Targeted Cancer Therapy Using Compounds Activated by Light
Abstract
Simple Summary
Abstract
1. Introduction
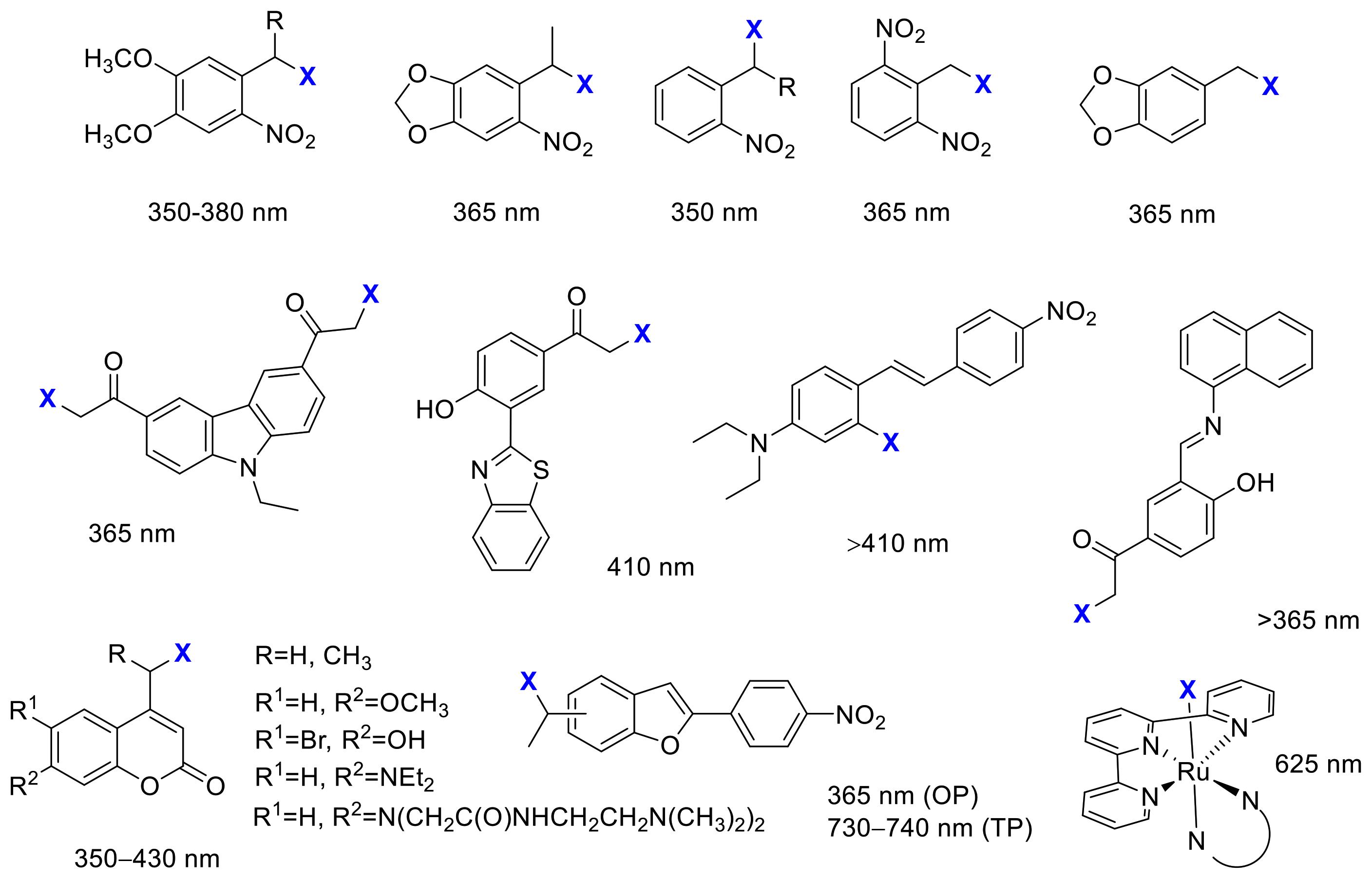
2. Irreversible Activation with Light: Photoremovable Protecting Groups (“Photocages”) for Antitumor Applications
2.1. Kinase Inhibitors
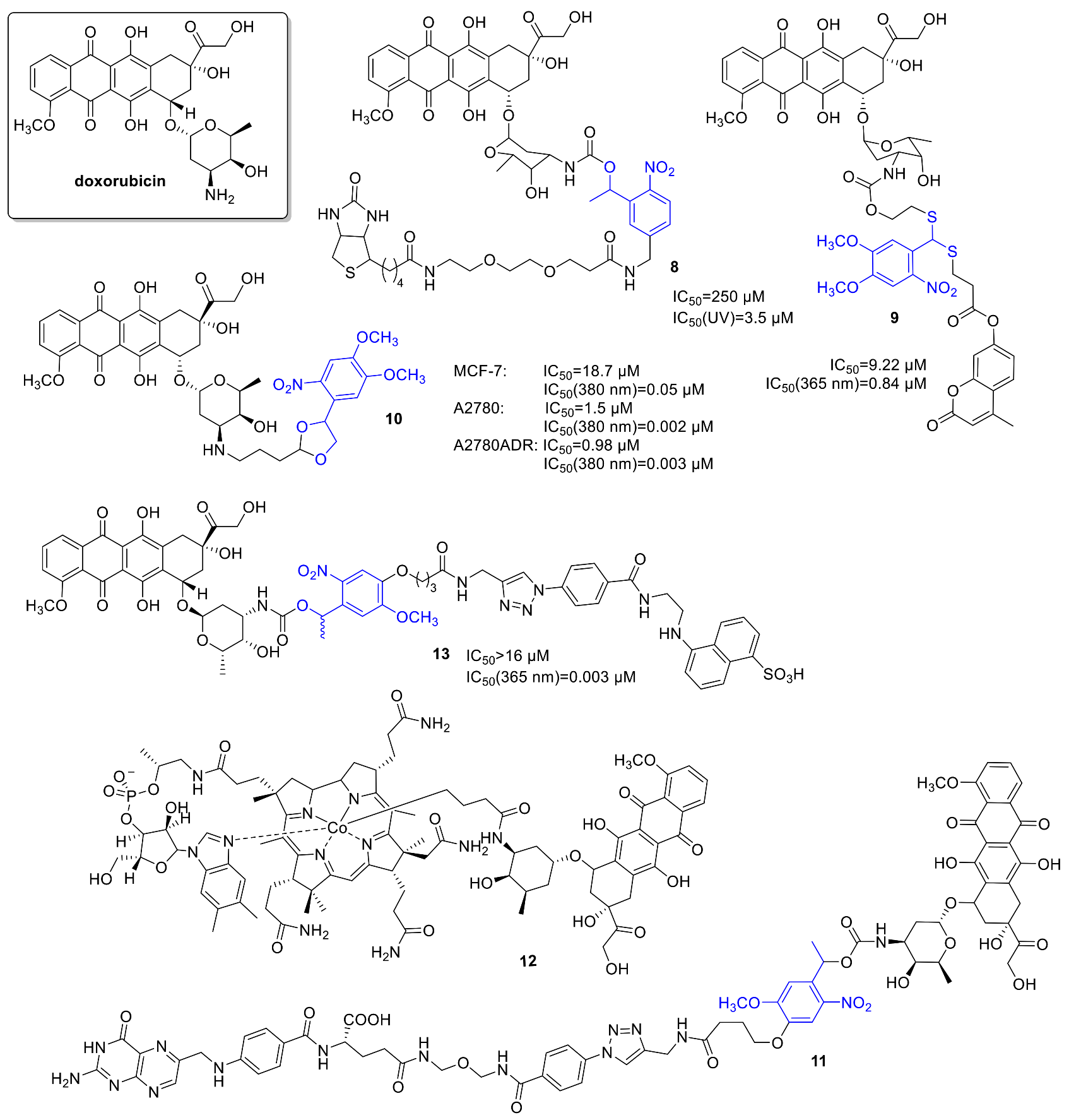
2.2. Anthracyclines
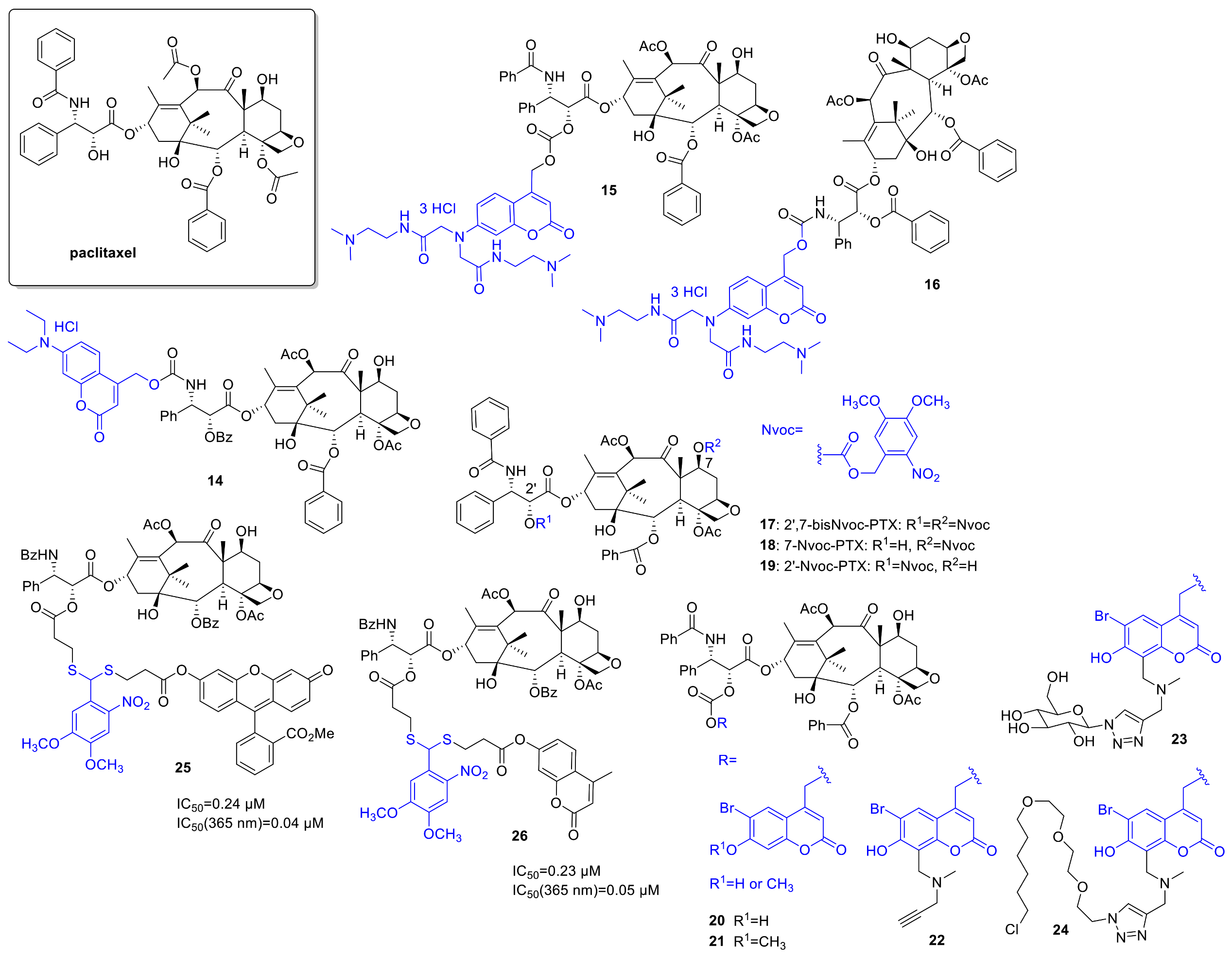
2.3. Tubulin (Dis)Assembly Inhibitors, Microtubule-Targeting Agents
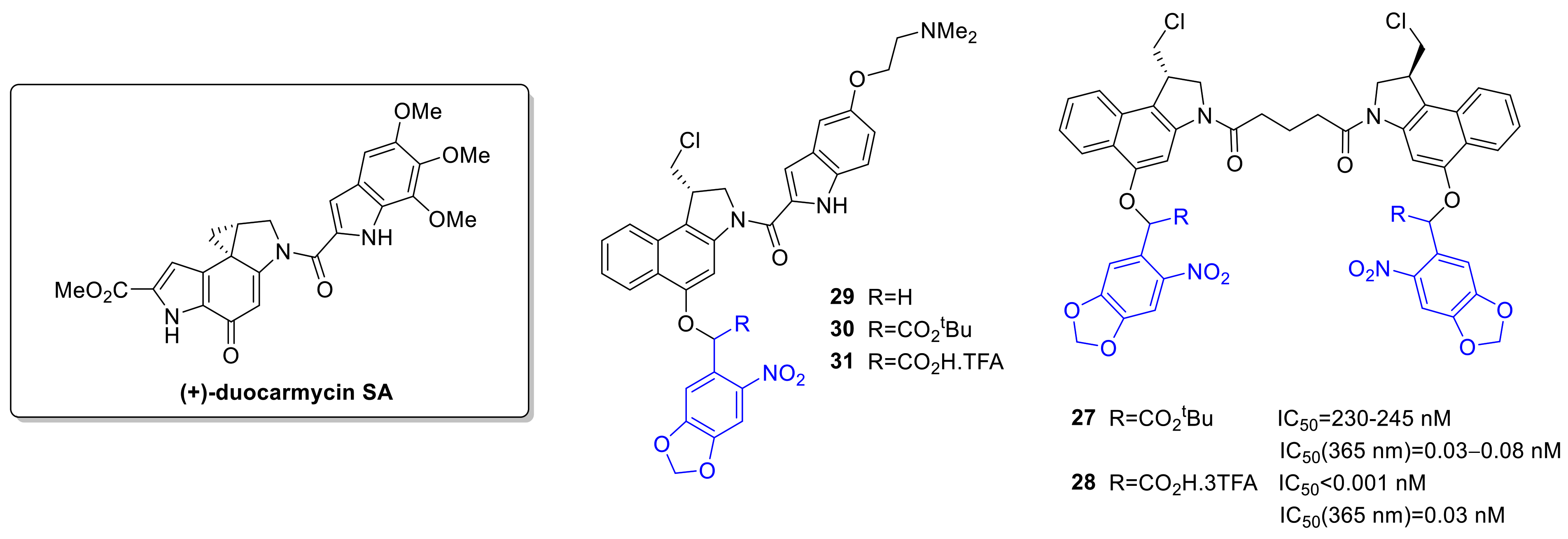
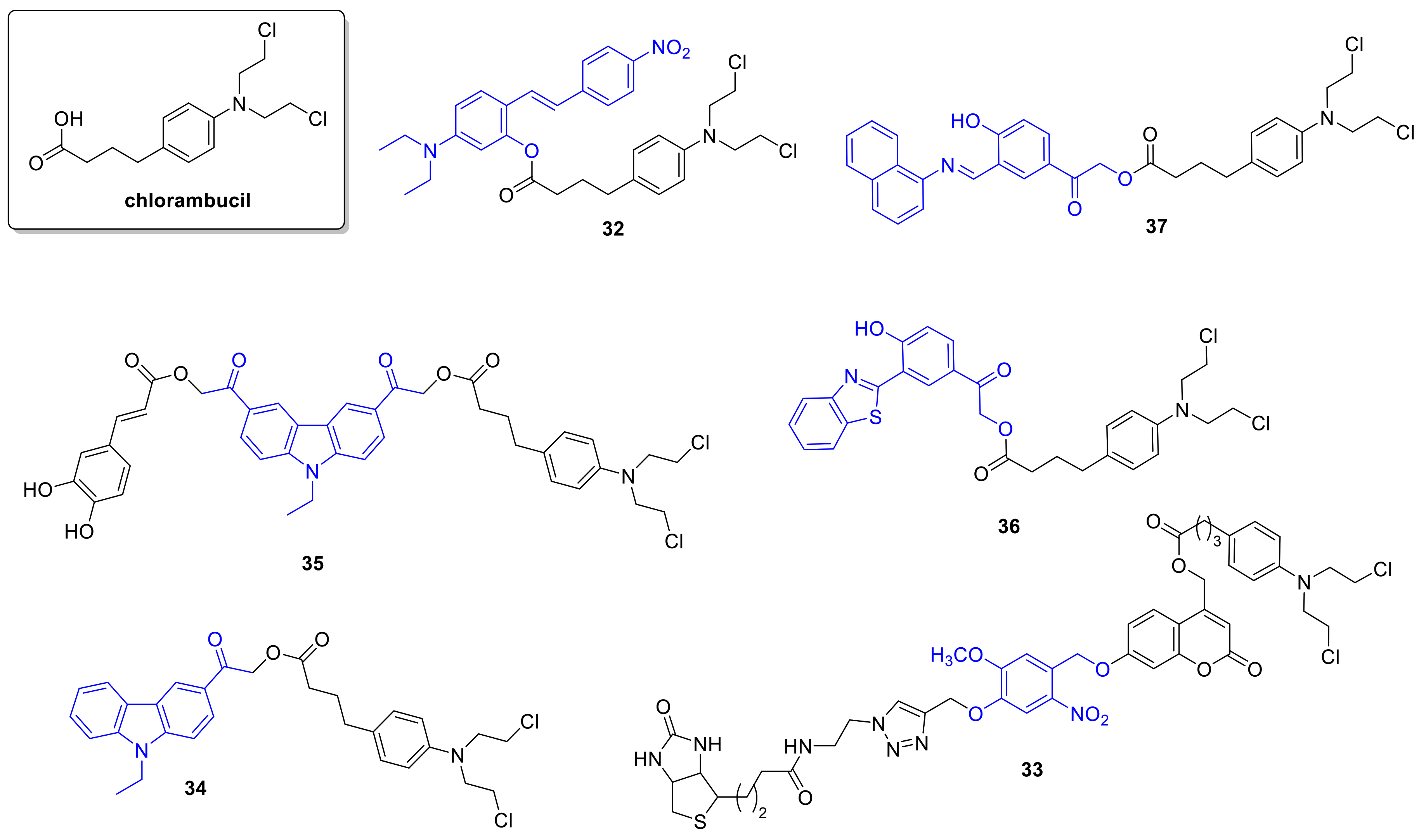
2.4. DNA Alkylating Agents
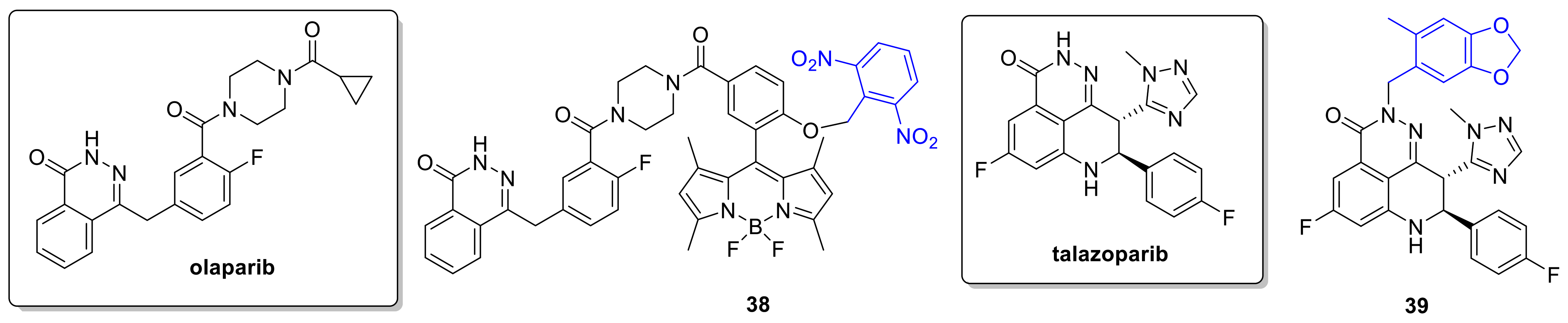
2.5. PARP Inhibitors
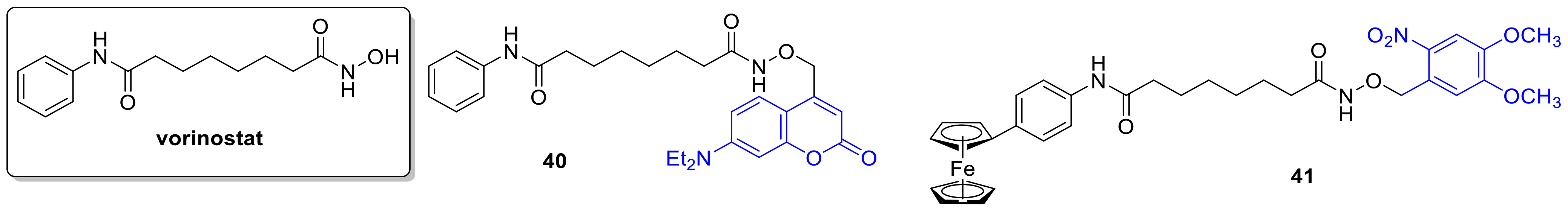
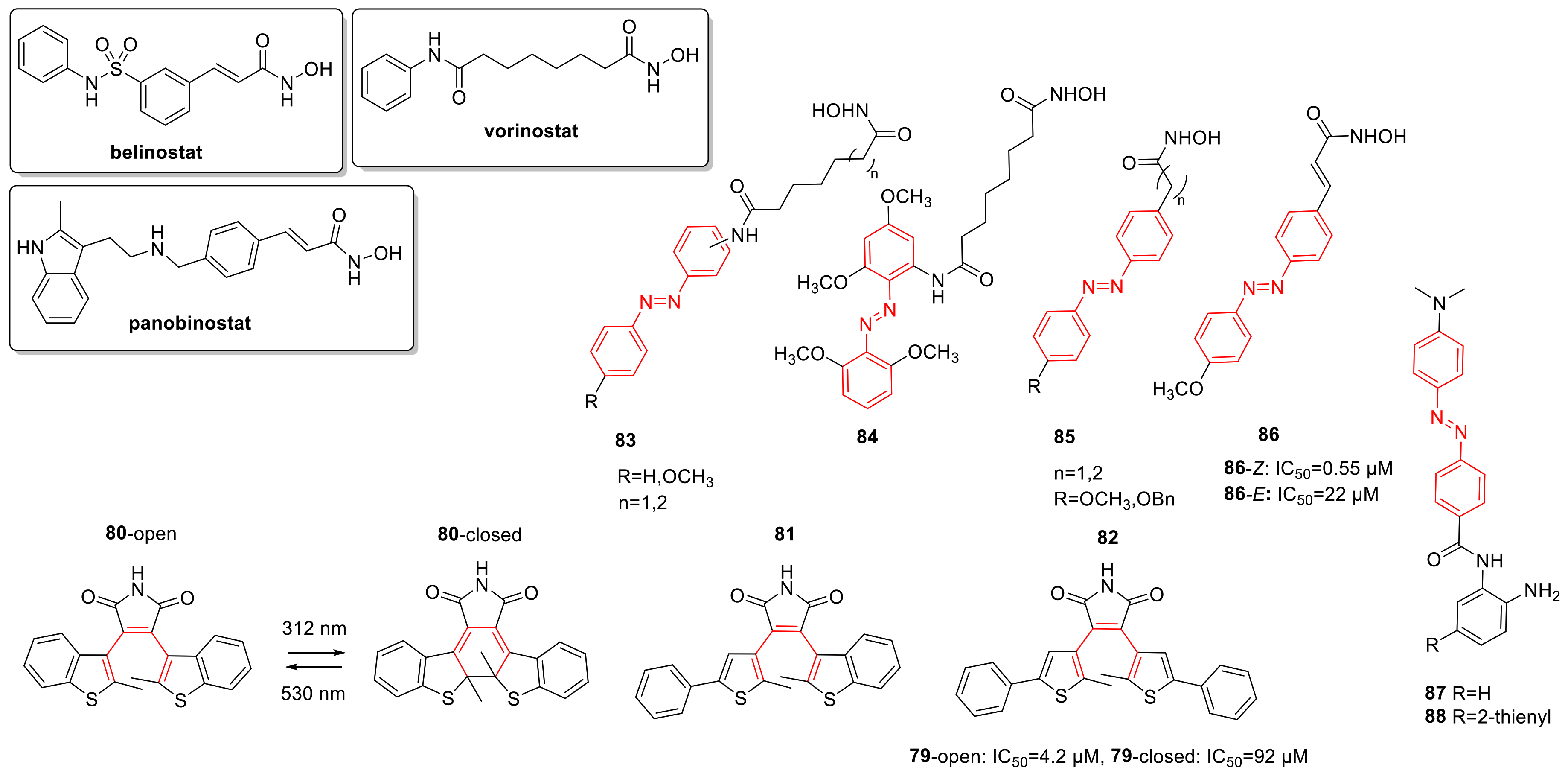
2.6. Histone Deacetylase Inhibitors
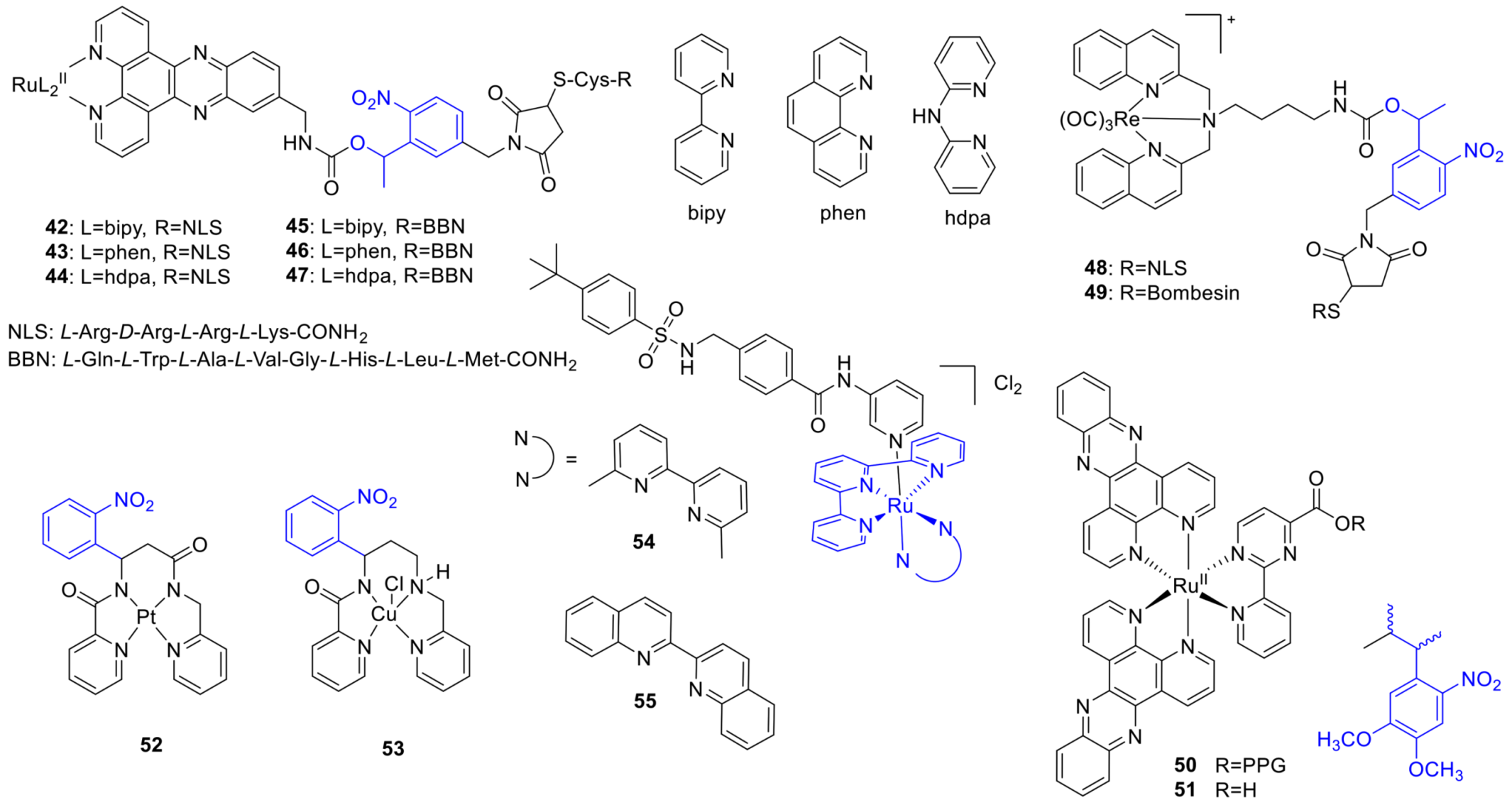
2.7. Metal Complexes
2.8. Nitroxide Donors
2.9. Thymidylate Synthase Inhibitors
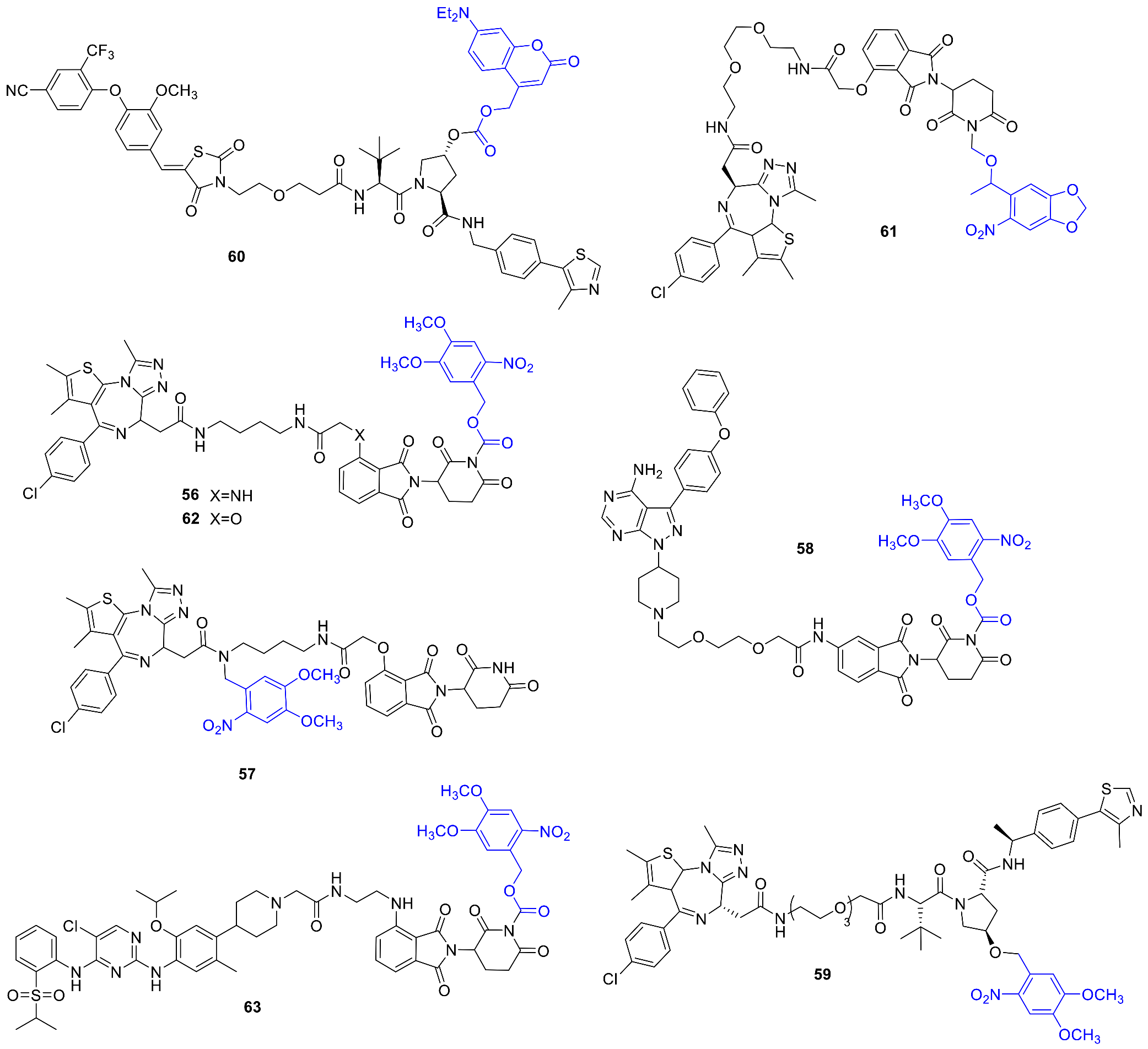
2.10. PROTACs (PROteolysis TArgeting Chimeras)
2.11. Modulators of p53 Signaling
3. Photoswitches for Antitumor Applications
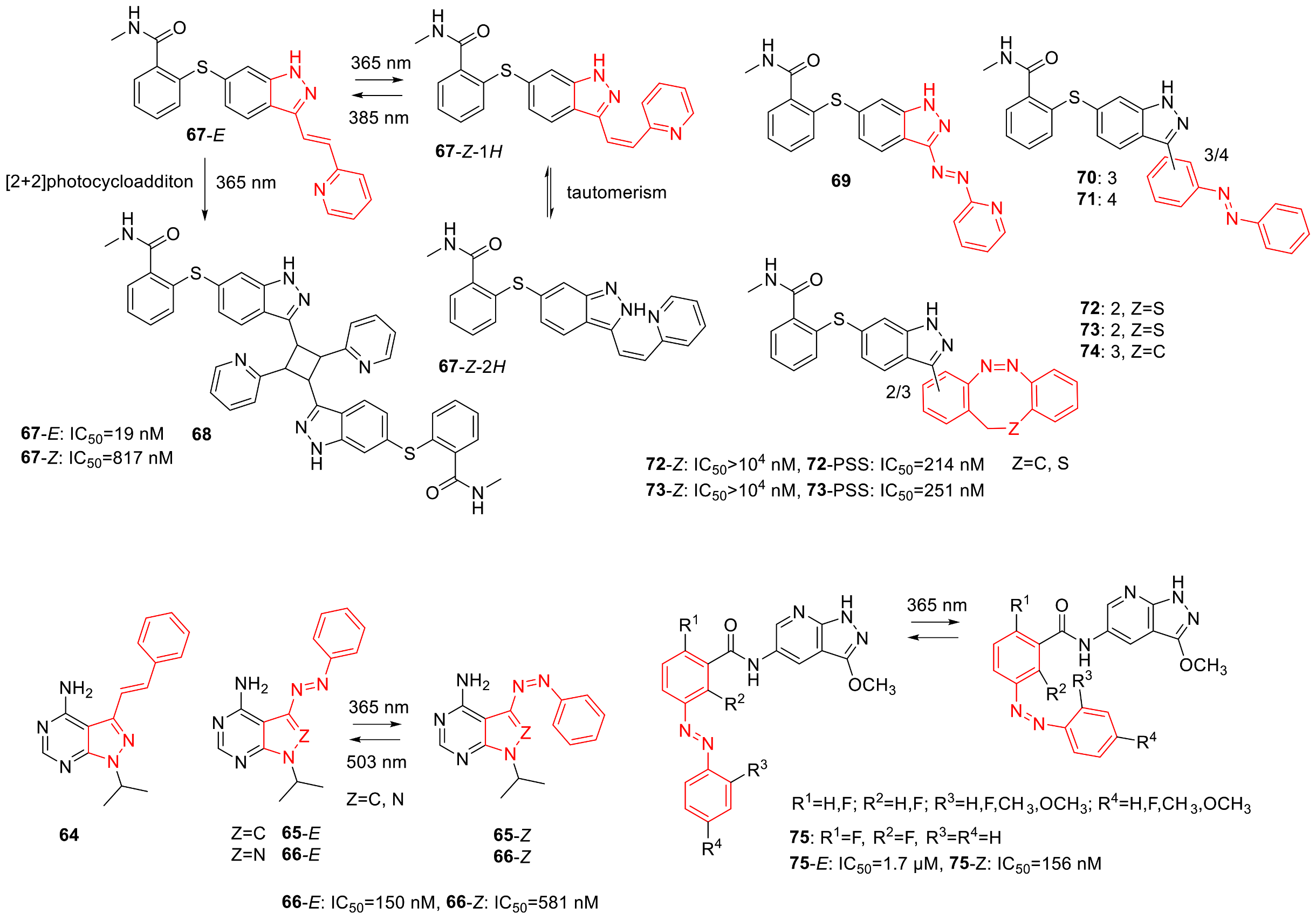
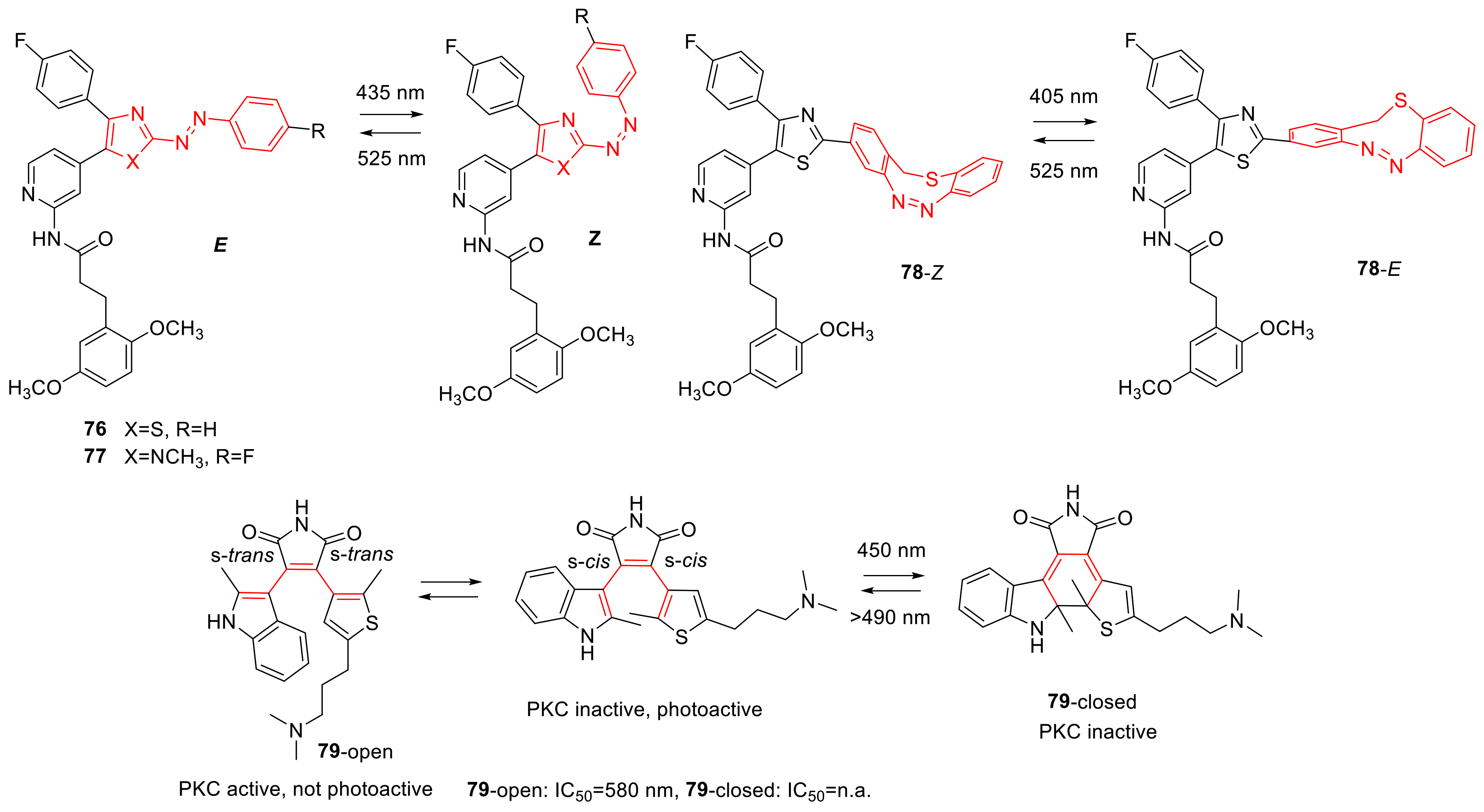
3.1. Photoswitchable Kinase Inhibitors
3.2. Photoswitchable Epigenetic Modulators
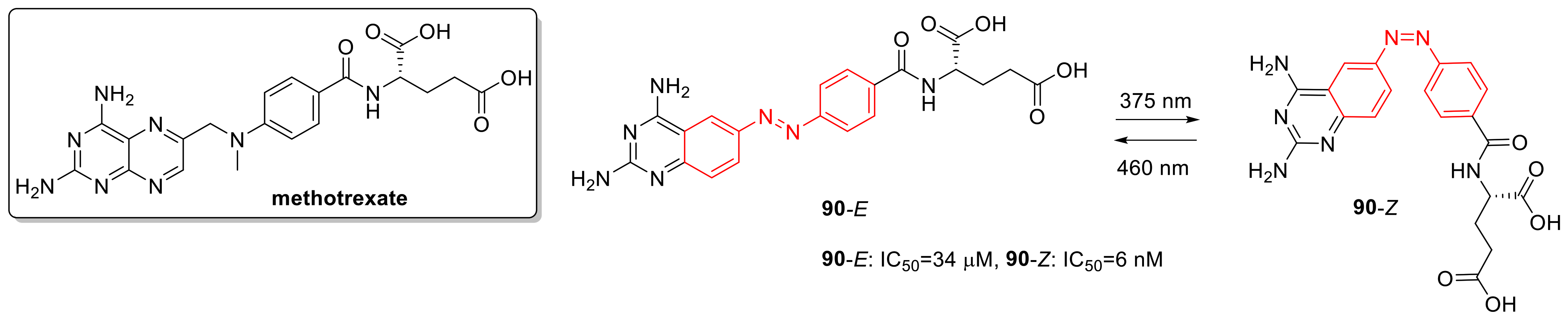
3.3. Photoswitchable Antimetabolites
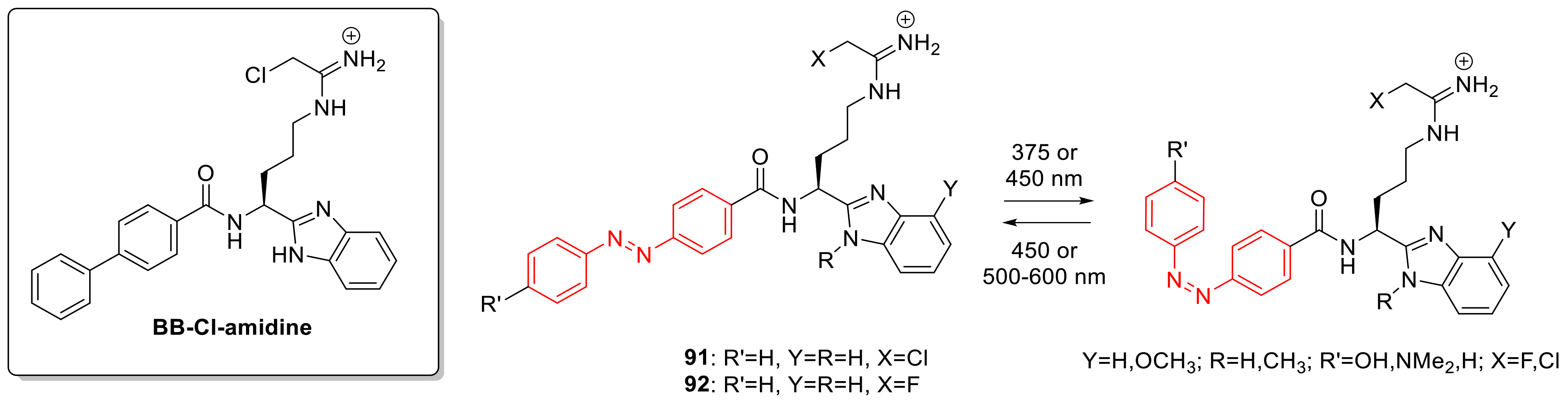
3.4. Photoswitchable PAD Inhibitors
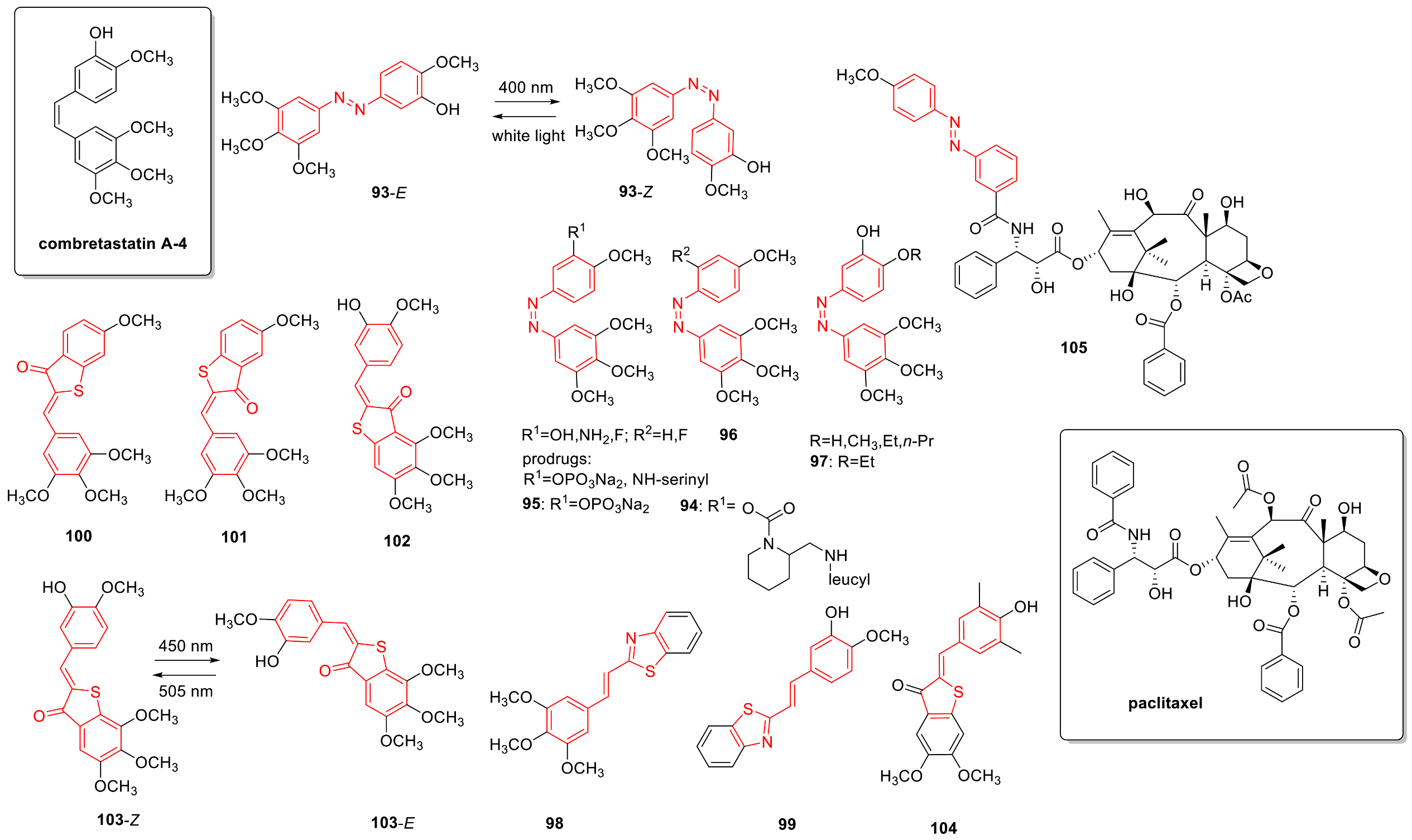
3.5. Microtubule-Targeting Agents
3.6. Photoswitchable F-Actin Modulating Agents
3.7. Photoswitchable Metal Complexes
3.8. Photoswitchable Proteasome Inhibitors
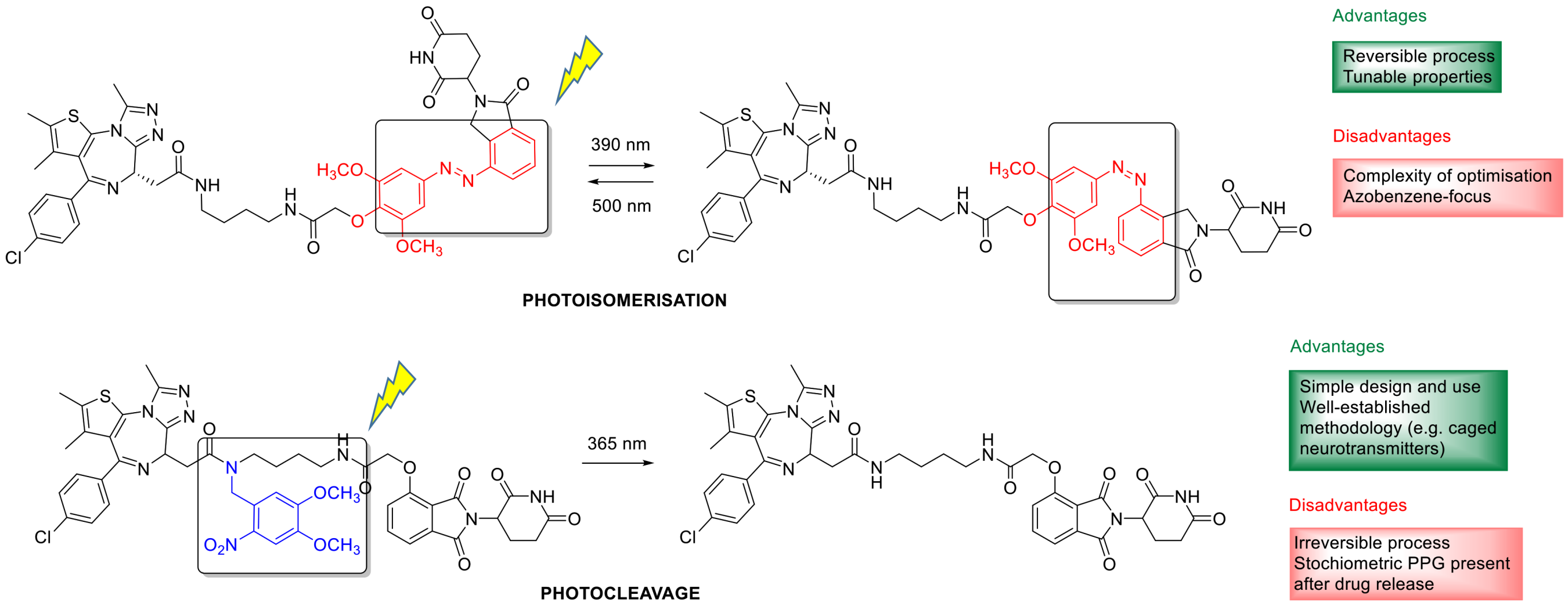
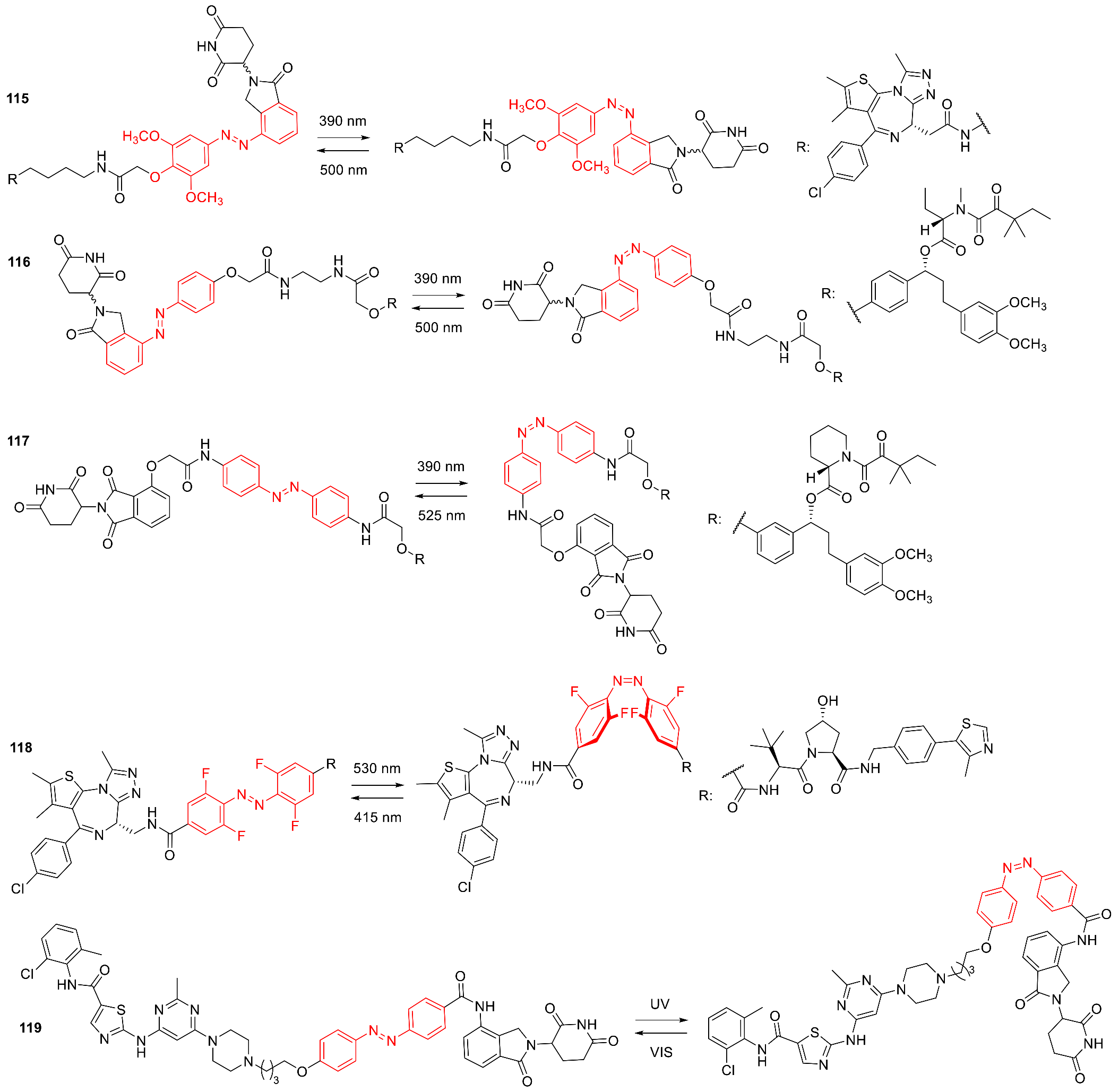
3.9. Photoswitchable PROTACs
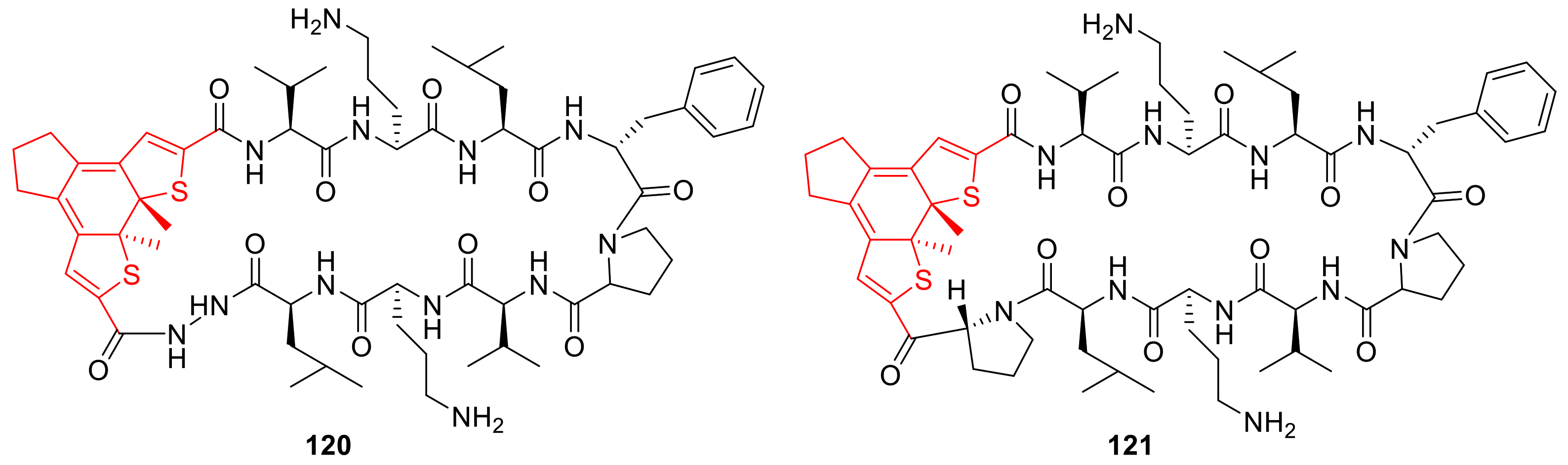
3.10. Photoswitchable Peptidomimetics
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassett, M.J.; O’Malley, A.J.; Pakes, J.R.; Newhouse, J.P.; Earle, C.C. Frequency and Cost of Chemotherapy-Related Serious Adverse Effects in a Population Sample of Women with Breast Cancer. J. Natl. Cancer Inst. 2006, 98, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef]
- Karimi, M.; Zangabad, P.S.; Baghaee-Ravari, S.; Ghazadeh, M.; Mirshekari, H.; Hamblin, M.R. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. J. Am. Chem. Soc. 2017, 139, 4584–4610. [Google Scholar] [CrossRef]
- Hughes, R.M.; Marvin, C.M.; Rodgers, Z.L.; Ding, S.; Oien, N.P.; Smith, W.J.; Lawrence, D.S. Phototriggered Secretion of Membrane Compartmentalized Bioactive Agents. Angew. Chem. Int. Ed. 2016, 55, 16080–16083. [Google Scholar] [CrossRef]
- Shamay, Y.; Adar, L.; Ashkenasy, G.; David, A. Light induced drug delivery into cancer cells. Biomaterials 2011, 32, 1377–1386. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Q.; Deng, R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X.; et al. In Vitro and In Vivo Uncaging and Bioluminescence Imaging by Using Photocaged Upconversion Nanoparticles. Angew. Chem. Int. Ed. 2012, 51, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Chou, Y.-L.; Wang, S.-W.; Hung, S.-T.; Liau, M.-C.; Chao, Y.-J.; Su, C.-H.; Yeh, C.-S. Near-Infrared Light Photocontrolled Targeting, Bioimaging, and Chemotherapy with Caged Upconversion Nanoparticles in Vitro and in Vivo. ACS Nano 2013, 7, 8516–8528. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.-J. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef]
- Tong, R.; Chiang, H.H.; Kohane, D.S. Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 19048–19053. [Google Scholar] [CrossRef]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855. [Google Scholar] [CrossRef]
- Parthiban, C.; Sen, D.; Singh, N.P. Visible-Light -Triggered Fluorescent Organic Nanoparticles for Chemo-Photodynamic Therapy with Real-Time Cellular Imaging. ACS Appl. Nano Mater. 2018, 1, 6281–6288. [Google Scholar] [CrossRef]
- Lin, Q.; Bao, C.; Yang, Y.; Liang, Q.; Zhang, D.; Cheng, S.; Zhu, L. Highly Discriminating Photorelease of Anticancer Drugs Based on Hypoxia Activatable Phototrigger Conjugated Chitosan Nanoparticles. Adv. Mater. 2013, 25, 1981–1986. [Google Scholar] [CrossRef]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef]
- Fang, N.-C.; Cheng, F.-Y.; Ho, J.A.; Yeh, C.-S. Photocontrolled Targeted Drug Delivery: Photocaged Biologically Active Folic Acid as a Light-Responsive Tumor-Targeting Molecule. Angew. Chem. Int. Ed. 2012, 51, 8806–8810. [Google Scholar] [CrossRef]
- Croissant, J.; Maynadier, M.; Gallud, A.; N’Dongo, H.P.; Nyalosaso, J.L.; Derrien, G.; Charnay, C.; Durand, J.-O.; Raehm, L.; Serein-Spirau, F.; et al. Two-Photon-Triggered Drug Delivery in Cancer Cells Using Nanoimpellers. Angew. Chem. Int. Ed. 2013, 52, 13813–13817. [Google Scholar] [CrossRef]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Kobayashi, H.; Schnermann, M.J. Near-IR Light-Mediated Cleavage of Antibody-Drug Conjugates Using Cyanine Photocages. Angew. Chem. Int. Ed. 2015, 54, 13635–13638. [Google Scholar] [CrossRef]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Yamamoto, T.; Ivanic, J.; Kobayashi, H.; Schnermann, M.J. In Vivo Activation of Duocarmycin–Antibody Conjugates by Near-Infrared Light. ACS Cent. Sci. 2017, 3, 329–337. [Google Scholar] [CrossRef]
- Klán, P.; Sólomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Blanc, A.; Bochet, C.G. Wavelength-Controlled Orthogonal Photolysis of Protecting Groups. J. Org. Chem. 2002, 67, 5567–5577. [Google Scholar] [CrossRef]
- Priestman, M.A.; Sun, L.; Lawrence, D.S. Dual Wavelength Photoactivation of cAMP- and cGMP-Dependent Protein Kinase Signaling Pathways. ACS Chem. Biol. 2011, 6, 377–384. [Google Scholar] [CrossRef]
- Kantevari, S.; Matsuzaki, M.; Kanemoto, Y.; Kasai, H.; Ellis-Davies, G.C.R. Two-color, two-photon uncaging of glutamate and GABA. Nat. Methods 2009, 7, 123–125. [Google Scholar] [CrossRef]
- Menge, C.; Heckel, A. Coumarin-Caged dG for Improved Wavelength-Selective Uncaging of DNA. Org. Lett. 2011, 13, 4620–4623. [Google Scholar] [CrossRef]
- Hansen, M.J.; Velema, W.A.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Wavelength-selective cleavage of photoprotecting groups: Strategies and applications in dynamic systems. Chem. Soc. Rev. 2015, 44, 3358–3377. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Larson, D.R.; Lawrence, D.S. Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds. ACS Chem. Biol. 2009, 4, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Chiovini, B.; Pálfi, D.; Majoros, M.; Juhász, G.; Szalay, G.; Katona, G.; Szőri, M.; Frigyesi, O.; Haveland, L.C.; Szabó, G.; et al. Theoretical Design, Synthesis, and In Vitro Neurobiological Applications of a Highly Efficient Two-Photon Caged GABA Validated on an Epileptic Case. ACS Omega 2021, 6, 15029–15045. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Reeßing, F.; Szymanski, W. Beyond Photodynamic Therapy: Light-Activated Cancer Chemotherapy. Curr. Med. Chem. 2017, 24, 4905–4950. [Google Scholar] [CrossRef]
- Zindler, M.; Pinchuk, B.; Renn, C.; Horbert, R.; Döbber, A.; Peifer, C. Design, Synthesis, and Characterization of a Photoactivatable Caged Prodrug of Imatinib. ChemMedChem 2015, 10, 1335–1338. [Google Scholar] [CrossRef]
- Horbert, R.; Pinchuk, B.; Davies, P.; Alessi, D.; Peifer, C. Photoactivatable Prodrugs of Antimelanoma Agent Vemurafenib. ACS Chem. Biol. 2015, 10, 2099–2107. [Google Scholar] [CrossRef]
- Peifer, C.; Stoiber, T.; Unger, E.; Totzke, F.; Schächtele, C.; Marmé, D.; Brenk, R.; Klebe, G.; Schollmeyer, D.; Dannhardt, G. Design, Synthesis, and Biological Evaluation of 3,4-Diarylmaleimides as Angiogenesis Inhibitors. J. Med. Chem. 2006, 49, 1271–1281. [Google Scholar] [CrossRef]
- Pinchuk, B.; Horbert, R.; Döbber, A.; Kuhl, L.; Peifer, C. Photoactivatable Caged Prodrugs of VEGFR-2 Kinase Inhibitors. Molecules 2016, 21, 570. [Google Scholar] [CrossRef]
- Woods, J.A.; Ferguson, J.S.; Kalra, S.; Degabriele, A.; Gardner, J.; Logan, P.; Ferguson, J. The phototoxicity of vemurafenib: An investigation of clinical monochromator phototesting and in vitro phototoxicity testing. J. Photochem. Photobiol. B 2015, 151, 233–238. [Google Scholar] [CrossRef]
- Pinchuk, B.; Von Drathen, T.; Opel, V.; Peifer, C. Photoinduced Conversion of Antimelanoma Agent Dabrafenib to a Novel Fluorescent BRAFV600E Inhibitor. ACS Med. Chem. Lett. 2016, 7, 962–966. [Google Scholar] [CrossRef]
- Ibsen, S.; Zahavy, E.; Wrasdilo, W.; Berns, M.; Chan, M.; Esener, S. A Novel Doxorubicin Prodrug with Controllable Photolysis Activation for Cancer Chemotherapy. Pharm. Res. 2010, 27, 1848–1860. [Google Scholar] [CrossRef]
- Ibsen, S.; Zahavy, E.; Wrasidlo, W.; Hayashi, T.; Norton, J.; Su, Y.; Adams, S.; Esener, S. Localized in vivo activation of a photoactivatable doxorubicin prodrug in deep tumor tissue. Photochem. Photobiol. 2013, 89, 698–708. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Cannon, J.; Mukherjee, J.; Isham, D.; Gam, K.; Payne, M.; Yanik, S.A.; Baker, J.R., Jr.; Choi, S.K. A Thioacetal Photocage Designed for Dual Release: Application in the Quantitation of Therapeutic Release by Synchronous Reporter Decaging. ChemBioChem 2017, 18, 126–135. [Google Scholar] [CrossRef]
- Dupart, P.S.; Mitra, K.; Lyons, C.E.; Hartman, M.C.T. Photo-controlled delivery of a potent analogue of doxorubicin. Chem. Commun. 2019, 55, 5607–5610. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate Receptor-Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Dcona, M.M.; Sheldon, J.E.; Mitra, D.; Hartman, M.C.T. Light induced drug release from a folic acid-drug conjugate. Bioorganic Med. Chem. Lett. 2017, 27, 466–469. [Google Scholar] [CrossRef][Green Version]
- Choi, S.K.; Thomas, T.; Li, M.-H.; Kotlyar, A.; Desai, A.; Baker, J.R., Jr. Light-controlled release of caged doxorubicin from folate receptor-targeting PAMAM dendrimer nanoconjugate. Chem. Commun. 2010, 46, 2632–2634. [Google Scholar] [CrossRef]
- Shell, T.A.; Lawrence, D.S. Vitamin B12: A Tunable, Long Wavelength, Light-Responsive Platform for Launching Therapeutic Agents. Acc. Chem. Res. 2015, 48, 2866–2874. [Google Scholar] [CrossRef]
- Shell, T.A.; Shell, J.R.; Rodgers, Z.L.; Lawrence, D.S. Tunable Visible and Near-IR Photoactivation of Light-Responsive Compounds by Using Fluorophores as Light-Capturing Antennas. Angew. Chem. Int. Ed. 2014, 53, 875–878. [Google Scholar] [CrossRef]
- Dcona, M.M.; Mitra, D.; Goehe, R.W.; Gewirtz, D.A.; Lebman, D.A.; Hartman, M.C.T. Photocaged permeability: A new strategy for controlled drug release. Chem. Commun. 2012, 48, 4755–4757. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Noguchi, M.; Hirota, S.; Sohma, Y.; Kimura, T.; Hayashia, Y.; Kiso, Y. Development of first photoresponsive prodrug of paclitaxel. Bioorganic Med. Chem. Lett. 2006, 16, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, M.; Skwarczynski, M.; Prakash, H.; Hirota, S.; Kimura, T.; Hayashia, Y.; Kiso, Y. Development of novel water-soluble photocleavable protective group and its application for design of photoresponsive paclitaxel prodrugs. Bioorganic Med. Chem. 2008, 16, 5389–5397. [Google Scholar] [CrossRef] [PubMed]
- Gropeanu, R.A.; Baumann, H.; Ritz, S.; Mailänder, V.; Surrey, T.; Del Campo, A. Phototriggerable 2′,7-Caged Paclitaxel. PLoS ONE 2012, 7, e43657. [Google Scholar] [CrossRef]
- Suzuki, A.Z.; Sekine, R.; Takeda, S.; Aikawa, R.; Shiraishi, Y.; Hamaguchi, T.; Okuno, H.; Tamamura, H.; Furuta, T. A clickable caging group as a new platform for modular caged compounds with improved photochemical properties. Chem. Commun. 2019, 55, 451–454. [Google Scholar] [CrossRef]
- Döbber, A.; Phoa, A.F.; Abbassi, R.H.; Stringer, B.W.; Day, B.W.; Johns, T.G.; Abadleh, M.; Peifer, C.; Munoz, L. Development and Biological Evaluation of a Photoactivatable Small Molecule Microtubule-Targeting Agent. ACS Med. Chem. Lett. 2017, 8, 395–400. [Google Scholar] [CrossRef]
- Tietze, L.F.; Muller, M.; Duefert, S.-C.; Schmuck, K.; Schuberth, I. Photoactivatable Prodrugs of Highly Potent Duocarmycin Analogues for a Selective Cancer Therapy. Chem. Eur. J. 2013, 19, 1726–1731. [Google Scholar] [CrossRef]
- Paul, A.; Biswas, A.; Sinha, S.; Shah, S.S.; Bera, M.; Mandal, M.; Singh, N.D.P. Push–Pull Stilbene: Visible Light Activated Photoremovable Protecting Group for Alcohols and Carboxylic Acids with Fluorescence Reporting Employed for Drug Delivery. Org. Lett. 2019, 21, 2968–2972. [Google Scholar] [CrossRef]
- Karthik, S.; Kumar, B.N.P.; Gangopadhyay, M.; Mandal, M.; Singh, N.D.P. A targeted, image-guided and dually locked photoresponsive drug delivery system. J. Mater. Chem. B 2015, 3, 728–732. [Google Scholar] [CrossRef]
- Venkatesh, Y.; Rajesh, Y.; Karthik, S.; Chetan, A.C.; Mandal, M.; Jana, A.; Singh, N.D.P. Photocaging of Single and Dual (Similar or Different) Carboxylic and Amino Acids by Acetyl Carbazole and its Application as Dual Drug Delivery in Cancer Therapy. J. Org. Chem. 2016, 81, 11168–11175. [Google Scholar] [CrossRef]
- Barman, S.; Mukhopadhyay, S.K.; Biswas, S.; Nandi, S.; Gangopadhyay, M.; Dey, S.; Anoop, A.; Singh, N.D.P. A p -Hydroxyphenacyl-Benzothiazole-Chlorambucil Conjugate as a Real-Time-Monitoring Drug-Delivery System Assisted by Excited-State Intramolecular Proton Transfer. Angew. Chem. Int. Ed. 2016, 55, 4194–4198. [Google Scholar] [CrossRef]
- Singh, A.K.; Kundu, M.; Roy, S.; Roy, B.; Shah, A.S.S.; Nair, A.V.; Pal, B.; Mondal, M.; Singh, N.D.P. Two-photon responsive napthyl tagged p-hydroxyphenacyl based drug delivery system: Uncaging of anti-cancer drug in the phototherapeutic window with real-time monitoring. Chem. Commun. 2020, 56, 9986–9989. [Google Scholar] [CrossRef]
- Agasti, S.S.; Laughney, A.M.; Kohler, R.H.; Weissleder, R. Photoactivatable drug-caged fluorophore conjugate allows direct quantification of intracellular drug transport. Chem. Commun. 2013, 49, 11050–11052. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Xiao, D.; Liu, L.; Xie, F.; Li, W.; Sun, W.; Yang, X.; Zhou, X. Design, Synthesis, and In Vitro Evaluation of the Photoactivatable Prodrug of the PARP Inhibitor Talazoparib. Molecules 2020, 25, 407. [Google Scholar] [CrossRef]
- Ieda, N.; Yamada, S.; Kawaguchi, M.; Miyata, N.; Nakagawa, H. (7-Diethylaminocoumarin-4-yl)methyl ester of suberoylanilide hydroxamic acid as a caged inhibitor for photocontrol of histone deacetylase activity. Bioorganic Med. Chem. 2016, 24, 2789–2793. [Google Scholar] [CrossRef]
- Leonidova, A.; Mari, C.; Aebersold, C.; Gasser, G. Selective Photorelease of an Organometallic-Containing Enzyme Inhibitor. Organometallics 2016, 35, 851–854. [Google Scholar] [CrossRef]
- Bonnet, S. Why developing photoactivated chemotherapy? Dalton Trans. 2018, 47, 10330–10343. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Ferrari, S.; Gasser, G. Combination of Ru(ii) complexes and light: New frontiers in cancer therapy. Chem. Sci. 2015, 6, 2660–2686. [Google Scholar] [CrossRef]
- Mari, C.; Pierroz, V.; Leonidova, A.; Ferrari, S.; Gasser, G. Towards Selective Light-Activated RuII-Based Prodrug Candidates. Eur. J. Inorg. Chem. 2015, 23, 3879–3891. [Google Scholar] [CrossRef]
- Leonidova, A.; Pierroz, V.; Rubbiani, R.; Lan, Y.; Schmitz, A.G.; Kaech, A.; Sigel, R.K.O.; Ferrari, S.; Gasser, G. Photo-induced uncaging of a specific Re(i) organometallic complex in living cells. Chem. Sci. 2014, 5, 4044–4056. [Google Scholar] [CrossRef]
- Joshi, T.; Pierroz, V.; Mari, C.; Gemperle, L.; Ferrari, S.; Gasser, G. A Bis(dipyridophenazine)(2-(2-pyridyl)pyrimidine-4-carboxylic acid)ruthenium(II) Complex with Anticancer Action upon Photodeprotection. Angew. Chem. Int. Ed. 2014, 53, 2960–2963. [Google Scholar] [CrossRef]
- Ciesienski, K.L.; Hyman, L.M.; Yang, D.T.; Haas, K.L.; Dickens, M.G.; Holbrook, R.J.; Franz, K.J. A Photo-Caged Platinum(II) Complex That Increases Cytotoxicity upon Light Activation. Eur. J. Inorg. Chem. 2010, 15, 2224–2228. [Google Scholar] [CrossRef]
- Kumbhar, A.A.; Franks, A.T.; Butcher, R.J.; Franz, K.J. Light uncages a copper complex to induce nonapoptotic cell death. Chem. Commun. 2013, 49, 2460–2462. [Google Scholar] [CrossRef]
- Ciesienski, K.L.; Haas, K.L.; Franz, K.J. Development of next-generation photolabile copper cages with improved copper binding properties. Dalton Trans. 2010, 39, 9538–9546. [Google Scholar] [CrossRef] [PubMed]
- Lameijer, L.N.; Ernst, D.; Hopkins, S.L.; Meijer, M.S.; Askes, S.H.C.; Le Dévédec, S.E.; Bonnet, S. A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 11549–11553. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Abe, M.; Nishimura, Y.; Ishizaka, S.; Namba, M.; Nakashima, T.; Shimoji, K.; Hattori, N. Photochemical generation of the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical from caged nitroxides by near-infrared two-photon irradiation and its cytocidal effect on lung cancer cells. Beilstein J. Org. Chem. 2019, 15, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Peng, D.; Wang, B.; Long, L.; Guo, C.; Yuan, J. A Model for Light-Triggered Porphyrin Anticancer Prodrugs Based on ano-Nitrobenzyl Photolabile Group. Eur. J. Org. Chem. 2008, 2008, 793–796. [Google Scholar] [CrossRef]
- Kounde, C.S.; Tate, E. Photoactive Bifunctional Degraders: Precision Tools to Regulate Protein Stability. J. Med. Chem. 2020, 63, 15483–15493. [Google Scholar] [CrossRef]
- Nalawansha, D.A.; Crews, C.M. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem. Biol. 2020, 27, 998–1014. [Google Scholar] [CrossRef]
- Xue, G.; Wang, K.; Zhou, D.; Zhong, H.; Pan, Z. Light-Induced Protein Degradation with Photocaged PROTACs. J. Am. Chem. Soc. 2019, 141, 18370–18374. [Google Scholar] [CrossRef]
- Kounde, C.S.; Shchepinova, M.M.; Saunders, C.N.; Muelbaier, M.; Rackham, M.D.; Harling, J.D.; Tate, E.W. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chem. Commun. 2020, 56, 5532–5535. [Google Scholar] [CrossRef]
- Naro, Y.; Darrah, K.; Deiters, A. Optical Control of Small Molecule-Induced Protein Degradation. J. Am. Chem. Soc. 2020, 142, 2193–2197. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Ma, L.; He, Z.; Wang, D.; Liu, Y.; Lin, Q.; Zhang, T.; Gray, N.; Kaniskan, H.Ü.; et al. Light-induced control of protein destruction by opto-PROTAC. Sci. Adv. 2020, 6, eaay5154. [Google Scholar] [CrossRef]
- Hansen, M.J.; Feringa, F.M.; Kobauri, P.; Szymanski, W.; Medema, R.H.; Feringa, B.L. Photoactivation of MDM2 Inhibitors: Controlling Protein–Protein Interaction with Light. J. Am. Chem. Soc. 2018, 140, 13136–13141. [Google Scholar] [CrossRef]
- Kaufman, H.; Vratsanos, S.M.; Erlanger, B.F. Photoregulation of an Enzymic Process by Means of a Light-Sensitive Ligand. Science 1968, 162, 1487–1489. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef]
- Szymanski, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Welleman, I.M.; Hoorens, M.W.H.; Feringa, B.L.; Boersma, H.H.; Szymański, W. Photoresponsive molecular tools for emerging applications of light in medicine. Chem. Sci. 2020, 11, 11672–11691. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef]
- Ryan, A. Azoreductases in drug metabolism. Br. J. Pharmacol. 2017, 174, 2161–2173. [Google Scholar] [CrossRef]
- Ferreira, R.; Nilsson, J.R.; Solano, C.; Andréasson, J.; Grøtli, M. Design, Synthesis and Inhibitory Activity of Photoswitchable RET Kinase Inhibitors. Sci. Rep. 2015, 5, 9769. [Google Scholar] [CrossRef]
- Schmidt, D.; Rodat, T.; Heintze, L.; Weber, J.; Horbert, R.; Girreser, U.; Raeker, T.; Bußmann, L.; Kriegs, M.; Hartke, B.; et al. Axitinib: A Photoswitchable Approved Tyrosine Kinase Inhibitor. ChemMedChem 2018, 13, 2415–2426. [Google Scholar] [CrossRef]
- Heintze, L.; Schmidt, D.; Rodat, T.; Witt, L.; Ewert, J.; Kriegs, M.; Herges, R.; Peifer, C. Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib. Int. J. Mol. Sci. 2020, 21, 8961. [Google Scholar] [CrossRef]
- Hoorens, M.W.; Ourailidou, M.E.; Rodat, T.; van der Wouden, P.E.; Kobauri, P.; Kriegs, M.; Peifer, C.; Feringa, B.L.; Dekker, F.J.; Szymanski, W. Light-controlled inhibition of BRAFV600E kinase. Eur. J. Med. Chem. 2019, 179, 133–146. [Google Scholar] [CrossRef]
- Wenglowsky, S.; Ahrendt, K.A.; Buckmelter, A.J.; Feng, B.; Gloor, S.L.; Gradl, S.; Grina, J.; Hansen, J.D.; Laird, E.R.; Lunghofer, P.; et al. Pyrazolopyridine inhibitors of B-RafV600E. Part 2: Structure–activity relationships. Bioorganic Med. Chem. Lett. 2011, 21, 5533–5537. [Google Scholar] [CrossRef]
- Schehr, M.; Ianes, C.; Weisner, J.; Heintze, L.; Müller, M.P.; Pichlo, C.; Charl, J.; Brunstein, E.; Ewert, J.; Lehr, M.; et al. 2-Azo-, 2-diazocine-thiazols and 2-azo-imidazoles as photoswitchable kinase inhibitors: Limitations and pitfalls of the photoswitchable inhibitor approach. Photochem. Photobiol. Sci. 2019, 18, 1398–1407. [Google Scholar] [CrossRef]
- Halekotte, J.; Witt, L.; Ianes, C.; Krüger, M.; Bührmann, M.; Rauh, D.; Pichlo, C.; Brunstein, E.; Luxenburger, A.; Baumann, U.; et al. Optimized 4,5-Diarylimidazoles as Potent/Selective Inhibitors of Protein Kinase CK1 and Their Structural Relation to p38 MAPK. Molecules 2017, 22, 522. [Google Scholar] [CrossRef]
- Wilson, D.; Li, J.W.; Branda, N.R. Visible-Light-Triggered Activation of a Protein Kinase Inhibitor. ChemMedChem 2017, 12, 284–287. [Google Scholar] [CrossRef]
- Falenczyk, C.; Schiedel, M.; Karaman, B.; Rumpf, T.; Kuzmanovic, N.; Grøtli, M.; Sippl, W.; Jung, M.; Konig, B. Chromo-pharmacophores: Photochromic diarylmaleimide inhibitors for sirtuins. Chem. Sci. 2014, 5, 4794–4799. [Google Scholar] [CrossRef]
- Szymanski, W.; Ourailidou, M.E.; Velema, W.A.; Dekker, F.J.; Feringa, B.L. Light-Controlled Histone Deacetylase (HDAC) Inhibitors: Towards Photopharmacological Chemotherapy. Chem. Eur. J. 2015, 21, 16517–16524. [Google Scholar] [CrossRef]
- Reis, S.A.; Ghosh, B.; Hendricks, J.A.; Szantai-Kis, D.M.; Törk, L.; Ross, K.N.; Lamb, J.; Read-Button, W.; Zheng, B.; Wang, H.; et al. Light-controlled modulation of gene expression by chemical optoepigenetic probes. Nat. Chem. Biol. 2016, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Matera, C.; Gomila, A.M.J.; Camarero, N.; Libergoli, M.; Soler, C.; Gorostiza, P. Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy. J. Am. Chem. Soc. 2018, 140, 15764–15773. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Parelkar, S.S.; Nagar, M.; Thompson, P.R. Photochemical Control of Protein Arginine Deiminase (PAD) Activity. ACS Chem. Biol. 2018, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Nahaboo, W.; Reynders, M.; Nekolla, K.; Jalinot, P.; Hasserodt, J.; Rehberg, M.; Delattre, M.; Zahler, S.; Vollmar, A.; et al. Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death. Cell 2015, 162, 403–411. [Google Scholar] [CrossRef]
- Gaspari, R.; Prota, A.E.; Bargsten, K.; Cavalli, A.; Steinmetz, M.O. Structural Basis of cis—And trans—Combretastatin Binding to Tubulin. Chem 2017, 2, 102–113. [Google Scholar] [CrossRef]
- Engdahl, A.J.; Torres, E.A.; Lock, S.E.; Engdahl, T.B.; Mertz, P.S.; Streu, C.N. Synthesis, Characterization, and Bioactivity of the Photoisomerizable Tubulin Polymerization Inhibitor azo-Combretastatin A4. Org. Lett. 2015, 17, 4546–4549. [Google Scholar] [CrossRef]
- Sheldon, J.E.; Dcona, M.M.; Lyons, C.E.; Hackett, J.C.; Hartman, M.C.T. Photoswitchable anticancer activity via trans–cis isomerization of a combretastatin A-4 analog. Org. Biomol. Chem. 2016, 14, 40–49. [Google Scholar] [CrossRef]
- Rastogi, S.K.; Zhao, Z.; Barrett, S.L.; Shelton, S.D.; Zafferani, M.; Anderson, H.E.; Blumenthal, M.O.; Jones, L.R.; Wang, L.; Li, X.; et al. Photoresponsive azo-combretastatin A-4 analogues. Eur. J. Med. Chem. 2018, 143, 1–7. [Google Scholar] [CrossRef]
- Brown, C.; Rastogi, S.K.; Barrett, S.L.; Anderson, H.E.; Twichell, E.; Gralinski, S.; McDonald, A.; Brittain, W.J. Differential azobenzene solubility increases equilibrium cis/trans ratio in water. J. Photochem. Photobiol. A Chem. 2017, 336, 140–145. [Google Scholar] [CrossRef]
- Gao, L.; Meiring, J.C.; Kraus, Y.; Wranik, M.; Weinert, T.; Pritzl, S.D.; Bingham, R.; Ntouliou, E.; Jansen, K.I.; Olieric, N.; et al. A Robust, GFP-Orthogonal Photoswitchable Inhibitor Scaffold Extends Optical Control over the Microtubule Cytoskeleton. Cell Chem. Biol. 2021, 28, 228–241. [Google Scholar] [CrossRef]
- Sailer, A.; Ermer, F.; Kraus, Y.; Lutter, F.H.; Donau, C.; Bremerich, M.; Ahlfeld, J.; Thorn-Seshold, O. Hemithioindigos for Cellular Photopharmacology: Desymmetrised Molecular Switch Scaffolds Enabling Design Control over the Isomer-Dependency of Potent Antimitotic Bioactivity. ChemBioChem 2019, 20, 1305–1314. [Google Scholar] [CrossRef]
- Wiedbrauk, S.; Dube, H. Hemithioindigo—an emerging photoswitch. Tetrahedron Lett. 2015, 56, 4266–4274. [Google Scholar] [CrossRef]
- Sailer, A.; Ermer, F.; Kraus, Y.; Bingham, R.; Lutter, F.H.; Ahlfeld, J.; Thorn-Seshold, O. Potent hemithioindigo-based antimitotics photocontrol the microtubule cytoskeleton in cellulo. Beilstein J. Org. Chem. 2020, 16, 125–134. [Google Scholar] [CrossRef]
- Müller-Deku, A.; Meiring, J.C.M.; Loy, K.; Kraus, Y.; Heise, C.; Bingham, R.; Jansen, K.I.; Qu, X.; Bartolini, F.; Kapitein, L.C.; et al. Photoswitchable paclitaxel-based microtubule stabilisers allow optical control over the microtubule cytoskeleton. Nat. Commun. 2020, 11, 4640. [Google Scholar] [CrossRef]
- Mafy, N.N.; Matsuo, K.; Hiruma, S.; Uehara, R.; Tamaoki, N. Photoswitchable CENP-E Inhibitor Enabling the Dynamic Control of Chromosome Movement and Mitotic Progression. J. Am. Chem. Soc. 2020, 142, 1763–1767. [Google Scholar] [CrossRef]
- Borowiak, M.; Küllmer, F.; Gegenfurtner, F.; Peil, S.; Nasufovic, V.; Zahler, S.; Thorn-Seshold, O.; Trauner, D.; Arndt, H.-D. Optical Manipulation of F-Actin with Photoswitchable Small Molecules. J. Am. Chem. Soc. 2020, 142, 9240–9249. [Google Scholar] [CrossRef]
- Presa, A.; Brissos, R.F.; Caballero, A.B.; Borilovic, I.; Korrodi-Gregório, L.; Pérez-Tomás, R.; Roubeau, O.; Gamez, P. Photoswitching the Cytotoxic Properties of Platinum(II) Compounds. Angew. Chem. Int. Ed. 2015, 54, 4561–4565. [Google Scholar] [CrossRef]
- Presa, A.; Vázquez, G.; Barrios, L.A.; Roubeau, O.; Korrodi-Gregório, L.; Pérez-Tomás, R.; Gamez, P. Photoactivation of the Cytotoxic Properties of Platinum(II) Complexes through Ligand Photoswitching. Inorg. Chem. 2018, 57, 4009–4022. [Google Scholar] [CrossRef]
- Hansen, M.J.; Velema, W.A.; De Bruin, G.; Overkleeft, H.S.; Szymanski, W.; Feringa, B.L. Proteasome Inhibitors with Photocontrolled Activity. ChemBioChem 2014, 15, 2053–2057. [Google Scholar] [CrossRef]
- Blanco, B.; Palasis, K.A.; Adwal, A.; Callen, D.F.; Abell, A.D. Azobenzene-containing photoswitchable proteasome inhibitors with selective activity and cellular toxicity. Bioorganic Med. Chem. 2017, 25, 5050–5054. [Google Scholar] [CrossRef] [PubMed]
- Reynders, M.; Matsuura, B.S.; Bérouti, M.; Simoneschi, D.; Marzio, A.; Pagano, M.; Trauner, D. PHOTACs enable optical control of protein degradation. Sci. Adv. 2020, 6, eaay5064. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, P.; Samarasinghe, K.T.G.; Crews, C.M.; Carreira, E.M. Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Cent. Sci. 2019, 5, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Lu, M.-C.; Wang, Y.; Shan, W.-X.; Wang, X.-Y.; You, Q.-D.; Jiang, Z.-Y. Azo-PROTAC: Novel Light-Controlled Small-Molecule Tool for Protein Knockdown. J. Med. Chem. 2020, 63, 4644–4654. [Google Scholar] [CrossRef]
- Albert, L.; Vázquez, O. Photoswitchable peptides for spatiotemporal control of biological functions. Chem. Commun. 2019, 55, 10192–10213. [Google Scholar] [CrossRef]
- Albert, L.; Xu, J.; Wan, R.; Srinivasan, V.; Dou, Y.; Vázquez, O. Controlled inhibition of methyltransferases using photoswitchable peptidomimetics: Towards an epigenetic regulation of leukemia. Chem. Sci. 2017, 8, 4612–4618. [Google Scholar] [CrossRef]
- Babii, O.; Afonin, S.; Garmanchuk, L.V.; Nikulina, V.V.; Nikolaienko, T.V.; Storozhuk, O.V.; Shelest, D.V.; Dasyukevich, O.I.; Ostapchenko, L.I.; Iurchenko, V.; et al. Direct Photocontrol of Peptidomimetics: An Alternative to Oxygen-Dependent Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2016, 55, 5493–5496. [Google Scholar] [CrossRef]
- Babii, O.; Afonin, S.; Schober, T.; Garmanchuk, L.V.; Ostapchenko, L.I.; Yurchenko, V.; Zozulya, S.; Tarasov, O.; Pishel, I.; Ulrich, A.S.; et al. Peptide drugs for photopharmacology: How much of a safety advantage can be gained by photocontrol? Future Drug Discov. 2020, 2, FDD28. [Google Scholar] [CrossRef]
- Said, S.S.; Campbell, S.; Hoare, T. Externally Addressable Smart Drug Delivery Vehicles: Current Technologies and Future Directions. Chem. Mater. 2019, 31, 4971–4989. [Google Scholar] [CrossRef]
- Cheong, W.-F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Salvador, M.A.; Almeida, P.; Reis, L.V.; Santos, P.F. Near-infrared absorbing delocalized cationic azo dyes. Dye. Pigment. 2009, 82, 118–123. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Bléger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as Readily Synthesized Photoswitches Offering Nearly Quantitative Two-Way Isomerization with Visible Light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef]
- Sadovski, O.; Beharry, A.A.; Zhang, F.; Woolley, G.A. Spectral Tuning of Azobenzene Photoswitches for Biological Applications. Angew. Chem. Int. Ed. 2009, 48, 1484–1486. [Google Scholar] [CrossRef]
- Samanta, S.; Beharry, A.A.; Sadovski, O.; McCormick, T.M.; Babalhavaeji, A.; Tropepe, V.; Woolley, G.A. Photoswitching Azo Compounds in Vivo with Red Light. J. Am. Chem. Soc. 2013, 135, 9777–9784. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Collins, C.V.; Jarrah, K.; Sadovski, O.; Dai, Q.; Woolley, G.A. Near-Infrared Photoswitching of Azobenzenes under Physiological Conditions. J. Am. Chem. Soc. 2017, 139, 13483–13486. [Google Scholar] [CrossRef]
- Samanta, S.; McCormick, T.M.; Schmidt, S.K.; Seferos, D.S.; Woolley, G.A. Robust visible light photoswitching with ortho-thiol substituted azobenzenes. Chem. Commun. 2013, 49, 10314–10316. [Google Scholar] [CrossRef]
- Samanta, S.; Babalhavaeji, A.; Dong, M.; Woolley, G.A. Photoswitching ofortho-Substituted Azonium Ions by Red Light in Whole Blood. Angew. Chem. Int. Ed. 2013, 52, 14127–14130. [Google Scholar] [CrossRef]
- Yang, Y.; Hughes, R.P.; Aprahamian, I. Visible Light Switching of a BF2-Coordinated Azo Compound. J. Am. Chem. Soc. 2012, 134, 15221–15224. [Google Scholar] [CrossRef]
- Yang, Y.; Hughes, R.P.; Aprahamian, I. Near-Infrared Light Activated Azo-BF2 Switches. J. Am. Chem. Soc. 2014, 136, 13190–13193. [Google Scholar] [CrossRef]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly Efficient Reversible Z−E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(nπ*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef]
- Schehr, M.; Hugenbusch, D.; Moje, T.; Näther, C.; Herges, R. Synthesis of mono-functionalized S-diazocines via intramolecular Baeyer–Mills reactions. Beilstein J. Org. Chem. 2018, 14, 2799–2804. [Google Scholar] [CrossRef]
- Klaue, K.; Garmshausen, Y.; Hecht, S. Taking Photochromism beyond Visible: Direct One-Photon NIR Photoswitches Operating in the Biological Window. Angew. Chem. Int. Ed. 2018, 57, 1414–1417. [Google Scholar] [CrossRef]
- Umeda, N.; Takahashi, H.; Kamiya, M.; Ueno, T.; Komatsu, T.; Terai, T.; Hanaoka, K.; Nagano, T.; Urano, Y. Boron Dipyrromethene As a Fluorescent Caging Group for Single-Photon Uncaging with Long-Wavelength Visible Light. ACS Chem. Biol. 2014, 9, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.P.; Syed, A.; Beck, C.L.; Albright, T.R.; Mahoney, K.M.; Unash, R.; Smith, E.A.; Winter, A.H. BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. J. Am. Chem. Soc. 2015, 137, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Slanina, T.; Shrestha, P.; Palao, E.; Kand, D.; Peterson, J.A.; Dutton, A.S.; Rubinstein, N.; Weinstain, R.; Winter, A.H.; Klán, P. In Search of the Perfect Photocage: Structure–Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168–15175. [Google Scholar] [CrossRef] [PubMed]
- Gorka, A.P.; Nani, R.R.; Zhu, J.; Mackem, S.; Schnermann, M.J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, L.; Wang, Z.; Hua, Y.; Zhang, D.; Bao, B.; Bao, C.; Gong, X.; Zhu, L. Coumarin Photocaging Groups Modified with an Electron-Rich Styryl Moiety at the 3-Position: Long-Wavelength Excitation, Rapid Photolysis, and Photobleaching. Angew. Chem. Int. Ed. 2018, 57, 3722–3726. [Google Scholar] [CrossRef]
- Warther, D.; Gug, S.; Specht, A.; Bolze, F.; Nicoud, J.-F.; Mourot, A.; Goeldner, M. Two-photon uncaging: New prospects in neuroscience and cellular biology. Bioorganic Med. Chem. 2010, 18, 7753–7758. [Google Scholar] [CrossRef]
- Gug, S.; Bolze, F.; Specht, A.; Bourgogne, C.; Goeldner, M.; Nicoud, J.-F. Molecular Engineering of Photoremovable Protecting Groups for Two-Photon Uncaging. Angew. Chem. Int. Ed. 2008, 47, 9525–9529. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, Z.; Hooker, J.; Moore, A. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol. Imaging Biol. 2012, 14, 156–162. [Google Scholar] [CrossRef]
- Tochitsky, I.; Trautman, J.; Gallerani, N.; Malis, J.G.; Kramer, R.H. Restoring visual function to the blind retina with a potent, safe and long-lasting photoswitch. Sci. Rep. 2017, 7, 45487. [Google Scholar] [CrossRef]
- Beharry, A.A.; Wong, L.; Tropepe, V.; Woolley, G.A. Fluorescence Imaging of Azobenzene Photoswitching In Vivo. Angew. Chem. Int. Ed. 2011, 50, 1325–1327. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; Van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Reeßing, F.; Stuart, M.; Samplonius, D.F.; Dierckx, R.; Feringa, B.L.; Helfrich, W.; Szymanski, W. A light-responsive liposomal agent for MRI contrast enhancement and monitoring of cargo delivery. Chem. Commun. 2019, 55, 10784–10787. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconj. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunkel, P.; Ilaš, J. Targeted Cancer Therapy Using Compounds Activated by Light. Cancers 2021, 13, 3237. https://doi.org/10.3390/cancers13133237
Dunkel P, Ilaš J. Targeted Cancer Therapy Using Compounds Activated by Light. Cancers. 2021; 13(13):3237. https://doi.org/10.3390/cancers13133237
Chicago/Turabian StyleDunkel, Petra, and Janez Ilaš. 2021. "Targeted Cancer Therapy Using Compounds Activated by Light" Cancers 13, no. 13: 3237. https://doi.org/10.3390/cancers13133237
APA StyleDunkel, P., & Ilaš, J. (2021). Targeted Cancer Therapy Using Compounds Activated by Light. Cancers, 13(13), 3237. https://doi.org/10.3390/cancers13133237





