic Sarcomas. Sci. Rep. 2017, 7, 1312. [Google Scholar] [CrossRef] [Green Version]
- Ragone, C.; Minopoli, M.; Ingangi, V.; Botti, G.; Fratangelo, F.; Pessi, A.; Stoppelli, M.P.; Ascierto, P.A.; Ciliberto, G.; Motti, M.L.; et al. Targeting the Cross-Talk between Urokinase Receptor and Formyl Peptide Receptor Type 1 to Prevent Invasion and Trans-Endothelial Migration of Melanoma Cells. J. Exp. Clin. Cancer Res. 2017, 36, 180. [Google Scholar] [CrossRef] [Green Version]
- Pollard, J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of Tumor-Associated Macrophages in Neoplastic Progression and Immune Surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Dancsok, A.R.; Setsu, N.; Gao, D.; Blay, J.-Y.; Thomas, D.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Expression of Lymphocyte Immunoregulatory Biomarkers in Bone and Soft-Tissue Sarcomas. Mod. Pathol. 2019, 32, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Hemminger, J.A.; Ewart Toland, A.; Scharschmidt, T.J.; Mayerson, J.L.; Kraybill, W.G.; Guttridge, D.C.; Iwenofu, O.H. The Cancer-Testis Antigen NY-ESO-1 Is Highly Expressed in Myxoid and Round Cell Subset of Liposarcomas. Mod. Pathol. 2013, 26, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, S.; Jungbluth, A.; Cassian Yee, M.; Hoch, B.L. NY-ESO-1 Is a Ubiquitous Immunotherapeutic Target Antigen for Patients with Myxoid/Round Cell Liposarcoma. Cancer 2012, 118, 4564–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, E.; Nagata, Y.; Gnjatic, S.; Wada, H.; Stockert, E.; Karbach, J.; Dunbar, P.R.; Lee, S.Y.; Jungbluth, A.; Jäger, D.; et al. Monitoring CD8 T Cell Responses to NY-ESO-1: Correlation of Humoral and Cellular Immune Responses. Proc. Natl. Acad. Sci. USA 2000, 97, 4760–4765. [Google Scholar] [CrossRef] [Green Version]
- Klaver, Y.; Rijnders, M.; Oostvogels, A.; Wijers, R.; Smid, M.; Grünhagen, D.; Verhoef, C.; Sleijfer, S.; Lamers, C.; Debets, R. Differential Quantities of Immune Checkpoint-Expressing CD8 T Cells in Soft Tissue Sarcoma Subtypes. J. Immunother. Cancer 2020, 8, e000271. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Du, M.; Roy, K.M.; Zhong, L.; Shen, Z.; Meyers, H.E.; Nichols, R.C. VEGF Gene Expression Is Regulated Post-Transcriptionally in Macrophages. FEBS J. 2006, 273, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Lamagna, C.; Aurrand-Lions, M.; Imhof, B.A. Dual Role of Macrophages in Tumor Growth and Angiogenesis. J. Leukoc. Biol. 2006, 80, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of Tumor-Associated Macrophages in Tumor Progression and Invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef]
- Nabeshima, A.; Matsumoto, Y.; Fukushi, J.; Iura, K.; Matsunobu, T.; Endo, M.; Fujiwara, T.; Iida, K.; Fujiwara, Y.; Hatano, M.; et al. Tumour-Associated Macrophages Correlate with Poor Prognosis in Myxoid Liposarcoma and Promote Cell Motility and Invasion via the HB-EGF-EGFR-PI3K/Akt Pathways. Br. J. Cancer 2015, 112, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of Tumor-Associated Macrophages and Neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.E.; Hingorani, S.R.; Mick, R.; Combs, C.; Tuveson, D.A.; Vonderheide, R.H. Dynamics of the Immune Reaction to Pancreatic Cancer from Inception to Invasion. Cancer Res. 2007, 67, 9518–9527. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Harper, K.L.; Sosa, M.S.; Entenberg, D.; Hosseini, H.; Cheung, J.F.; Nobre, R.; Avivar-Valderas, A.; Nagi, C.; Girnius, N.; Davis, R.J.; et al. Mechanism of Early Dissemination and Metastasis in Her2+ Mammary Cancer. Nature 2016, 540, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Van Overmeire, E.; Laoui, D.; Keirsse, J.; Van Ginderachter, J.A.; Sarukhan, A. Mechanisms Driving Macrophage Diversity and Specialization in Distinct Tumor Microenvironments and Parallelisms with Other Tissues. Front. Immunol. 2014, 5, 127. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage Activation and Polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Healey, J.; Ogura, K.; Yoshida, A.; Kondo, H.; Hata, T.; Kure, M.; Tazawa, H.; Nakata, E.; Kunisada, T.; et al. Role of Tumor-Associated Macrophages in Sarcomas. Cancers 2021, 13, 1086. [Google Scholar] [CrossRef]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the CCL2/CCR2 Axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Xian, M.; Xiang, S.; Xiang, D.; Shao, X.; Wang, J.; Cao, J.; Yang, X.; Yang, B.; Ying, M.; et al. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol. Res. 2017, 5, 547–559. [Google Scholar] [CrossRef] [Green Version]
- Minopoli, M.; Polo, A.; Ragone, C.; Ingangi, V.; Ciliberto, G.; Pessi, A.; Sarno, S.; Budillon, A.; Costantini, S.; Carriero, M.V. Structure-Function Relationship of an Urokinase Receptor-Derived Peptide Which Inhibits the Formyl Peptide Receptor Type 1 Activity. Sci. Rep. 2019, 9, 12169. [Google Scholar] [CrossRef] [PubMed]
| Myxoid Liposarcomas | Primary | 47 (94%) |
| Recurrence | 1 (2%) | |
| Metastasis | 2 (4%) | |
| Age (years) | Mean | 51.66 |
| <60 | 33 (66%) | |
| >60 | 17 (34%) | |
| Sex | Male | 27 (54%) |
| Female | 23 (46%) | |
| Size (cm) | Mean | 138.302 |
| <10 cm | 14 (28%) | |
| >10 cm | 30 (60%) | |
| Unknown | 6 (12%) | |
| Tumor location | Axilla | 3 (6%) |
| Chest wall | 1 (2%) | |
| Abdomen | 1 (2%) | |
| Pelvis | 1 (2%) | |
| Gluteus | 2 (4%) | |
| Thigh | 29 (58%) | |
| Knee | 1 (2%) | |
| Leg | 12 (24%) | |
| Histological grade | Low | 26 (52%) |
| High | 24 (48%) | |
| Follow up (ten years) | None | 18 (36%) |
| Recurrence | 25 (50%) | |
| Unknown | 7 (14%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minopoli, M.; Sarno, S.; Cannella, L.; Tafuto, S.; Scognamiglio, G.; Gallo, M.; Fazioli, F.; Azzaro, R.; Apice, G.; De Angelis, B.; et al. Crosstalk between Macrophages and Myxoid Liposarcoma Cells Increases Spreading and Invasiveness of Tumor Cells. Cancers 2021, 13, 3298. https://doi.org/10.3390/cancers13133298
Minopoli M, Sarno S, Cannella L, Tafuto S, Scognamiglio G, Gallo M, Fazioli F, Azzaro R, Apice G, De Angelis B, et al. Crosstalk between Macrophages and Myxoid Liposarcoma Cells Increases Spreading and Invasiveness of Tumor Cells. Cancers. 2021; 13(13):3298. https://doi.org/10.3390/cancers13133298
Chicago/Turabian StyleMinopoli, Michele, Sabrina Sarno, Lucia Cannella, Salvatore Tafuto, Gosuè Scognamiglio, Michele Gallo, Flavio Fazioli, Rosa Azzaro, Gaetano Apice, Biagio De Angelis, and et al. 2021. "Crosstalk between Macrophages and Myxoid Liposarcoma Cells Increases Spreading and Invasiveness of Tumor Cells" Cancers 13, no. 13: 3298. https://doi.org/10.3390/cancers13133298
APA StyleMinopoli, M., Sarno, S., Cannella, L., Tafuto, S., Scognamiglio, G., Gallo, M., Fazioli, F., Azzaro, R., Apice, G., De Angelis, B., Tamborini, E., Garofalo, C., Pignochino, Y., Mercatali, L., Ibrahim, T., Falcioni, R., Valenti, B., Maestro, R., Scotlandi, K., ... Carriero, M. V. (2021). Crosstalk between Macrophages and Myxoid Liposarcoma Cells Increases Spreading and Invasiveness of Tumor Cells. Cancers, 13(13), 3298. https://doi.org/10.3390/cancers13133298












