Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnosis
2.1. Pathological Diagnosis of GIST
2.2. Molecular Aspects of GIST
2.3. Clinical Diagnosis of GIST
2.4. Tissue Acquisition for Pathological Diagnosis
3. Surgery
3.1. Surgical Therapy of Primary GISTs
3.2. Surgical Therapy of Small GISTs
3.3. Laparoscopic Surgery for GISTs
3.4. Risk Evaluation in GIST
4. Medical Therapy
4.1. Medical Therapy for Metastatic/Recurrent GISTs
4.2. Newly Emerging Therapy: New TKIs and Drugs for the NTRK Fusion
4.3. Developing Therapy
5. Conclusions
Authors Contribution
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFIP | Armed Forces Institute of Pathology |
| CSF1R | macrophage colony-stimulating-factor 1 receptor |
| CTLA4 | cytotoxic T-lymphocyte-associated antigen 4 |
| DFS | disease-free survival |
| EGJ | esophagogastric junction |
| EMR | endoscopic mucosal resection |
| ESD | endoscopic submucosal dissection |
| ESEG | European Society of Gastrointestinal Endoscopy |
| ESMO | European Society for Medical Oncology |
| EUS | endoscopic ultrasonography |
| EUS-FNA | EUS-guided fine needle aspiration |
| FDA | Food and Drug Administration |
| FLT3 | FMS-like tyrosine kinase 3 |
| GI tract | gastrointestinal tract |
| GIST | gastrointestinal stromal tumor |
| GPR20 | G protein-coupled receptor 20 |
| HIF-1α | hypoxia-inducible factor-1α |
| HR | hazard ratio |
| IGF1R | insulin growth factor-1 receptor |
| IHC | immunohistochemistry |
| IMT | inflammatory myofibroblastic tumor |
| LECS | laparoscopic endoscopic cooperative surgery |
| MIAB | mucosal incision-assisted biopsy |
| NCCN | National Comprehensive Cancer Network |
| NIH | National Institutes of Health |
| OS | overall survival |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death-ligand 1 |
| PEComa | perivascular epithelioid cell tumor |
| PFS | progression-free survival |
| RFS | recurrence-free survival |
| ROSE | rapid on-site evaluation |
| RR | response rate |
| RTK | receptor tyrosine kinase |
| SDH | succinate dehydrogenase |
| SFT | solitary fibrous tumor |
| SMT | submucosal tumor |
| TKI | tyrosine kinase inhibitors |
| Tx | therapy |
| VEGFR | vascular endothelial growth factor receptor |
| SSTR2 | somatostatin receptor 2 |
References
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer 2011, 11, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Antonescu, C.R.; DeMatteo, R.P.; Ganjoo, K.N.; Maki, R.G.; Pisters, P.W.; Raut, C.P.; Riedel, R.F.; Schuetze, S.; et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc. Netw. 2010, 8 (Suppl. S2), S1–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Kang, Y.K.; Nishida, T.; von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Nishida, T.; Doi, T.; Naito, Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother. 2014, 15, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.L.; Heinrich, M.C.; Bauer, S.; George, S. Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann. Oncol. 2018, 29, 2037–2045. [Google Scholar] [CrossRef]
- Vanden Bempt, I.; Vander Borght, S.; Sciot, R.; Spans, L.; Claerhout, S.; Brems, H.; Lehnert, S.; Dehaspe, L.; Fransis, S.; Neuville, B.; et al. Comprehensive targeted next-generation sequencing approach in the molecular diagnosis of gastrointestinal stromal tumor. Genes Chromosomes Cancer 2021, 60, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Janeway, K.A.; Kim, S.Y.; Lodish, M.; Nosé, V.; Rustin, P.; Gaal, J.; Dahia, P.L.; Liegl, B.; Ball, E.R.; Raygada, M.; et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 314–318. [Google Scholar] [CrossRef] [Green Version]
- Huss, S.; Pasternack, H.; Ihle, M.A.; Merkelbach-Bruse, S.; Heitkötter, B.; Hartmann, W.; Trautmann, M.; Gevensleben, H.; Büttner, R.; Schildhaus, H.U.; et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum. Pathol. 2017, 62, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Hohenberger, P.; Corless, C.L. Gastrointestinal stromal tumour. Lancet 2013, 382, 973–983. [Google Scholar] [CrossRef]
- Nishida, T.; Goto, O.; Raut, C.P.; Yahagi, N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016, 122, 3110–3118. [Google Scholar] [CrossRef] [Green Version]
- Kawanowa, K.; Sakuma, Y.; Sakurai, S.; Hishima, T.; Iwasaki, Y.; Saito, K.; Hosoya, Y.; Nakajima, T.; Funata, N. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum. Pathol. 2006, 37, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Krasinskas, A.M.; Hofstetter, W.L.; Swisher, S.G.; Wu, T.T. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am. J. Surg. Pathol. 2007, 31, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Wünsch, P.H.; Dirnhofer, S.; Bihl, M.P.; Terracciano, L.M.; Tornillo, L. Microscopic gastrointestinal stromal tumors in esophageal and intestinal surgical resection specimens: A clinicopathologic, immunohistochemical, and molecular study of 19 lesions. Am. J. Surg. Pathol. 2008, 32, 867–873. [Google Scholar] [CrossRef]
- Rossi, S.; Gasparotto, D.; Toffolatti, L.; Pastrello, C.; Gallina, G.; Marzotto, A.; Sartor, C.; Barbareschi, M.; Cantaloni, C.; Messerini, L.; et al. Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am. J. Surg. Pathol. 2010, 34, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Hedenbro, J.L.; Ekelund, M.; Wetterberg, P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg. Endosc. 1991, 5, 20–23. [Google Scholar] [CrossRef]
- Nishida, T.; Hirota, S.; Taniguchi, M.; Hashimoto, K.; Isozaki, K.; Nakamura, H.; Kanakura, Y.; Tanaka, T.; Takabayashi, A.; Matsuda, H.; et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat. Genet. 1998, 19, 323–324. [Google Scholar] [CrossRef]
- Chompret, A.; Kannengiesser, C.; Barrois, M.; Terrier, P.; Dahan, P.; Tursz, T.; Lenoir, G.M.; Bressac-De Paillerets, B. PDGFRA germline mutation in a family with multiplecases of gastrointestinal stromal tumor. Gastroenterology 2004, 126, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Pasini, B.; McWhinney, S.R.; Bei, T.; Matyakhina, L.; Stergiopoulos, S.; Muchow, M.; Boikos, S.A.; Ferrando, B.; Pacak, K.; Assie, G.; et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur. J. Hum. Genet. 2008, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yantiss, R.K.; Rosenberg, A.E.; Sarran, L.; Besmer, P.; Antonescu, C.R. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: A pathologic and molecular study. Mod. Pathol. 2005, 18, 475–484. [Google Scholar] [CrossRef]
- Nishida, T.; Tsujimoto, M.; Takahashi, T.; Hirota, S.; Blay, J.Y.; Wataya-Kaneda, M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J. Gastroenterol. 2016, 51, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Haller, F.; Schulten, H.J.; Armbrust, T.; Langer, C.; Gunawan, B.; Füzesi, L. Multicentric sporadic gastrointestinal stromal tumors (GISTs) of the stomach with distinct clonal origin: Differential diagnosis to familial and syndromal GIST variants and peritoneal metastasis. Am. J. Surg. Pathol. 2007, 31, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Ma, X.L.; Yang, L.X.; Zhao, W.Y.; Tu, L.; Zhuang, C.; Ni, B.; Liu, Q.; Wang, M.; Cao, H. Clinicopathologic characteristics, diagnostic clues, and prognoses of patients with multiple sporadic gastrointestinal stromal tumors: A case series and review of the literature. Diagn. Pathol. 2020, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S. Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl. Gastroenterol. Hepatol. 2018, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Dei Tos, A.P.; Hornick, J.L.; Miettinen, M.; Wanless, I.R.; Wardelmann, E. Gastrointestinal Stromal Tumour. In WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; IARC Press: Lyon, France, 2020; pp. 216–221. [Google Scholar]
- Charville, G.W.; Longacre, T.A. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv. Anat. Pathol. 2017, 24, 336–353. [Google Scholar] [CrossRef]
- Corless, C.L. Gastrointestinal stromal tumors: What do we know now? Mod. Pathol. 2014, 27 (Suppl. S1), S1–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, P.; Chen, Y.; Zhang, L.; Guo, X.; Wongvipat, J.; Shamu, T.; Fletcher, J.A.; Dewell, S.; Maki, R.G.; Zheng, D.; et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature 2010, 467, 849–853. [Google Scholar] [CrossRef] [Green Version]
- Rubin, B.P.; Heinrich, M.C. Genotyping and immunohistochemistry of gastrointestinal stromal tumors: An update. Semin. Diagn. Pathol. 2015, 32, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Corless, C.L.; Duensing, A.; McGreevey, L.; Chen, C.J.; Joseph, N.; Singer, S.; Griffith, D.J.; Haley, A.; Town, A.; et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003, 299, 708–710. [Google Scholar] [CrossRef]
- Agaram, N.P.; Wong, G.C.; Guo, T.; Maki, R.G.; Singer, S.; Dematteo, R.P.; Besmer, P.; Antonescu, C.R. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer 2008, 47, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskoski, R., Jr. Structure and regulation of Kit protein-tyrosine kinase—The stem cell factor receptor. Biochem. Biophys. Res. Commun. 2005, 338, 1307–1315. [Google Scholar] [CrossRef]
- Corless, C.L.; McGreevey, L.; Haley, A.; Town, A.; Heinrich, M.C. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am. J. Pathol. 2002, 160, 1567–1572. [Google Scholar] [CrossRef] [Green Version]
- Manley, P.N.; Abu-Abed, S.; Kirsch, R.; Hawrysh, A.; Perrier, N.; Feilotter, H.; Pollett, A.; Riddell, R.H.; Hookey, L.; Walia, J.S. Familial PDGFRA-mutation syndrome: Somatic and gastrointestinal phenotype. Hum. Pathol. 2018, 76, 52–57. [Google Scholar] [CrossRef]
- Bosbach, B.; Rossi, F.; Yozgat, Y.; Loo, J.; Zhang, J.Q.; Berrozpe, G.; Warpinski, K.; Ehlers, I.; Veach, D.; Kwok, A.; et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA 2017, 114, E8448–E8457. [Google Scholar] [CrossRef] [Green Version]
- Shi, E.; Chmielecki, J.; Tang, C.M.; Wang, K.; Heinrich, M.C.; Kang, G.; Corless, C.L.; Hong, D.; Fero, K.E.; Murphy, J.D.; et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J. Transl. Med. 2016, 14, 339. [Google Scholar] [CrossRef] [Green Version]
- Atiq, M.A.; Davis, J.L.; Hornick, J.L.; Dickson, B.C.; Fletcher, C.D.M.; Fletcher, J.A.; Folpe, A.L.; Mariño-Enríquez, A. Mesenchymal tumors of the gastrointestinal tract with NTRK rearrangements: A clinicopathological, immunophenotypic, and molecular study of eight cases, emphasizing their distinction from gastrointestinal stromal tumor (GIST). Mod. Pathol. 2021, 34, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.G.; Blay, J.Y.; Demetri, G.D.; Fletcher, J.A.; Joensuu, H.; Martín-Broto, J.; Nishida, T.; Reichardt, P.; Schöffski, P.; Trent, J.C. Key Issues in the Clinical Management of Gastrointestinal Stromal Tumors: An Expert Discussion. Oncologist 2015, 20, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falchook, G.S.; Trent, J.C.; Heinrich, M.C.; Beadling, C.; Patterson, J.; Bastida, C.C.; Blackman, S.C.; Kurzrock, R. BRAF mutant gastrointestinal stromal tumor: First report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget 2013, 4, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Bergqvist, C.; Wolkenstein, P. MEK inhibitors in RASopathies. Curr. Opin. Oncol. 2021, 33, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Hirota, S.; Yanagisawa, A.; Sugino, Y.; Minami, M.; Yamamura, Y.; Otani, Y.; Shimada, Y.; Takahashi, F.; Kubota, T.; et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int. J. Clin. Oncol. 2008, 13, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.W.; Jung, D.H.; Kim, J.S.; Shin, Y.R.; Choi, S.H.; Kim, B.W. CT Versus Endoscopic Ultrasound for Differentiating Small (2–5 cm) Gastrointestinal Stromal Tumors From Leiomyomas. AJR Am. J. Roentgenol. 2019, 213, 586–591. [Google Scholar] [CrossRef]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Casper, E.S.; Conrad, E.U., 3rd; DeLaney, T.F.; Ganjoo, K.N.; George, S.; et al. Gastrointestinal stromal tumors, version 2.2014. J. Natl. Compr. Canc. Netw. 2014, 12, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Akahoshi, K.; Oya, M.; Koga, T.; Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World. J. Gastroenterol. 2018, 24, 2806–2817. [Google Scholar] [CrossRef]
- Zhang, X.C.; Li, Q.L.; Yu, Y.F.; Yao, L.Q.; Xu, M.D.; Zhang, Y.Q.; Zhong, Y.S.; Chen, W.F.; Zhou, P.H. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: A meta-analysis. Surg. Endosc. 2016, 30, 2431–2441. [Google Scholar] [CrossRef]
- Cazacu, I.M.; Singh, B.S.; Chavez, A.A.L.; Koduru, P.; Ejaz, S.; Weston, B.R.; Ross, W.A.; Lee, J.H.; Roy-Chowdhuri, S.; Bhutani, M.S. EUS and EUS-guided FNA/core biopsies in the evaluation of subepithelial lesions of the lower gastrointestinal tract: 10-year experience. Endosc. Ultrasound 2020, 9, 329–336. [Google Scholar] [PubMed]
- Tamura, T.; Yamashita, Y.; Ueda, K.; Kawaji, Y.; Itonaga, M.; Murata, S.I.; Yamamoto, K.; Yoshida, T.; Maeda, H.; Maekita, T.; et al. Rapid On-Site Evaluation by Endosonographers during Endoscopic Ultrasonography-Guided Fine-Needle Aspiration for Diagnosis of Gastrointestinal Stromal Tumors. Clin. Endosc. 2017, 50, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osoegawa, T.; Minoda, Y.; Ihara, E.; Komori, K.; Aso, A.; Goto, A.; Itaba, S.; Ogino, H.; Nakamura, K.; Harada, N.; et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: A randomized cross-over study. Digest. Endosc. 2019, 31, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedenström, P.; Marschall, H.U.; Nilsson, B.; Demir, A.; Lindkvist, B.; Nilsson, O.; Sadik, R. High clinical impact and diagnostic accuracy of EUS-guided biopsy sampling of subepithelial lesions: A prospective, comparative study. Surg. Endosc. 2018, 32, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.H.; Pan, K.T.; Chu, S.Y.; Chen, C.M.; Hsu, M.Y.; Hung, C.F.; Tseng, J.H. Safety and efficacy of image-guided percutaneous biopsies in the diagnosis of gastrointestinal stromal tumors. Clin. Imaging 2012, 36, 19–23. [Google Scholar] [CrossRef]
- Eriksson, M.; Reichardt, P.; Sundby Hall, K.; Schütte, J.; Cameron, S.; Hohenberger, P.; Bauer, S.; Leinonen, M.; Reichardt, A.; Rejmyr Davis, M.; et al. Needle biopsy through the abdominal wall for the diagnosis of gastrointestinal stromal tumour—Does it increase the risk for tumour cell seeding and recurrence? Eur. J. Cancer 2016, 59, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumonceau, J.M.; Polkowski, M.; Larghi, A.; Vilmann, P.; Giovannini, M.; Frossard, J.L.; Heresbach, D.; Pujol, B.; Fernández-Esparrach, G.; Vazquez-Sequeiros, E.; et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2011, 43, 897–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, D.H.; Ryu, M.H.; Kim, K.M.; Yang, H.K.; Sawaki, A.; Hirota, S.; Zheng, J.; Zhang, B.; Tzen, C.Y.; Yeh, C.N.; et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res. Treat. 2016, 48, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- McCarter, M.D.; Antonescu, C.R.; Ballman, K.V.; Maki, R.G.; Pisters, P.W.; Demetri, G.D.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; McCall, L.; et al. Microscopically positive margins for primary gastrointestinal stromal tumors: Analysis of risk factors and tumor recurrence. J. Am. Coll. Surg. 2012, 215, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Cavnar, M.J.; Seier, K.; Curtin, C.; Balachandran, V.P.; Coit, D.G.; Yoon, S.S.; Crago, A.M.; Strong, V.E.; Tap, W.D.; Gönen, M.; et al. Outcome of 1000 Patients with Gastrointestinal Stromal Tumor (GIST) Treated by Surgery in the Pre- and Post-imatinib Eras. Ann. Surg. 2021, 273, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Hølmebakk, T.; Raut, C.P.; Rutkowski, P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann. Surg Oncol 2019, 26, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv68–iv78. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, C.E.; Lai, C.C.; Chen, J.S.; Tsai, C.Y.; Cheng, C.T.; Yeh, T.S.; Yeh, C.N. Prospective Evaluation of Neoadjuvant Imatinib Use in Locally Advanced Gastrointestinal Stromal Tumors: Emphasis on the Optimal Duration of Neoadjuvant Imatinib Use, Safety, and Oncological Outcome. Cancers 2019, 11, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurokawa, Y.; Yang, H.K.; Cho, H.; Ryu, M.H.; Masuzawa, T.; Park, S.R.; Matsumoto, S.; Lee, H.J.; Honda, H.; Kwon, O.K.; et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br. J. Cancer 2017, 117, 25–32. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Wang, L.; Balachandran, V.P.; Antonescu, C.R.; Tap, W.D.; Keohan, M.; Singer, S.; Temple, L.; Nash, G.M.; Weiser, M.R.; et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann. Surg. Oncol. 2017, 24, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Q.; Blanke, C.D.; Demetri, G.D.; Heinrich, M.C.; Watson, J.C.; Hoffman, J.P.; Okuno, S.; Kane, J.M.; von Mehren, M.; et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: Long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann. Surg. Oncol. 2012, 19, 1074–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassos, N.; Jakob, J.; Kähler, G.; Reichardt, P.; Marx, A.; Dimitrakopoulou-Strauss, A.; Rathmann, N.; Wardelmann, E.; Hohenberger, P. Preservation of Organ Function in Locally Advanced Non-Metastatic Gastrointestinal Stromal Tumors (GIST) of the Stomach by Neoadjuvant Imatinib Therapy. Cancers 2021, 13, 586. [Google Scholar] [CrossRef]
- Joensuu, H.; Vehtari, A.; Riihimäki, J.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Cirilli, C.; Braconi, C.; et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012, 13, 265–274. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Takahashi, T.; Muguruma, K.; Toyokawa, T.; Kusanagi, H.; Omori, T.; Masuzawa, T.; Tanaka, K.; Hirota, S.; Nishida, T. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: Indications for adjuvant therapy. Gastric Cancer 2015, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Coe, T.M.; Fero, K.E.; Fanta, P.T.; Mallory, R.J.; Tang, C.M.; Murphy, J.D.; Sicklick, J.K. Population-Based Epidemiology and Mortality of Small Malignant Gastrointestinal Stromal Tumors in the USA. J. Gastrointest. Surg. 2016, 20, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishitani, A.; Hirota, S.; Nishida, T.; Isozaki, K.; Hashimoto, K.; Nakagomi, N.; Matsuda, H. Differential expression of connexin 43 in gastrointestinal stromal tumours of gastric and small intestinal origin. J. Pathol. 2005, 206, 377–382. [Google Scholar] [CrossRef]
- Haller, F.; Happel, N.; Schulten, H.J.; von Heydebreck, A.; Schwager, S.; Armbrust, T.; Langer, C.; Gunawan, B.; Doenecke, D.; Füzesi, L. Site-dependent differential KIT and PDGFRA expression in gastric and intestinal gastrointestinal stromal tumors. Mod. Pathol. 2007, 20, 1103–1111. [Google Scholar] [CrossRef]
- Meng, Y.; Li, W.; Han, L.; Zhang, Q.; Gong, W.; Cai, J.; Li, A.; Yan, Q.; Lai, Q.; Yu, J.; et al. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J. Gastroenterol. Hepatol. 2017, 32, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Bang, B.W.; Kwon, K.S.; Shin, Y.W.; Kim, H.K. Endoscopic Enucleation Is Effective and Relatively Safe in Small Gastric Subepithelial Tumors Originating from Muscularis Propria. Dig. Dis. Sci. 2019, 64, 524–531. [Google Scholar] [CrossRef]
- Li, B.; Chen, T.; Qi, Z.P.; Yao, L.Q.; Xu, M.D.; Shi, Q.; Cai, S.L.; Sun, D.; Zhou, P.H.; Zhong, Y.S. Efficacy and safety of endoscopic resection for small submucosal tumors originating from the muscularis propria layer in the gastric fundus. Surg. Endosc. 2019, 33, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.Q.; Chai, N.L.; Li, H.K.; Lu, Z.S.; Feng, X.X.; Zhang, W.G.; Liu, S.Z.; Linghu, E.Q. Endoscopic submucosal excavation and endoscopic full-thickness resection for gastric schwannoma: Five-year experience from a large tertiary center in China. Surg. Endosc. 2020, 34, 4943–4949. [Google Scholar] [CrossRef]
- Ye, L.S.; Li, Y.; Liu, W.; Yao, M.H.; Khan, N.; Hu, B. Clinical course of suspected small gastrointestinal stromal tumors in the stomach. World J. Gastrointest. Surg. 2020, 12, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.G.; Chung, S.J.; Kang, H.Y.; Yang, S.Y.; Kim, Y.S. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy 2015, 47, 675–679. [Google Scholar] [CrossRef]
- Shen, C.; Wang, C.; He, T.; Cai, Z.; Yin, X.; Yin, Y.; Lu, D.; Zhang, B.; Zhou, Z. Long-term survival among patients with gastrointestinal stromal tumors diagnosed after another malignancy: A SEER population-based study. World J. Surg. Oncol. 2020, 18, 88. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; Fan, X.; Zhao, X.; Zhu, S.; Guo, M.; Liu, Z.; Yang, X.; Han, Y. A multivariate prediction model for high malignancy potential gastric GI stromal tumors before endoscopic resection. Gastrointest. Endosc. 2020, 91, 813–822. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Jia, Y.; Ye, C.; Lu, Y.; Chen, C.; Shen, J.; Chen, Y.; Zhao, W.; Wang, L.; et al. Laparoscopic surgery versus open resection in patients with gastrointestinal stromal tumors: An updated systematic review and meta-analysis. Am. J. Surg. 2017, 214, 538–546. [Google Scholar] [CrossRef]
- Hiki, N.; Nunobe, S.; Matsuda, T.; Hirasawa, T.; Yamamoto, Y.; Yamaguchi, T. Laparoscopic endoscopic cooperative surgery. Dig. Endosc. 2015, 27, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiki, N.; Nunobe, S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann. Gastroenterol. Surg. 2019, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wu, X.; Wu, T.; Wu, Q.; Liu, Z.; Liu, C.; Li, S.; Chen, T. Meta-analysis of laparoscopic vs. open resection of gastric gastrointestinal stromal tumors. PLoS ONE 2017, 12, e0177193. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Feng, F.; Guo, M.; Cai, L.; Liu, Z.; Liu, S.; Xiao, S.; Zheng, G.; Xu, G.; Zhang, H. Meta-analysis comparing laparoscopic versus open resection for gastric gastrointestinal stromal tumors larger than 5 cm. BMC Cancer 2017, 17, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.X.; Gao, Y.H.; Xi, H.Q.; Cai, A.Z.; Zhang, K.C.; Li, J.Y.; Wei, B.; Chen, L. Comparison between laparoscopic and open surgery for large gastrointestinal stromal tumors: A meta-analysis. World J. Gastrointest. Oncol. 2018, 10, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Gastrointestinal stromal tumors: Risk assessment and adjuvant therapy. Hematol. Oncol. Clin. N. Am. 2013, 27, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin. Diagn. Pathol. 2006, 23, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Gönen, M.; Gutiérrez, A.; Broto, J.M.; García-del-Muro, X.; Smyrk, T.C.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: A retrospective analysis. Lancet Oncol. 2009, 10, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Rutkowski, P.; Nishida, T.; Steigen, S.E.; Brabec, P.; Plank, L.; Nilsson, B.; Braconi, C.; Bordoni, A.; Magnusson, M.K.; et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J. Clin. Oncol. 2015, 33, 634–642. [Google Scholar] [CrossRef]
- Hølmebakk, T.; Bjerkehagen, B.; Boye, K.; Bruland, Ø.; Stoldt, S.; Sundby Hall, K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br. J. Surg. 2016, 103, 684–691. [Google Scholar] [CrossRef]
- Nishida, T.; Cho, H.; Hirota, S.; Masuzawa, T.; Chiguchi, G.; Tsujinaka, T.; Kinki GIST Study Group. Clinicopathological Features and Prognosis of Primary GISTs with Tumor Rupture in the Real World. Ann. Surg. Oncol. 2018, 25, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Hølmebakk, T.; Hompland, I.; Bjerkehagen, B.; Stoldt, S.; Bruland, Ø.S.; Hall, K.S.; Boye, K. Recurrence-Free Survival After Resection of Gastric Gastrointestinal Stromal Tumors Classified According to a Strict Definition of Tumor Rupture: A Population-Based Study. Ann. Surg. Oncol. 2018, 25, 1133–1139. [Google Scholar] [CrossRef]
- Casali, P.G.; Le Cesne, A.; Poveda Velasco, A.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.R.; Italiano, A.; Gelderblom, H.; et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated with Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2015, 33, 4276–4283. [Google Scholar] [PubMed] [Green Version]
- Joensuu, H.; Eriksson, M.; Sundby Hall, K.; Reichardt, A.; Hermes, B.; Schütte, J.; Cameron, S.; Hohenberger, P.; Jost, P.J.; Al-Batran, S.E.; et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients with High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol. 2020, 6, 1241–1246. [Google Scholar] [CrossRef]
- Raut, C.P.; Espat, N.J.; Maki, R.G.; Araujo, D.M.; Trent, J.; Williams, T.F.; Purkayastha, D.D.; DeMatteo, R.P. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients with Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018, 4, e184060. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S.; Casali, P.G. Adjuvant Imatinib for GI Stromal Tumors: When and For How Long? J. Clin. Oncol. 2016, 34, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef]
- Reichardt, P.; Demetri, G.D.; Gelderblom, H.; Rutkowski, P.; Im, S.A.; Gupta, S.; Kang, Y.K.; Schöffski, P.; Schuette, J.; Soulières, D.; et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer 2016, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Serrano, C.; Mariño-Enríquez, A.; Tao, D.L.; Ketzer, J.; Eilers, G.; Zhu, M.; Yu, C.; Mannan, A.M.; Rubin, B.P.; Demetri, G.D.; et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer 2019, 120, 612–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.B.; Fletcher, J.A.; et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef]
- George, S.; Wang, Q.; Heinrich, M.C.; Corless, C.L.; Zhu, M.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; Choy, E.; Tap, W.D.; et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J. Clin. Oncol. 2012, 30, 2401–2407. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.N.; Chen, M.H.; Chen, Y.Y.; Yang, C.Y.; Yen, C.C.; Tzen, C.Y.; Chen, L.T.; Chen, J.S. A phase II trial of regorafenib in patients with metastatic and/or a unresectable gastrointestinal stromal tumor harboring secondary mutations of exon 17. Oncotarget 2017, 8, 44121–44130. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.D.; Kaufman, M.D.; Lu, W.P.; Gupta, A.; Leary, C.B.; Wise, S.C.; Rutkoski, T.J.; Ahn, Y.M.; Al-Ani, G.; Bulfer, S.L.; et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 2019, 35, 738–751. [Google Scholar] [CrossRef]
- Kang, Y.K.; Suzanne, G.; Jones, R.L.; Rutkowski, P.; Shen, L.; Mir, O.; Patel, S.; Zhou, Y.; von Mehren, M.; Hohenberger, P.; et al. Avapritinib vs Regorafenib in Patients with Locally Advanced Unresectable or Metastatic Gastrointestinal Stromal Tumor (GIST): Efficacy and Safety Data from Phase 3 VOYAGER Study. In Proceedings of the CTOS 2020, Boca Raton, FL, USA, 18‒21 November 2020. Poster 114. [Google Scholar]
- Demetri, G.D.; Antonescu, C.R.; Bjerkehagen, B.; Bovée, J.V.M.G.; Boye, K.; Chacón, M.; Dei Tos, A.P.; Desai, J.; Fletcher, J.A.; Gelderblom, H.; et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: Expert recommendations from the World Sarcoma Network. Ann. Oncol. 2020, 31, 1506–1517. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Singh, A.S.; Chmielowski, B.; Hecht, J.R.; Rosen, L.S.; Chow, W.A.; Wang, X.; Brackert, S.; Adame, C.; Bovill, J.; Schink, E.; et al. A randomized phase II study of nivolumab monotherapy versus nivolumab combined with ipilimumab in advanced gastrointestinal stromal tumor (GIST). J. Clin. Oncol. 2019, 37, 11017. [Google Scholar] [CrossRef]
- Epacadostat Pembrolizumab in Patients with, G.I.S.T. Available online: https://clinicaltrials.gov/ct2/show/NCT03291054 (accessed on 8 May 2021).
- Wagner, A.J.; Chugh, R.; Rosen, L.S.; Morgan, J.A.; George, S.; Gordon, M.; Dunbar, J.; Normant, E.; Grayzel, D.; Demetri, G.D. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas. Clin. Cancer Res. 2013, 19, 6020–6029. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.C.; Bauer, T.M.; Lamar, R.; Joseph, M.; Penley, W.; Thompson, D.S.; Spigel, D.R.; Owera, R.; Lane, C.M.; Earwood, C.; et al. A Phase 2 Study of the Hsp90 Inhibitor AUY922 as Treatment for Patients with Refractory Gastrointestinal Stromal Tumors. Cancer Investig. 2016, 34, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, T.; Kurokawa, Y.; Sawaki, A.; Komatsu, Y.; Ozaka, M.; Takahashi, T.; Naito, Y.; Ohkubo, S.; Nishida, T. Efficacy and safety of TAS-116, an oral inhibitor of heat shock protein 90, in patients with metastatic or unresectable gastrointestinal stromal tumour refractory to imatinib, sunitinib and regorafenib: A phase II, single-arm trial. Eur. J. Cancer 2019, 121, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Honma, Y.; Kurokawa, Y.; Sawaki, A.; Naito, Y.; Iwagami, S.; Baba, H.; Komatsu, Y.; Nishida, T.; Doi, T. Randomized, double-blind, placebo-controlled, phase III trial of pimitespib (TAS-116), an oral inhibitor of heat shock protein 90 (HSP90), in patients with advanced gastrointestinal stromal tumor refractory to imatinib, sunitinib and regorafenib. J. Clin. Oncol. 2021, 39 (Suppl. S15), 11524. Available online: https://meetinglibrary.asco.org/record/195669/abstract (accessed on 7 June 2021). [CrossRef]
- Selinexor as a Single Agent and in Combination with Imatinib in Patients with Metastatic and/or Unresectable Gastrointestinal Stromal Tumors. (SeliGIST). Available online: https://clinicaltrials.gov/ct2/show/NCT04138381 (accessed on 8 May 2021).
- Iida, K.; Abdelhamid Ahmed, A.H.; Nagatsuma, A.K.; Shibutani, T.; Yasuda, S.; Kitamura, M.; Hattori, C.; Abe, M.; Hasegawa, J.; Iguchi, T.; et al. Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-in-Class Antibody-Drug Conjugate. Cancer Discov. 2021, 11, 1508–1523, Epub ahead of Print. & Clinical Trial of DS-6157a in Participants With Advanced Gastrointestinal Stromal Tumor (GIST). Available online: https://clinicaltrials.gov/ct2/show/NCT04276415 (accessed on 8 May 2021). [CrossRef] [PubMed]
- A Study of XmAb®18087 in Subjects With NET and GIST. Available online: https://clinicaltrials.gov/ct2/show/NCT03411 (accessed on 8 May 2021).

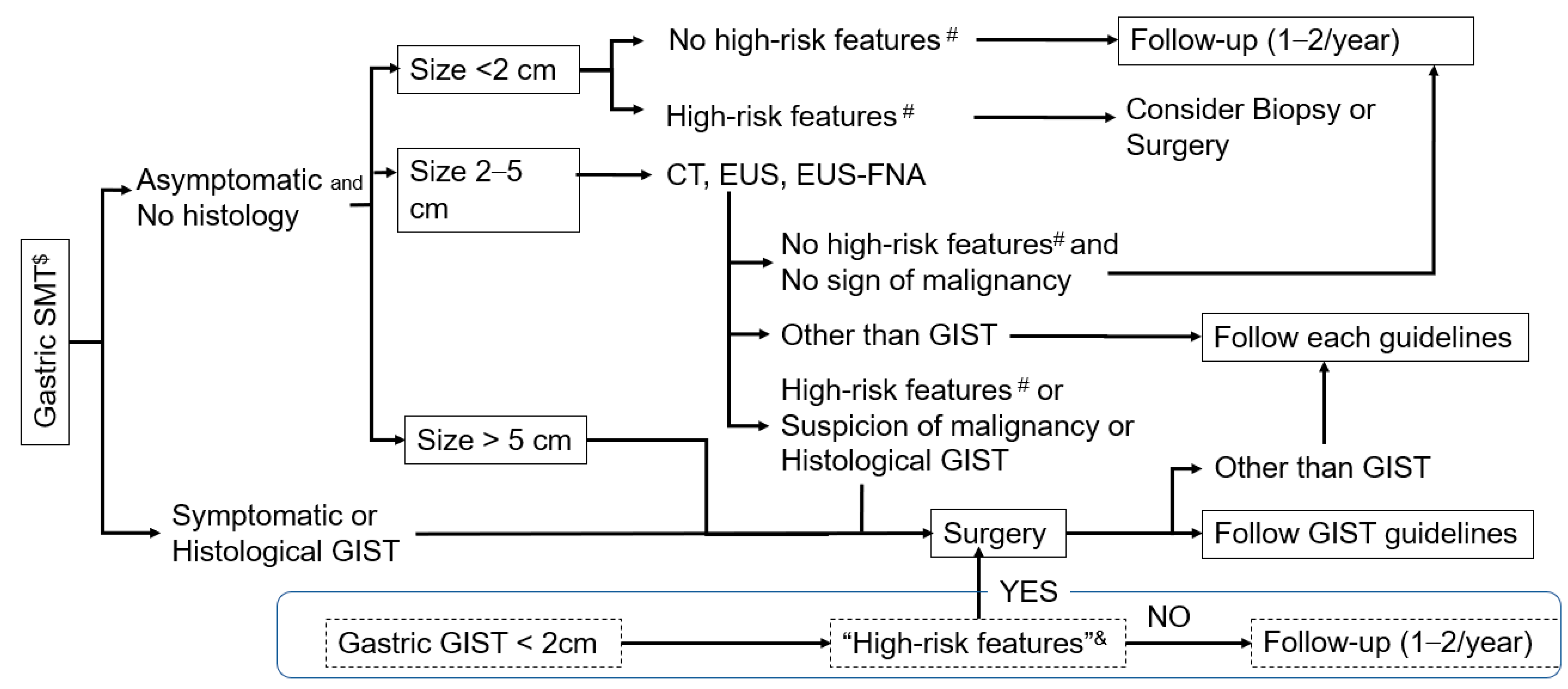
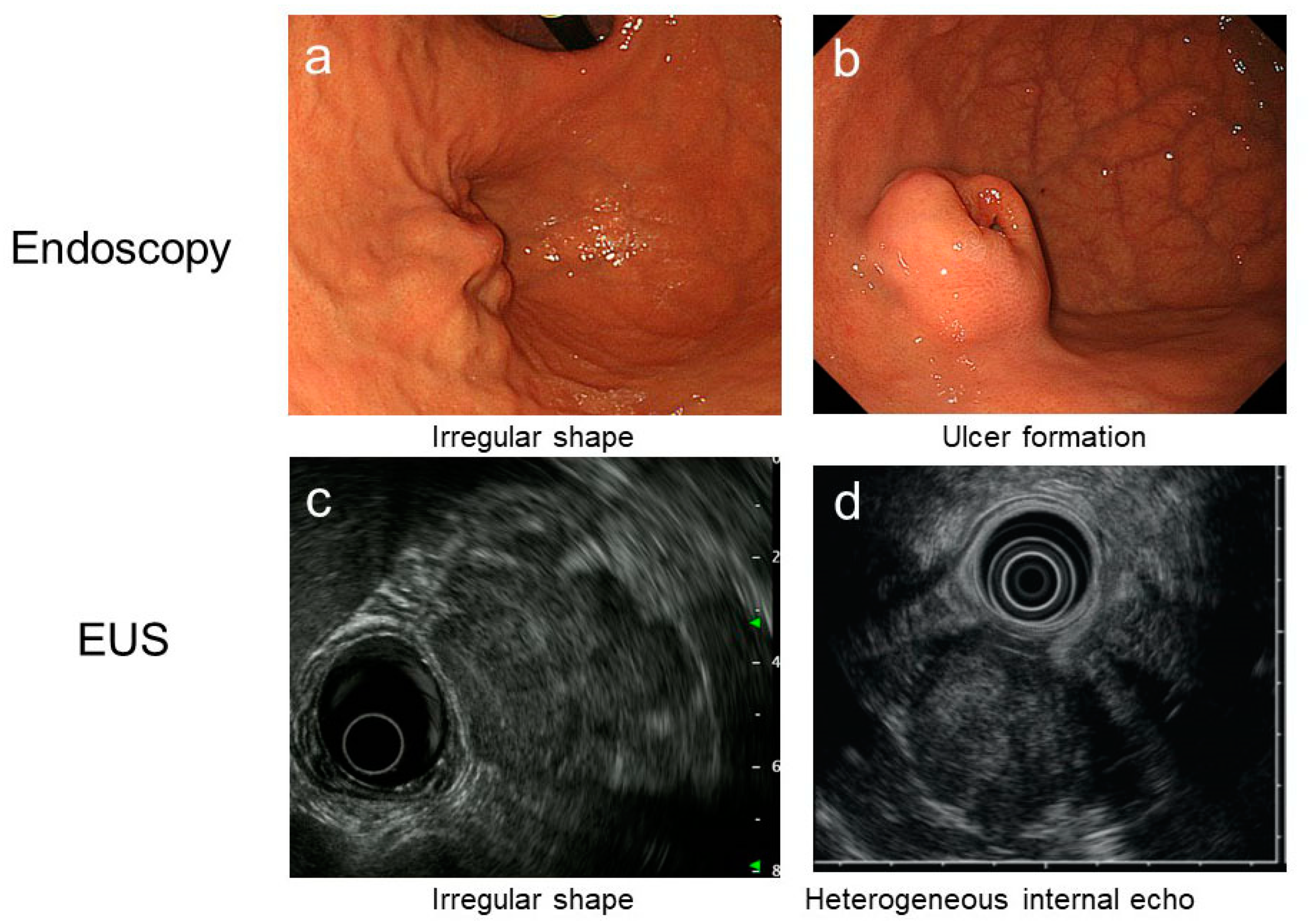
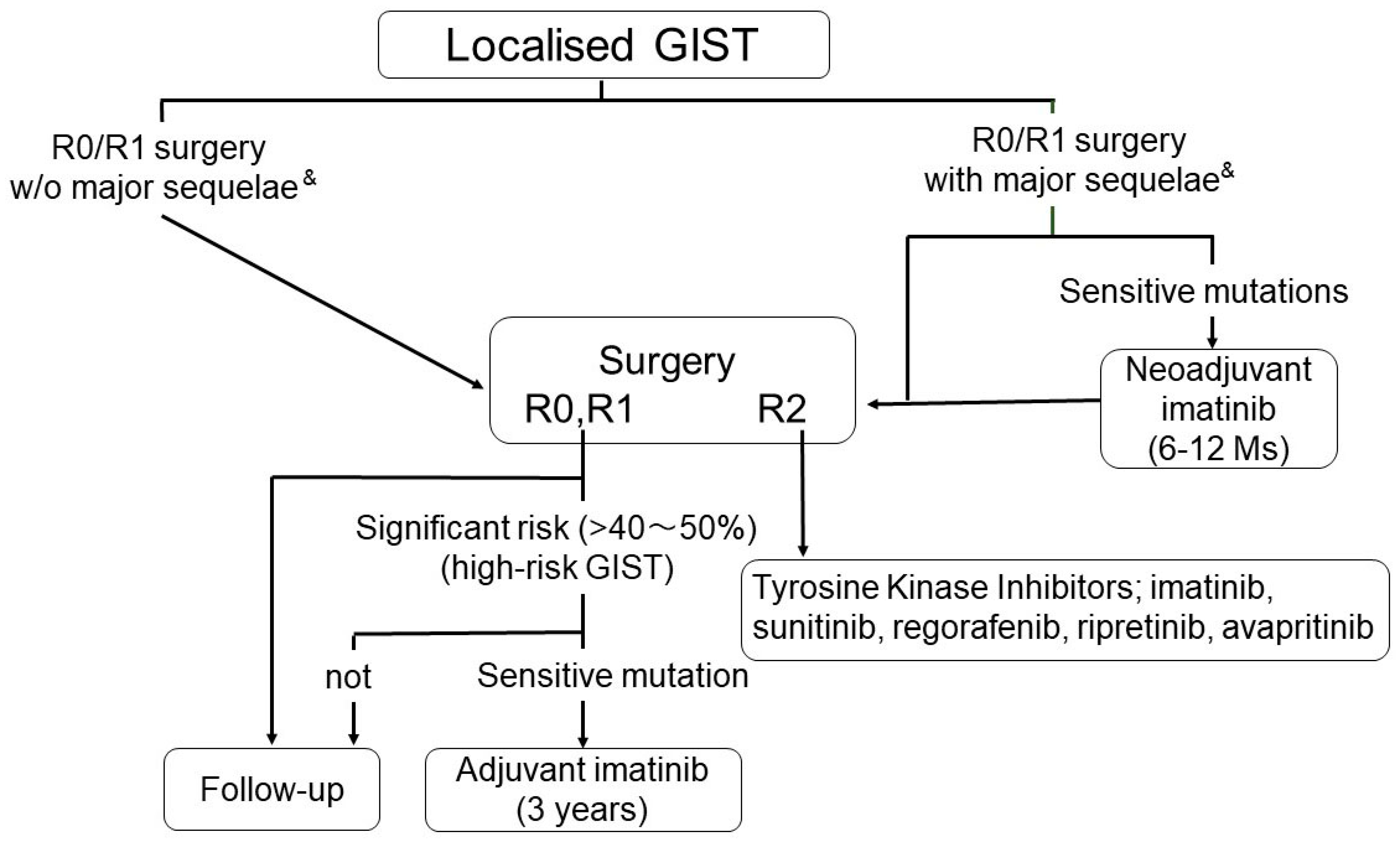
| Alterations | Estimated Frequency | Main Location | Characteristics | Sensitivity to Drugs & Potential Drugs | |||
|---|---|---|---|---|---|---|---|
| KIT mutations in the autoinhibited form | KIT mutation # | exon 9 (or exon 8), typically duplicated insertion of A502-Y503 codons | 5–10% | Small intestine | Spindle cell type Aggressive features | Less imatinib sensitive, sensitive to sunitinib, regorafenib, ripretinib, avapritinib | |
| exon 11 (deletions, missense, insertions etc.) | ~60% | All sites | Aggressive features with del 557-558, which is very sensitive to imatinib | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||
| exon 13 (K642E) | <1% | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| exon 17 (D820Y, N822K, Y823D) | 1% | Sensitive to imatinib, regorafenib, ripretinib, avapritinib, and less sensitive to sunitinib | |||||
| PDGFRA mutations in the autoinhibited form | PDGFRA mutation # | exon 12 (V561D etc.) | <1% | Stomach>>small intestine | Epithelioid cell type Indolent clinical course in main | Probably sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |
| exon 14 (N659K) | <1% | Probably sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| exon 18 (del, Y849H etc., other than D842V) | 1–2% | Sensitive to imatinib, sunitinib, regorafenib, ripretinib, avapritinib | |||||
| KIT or PDGFRA mutations in the activated form | PDGFRA exon 18 D842V, rarely KIT exon 17 D816V | ~10% | Stomach>>small intestine | Epithelioid cell type | D842V is resistant to imatinib, sunitinib, regorafenib. D842V is sensitive to avapritinib & ripretinib | ||
| No mutation in KIT and PDGFRA | SDHB-competent | NF1 mutation $ | 1–2% | Small intestine | Spindle cell type Generally indolent clinical course associated with Neurofibromatosis type I | not sensitive to available drugs | possibly sensitive to MEK inhibitors, such as selumetinib |
| BRAF mutation | <1% | Small intestine/stomach | Spindle cell type VE1-positive | possibly sensitive to BRAF inhibitors (e.g., vemurafenib, dabrafenib) | |||
| HRAS, NRAS or KRAS mutation | very rare | no data | no data | MEK inhibitors (e.g., trametinib) may possibly have some activities | |||
| Others including PIK3CA, CBL, ETV6–NTRK3 et al. | very rare | no data | no data | NTR-fusion is sensitive to entrectinib and larotrectinib | |||
| SDHB-deficient | SDHA, SDHB, SDHC or SDHD mutation (including Carney-Stratakis syndrome #) | ~3% | Stomach>>small intestine | Epithelioid cell type SDHB-negative Children/adolescent and young adult Frequent lymph node metastasis Indolent clinical course | not sensitive to available drugs VEGFR inhibitors may have temporary stabilizing effects | ||
| Loss of SDHB expression (including Carney Triad $) | <1% | Stomach | |||||
| Disease | Endoscopic Findings | EUS Findings | Pathological Features | |||
|---|---|---|---|---|---|---|
| Surface, Form, etc. | Major Location | Main Layer | Echo Findings | Morphology | IHC; Genetic Changes | |
| GIST | hemi-spherical, occasionally with delle or ulcer | body | proper muscle, rarely submucosa | hypoechoic, heterogenous with increased malinancy | spindle cell > epithelioid cell | KIT, DOG1; mutation in KIT or PDGFRA |
| Myogenic tumor & Leiomyoma | hemi-spherical, intact mucosa | near cardia | proper muscle, sometimes submucosa | round, hypoechoic, homogenous | spindle cell (eosinophilic cell) | Desmin, α-SMA |
| Schwanomma & neurogenic tumor | hemi-spherical, intact mucosa | body, lesser curvature | proper muscle, sometimes submucosa~deep mucosa | hypoechoic, homogenous~slightly heterogeneous | spindle cell, palisading, Verocay body, lymphoid cuff in Schwannoma | S-100, SOX10, NSE in neurogenic tumor |
| Heterotopic Pancreas | hill-shaped, intact mucosa, maybe dimple or aperture | antrum | submucosa | sometimes lobulated, ductal structure, heterogeneous internal echo, thickend proper muscle | Heimlich classification & | |
| Neuroendocrine tumor | hemi-spherical, mucosal color~yellowish~red, occasionally dimple | body | initially deep mucosa or submucosa | homogenous, heterogeneous with increased malinancy | epithelioid cell, organoid pattern | CD56, synaptophysin, chromogranin A, NSE |
| MALT lymphoma | various surface, multiple lesions | anywhere | deep mucosa~submucosa | beltlike~multiple round, hypoechoic, homogenous | Centrocyte-like, lymphoepithelial lesion, plasma cell differentiation | κ or λ chain; t(11;18)/API2-MALT1 |
| Malignant lymphoma | various surface, multiple lesions | anywhere | initially deep mucosa~submucosa | beltlike~advanced carcinoma-like, hypoechoic, homogenous | CD20+, CD79a+; t(3;14)/BCL6-IGH | |
| Lipoma & lipogenic tumor | hill-shaped to pedunculated, intact mucosa (~yellowish), cushion sign | antrum | submucosa | round~oval, hyperechoic | Lipoblast (spider-web cell) | MDM2, CDK4 in well differenciated liposarcoma |
| Granular cell tumor | hemi-spherical, molar-like appearance, intact~ivoly | body | submucosa | round, heterogenously hypoechoic | eosinophilic granules | S-100, SOX10, CD68 |
| inflammatory fibroid polyp (IFP) | pedunculated or penis-like, may with erosion/ulcer | antrum | deep mucosa~submucosa | hypoechoic, relatively homogeneous | perivascular fibrosis (onion skin pattern), eosinophil infiltration | CD34, α-SMA; mutations in PDGFRA |
| inflammatory myofibroblastic tumor (IMT) | hill-shaped, mucosal color | fornix~body | hypoechoic (not definite) | spindle cell & inflammatory cell infiltration | ALK, α-SMA, ALK-fusion, CD34, | |
| Solitary fibrous tumor (SFT) | n.d. | n.d. | n.d. | spindle cell, patternless pattern | CD34, nuclear STAT6, bcl2, CD99; NAB2-STAT6 fusion | |
| Glomus tumor | hemi-spherical, same color as mucosa | antrum | proper muscle | relatively hyperechoic~heterogenous | eosinophilic cell with oval nucleus | α-SMA |
| lymphangioma or cavenous hemangioma | flat-elavated, intact mucosa (whitish or dark-reddish, respectively), cushion sign | n.d. | deep mucosa~submucosa | aechoic~hyperechoic, multicystic | endothelial cells | CD31, CD34, Factor VIII in vascular tumor |
| PEComa | hemi-spherical~polypoid, intact mucosa | n.d. | submucosa | hypoechoic, homogenous | epithelioid cell with clear cytoplasm | α-SMA, HMB45, Melan A; LOH of TSC2 |
| Melanoma | pedunculated or lobular protrusion (may with melanosis), occasionally with erosion | n.d. | initially deep mucosa~submucosa | iso-hypoechoic, maybe regional lymph node metastasis | S-100, HMB45, Melan A, SOX10; mutations in BRAF ot KIT | |
| Desmoid | n.d. | n.d. | n.d. | spindle cell | nuclear β-catenin; alterations in CTNNB1 | |
| Metastatic tumor | bull’s eye~various, multiple lesions | n.d. | deep mucosa~submucosa | round~oval, hyperechoic & heterogenous | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, T.; Yoshinaga, S.; Takahashi, T.; Naito, Y. Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers 2021, 13, 3158. https://doi.org/10.3390/cancers13133158
Nishida T, Yoshinaga S, Takahashi T, Naito Y. Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers. 2021; 13(13):3158. https://doi.org/10.3390/cancers13133158
Chicago/Turabian StyleNishida, Toshirou, Shigetaka Yoshinaga, Tsuyoshi Takahashi, and Yoichi Naito. 2021. "Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors" Cancers 13, no. 13: 3158. https://doi.org/10.3390/cancers13133158
APA StyleNishida, T., Yoshinaga, S., Takahashi, T., & Naito, Y. (2021). Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers, 13(13), 3158. https://doi.org/10.3390/cancers13133158





