Interventions to Improve Sexual Health in Women Living with and Surviving Cancer: Review and Recommendations
Abstract
Simple Summary
Abstract
1. Introduction
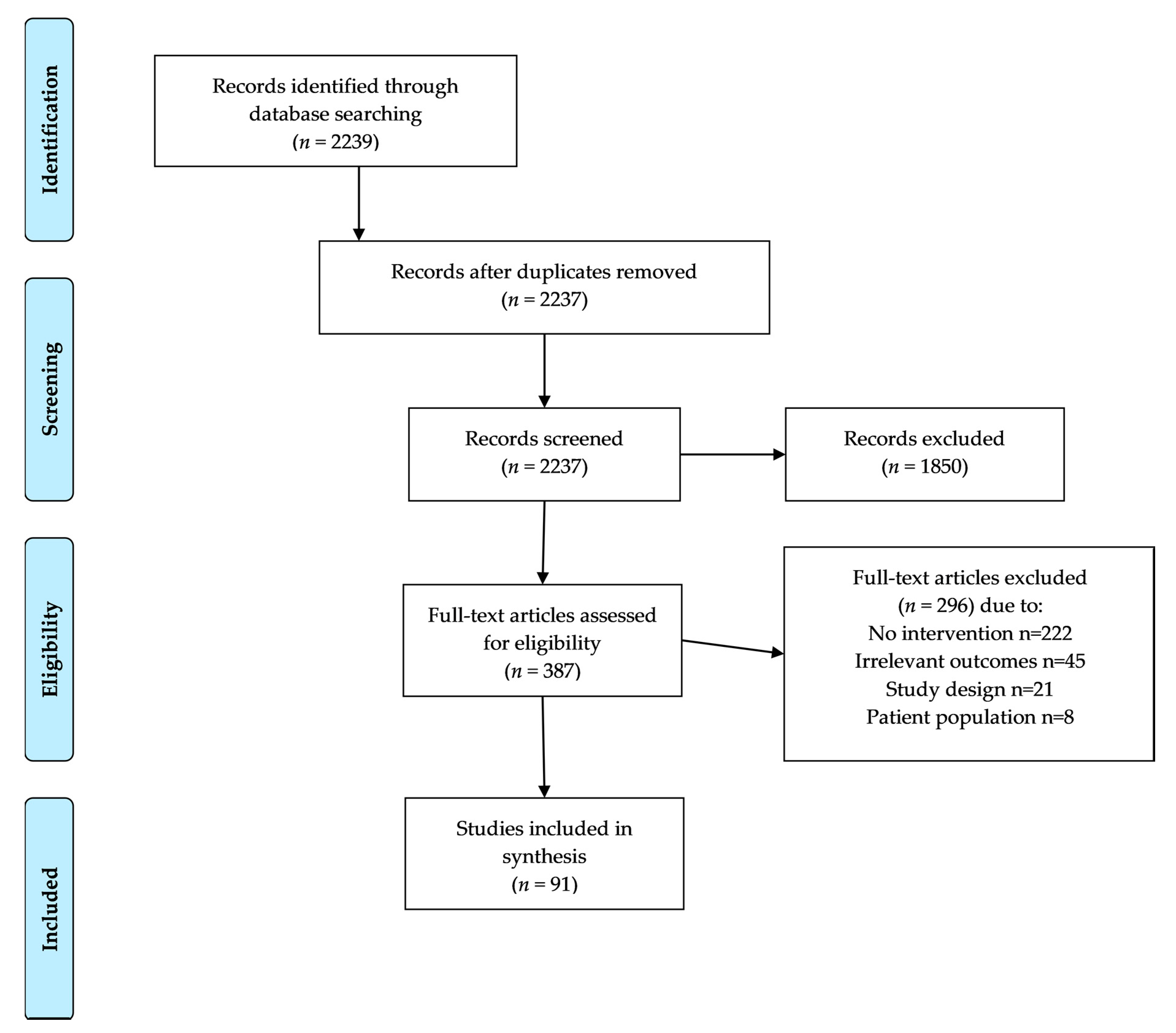
2. Materials and Methods
3. Results
3.1. General Sexual Concerns after Cancer
3.1.1. Psychoeducational and Psychotherapeutic Interventions
In-Person, Group Psychoeducational Interventions
In-Person, Individually Delivered Psychoeducational Interventions
Technology-Based Psychoeducational Interventions
Evaluation of Literature for Psychoeducational and Psychotherapeutic Interventions for General Sexual Function Concerns
3.1.2. Multimodal Sexual Medicine Clinics to Improve Sexual Health in Women with Cancer
3.1.3. Exercise Programs to Improve Sexual Health in Women with Cancer
3.1.4. Hormone Replacement in Women with Cancer with Low Sexual Desire
3.1.5. Complementary and Alternative Medicine Practices to Improve Sexual Health in Women with Cancer
3.1.6. Summary: General Sexual Function Interventions
3.2. Body Image Concerns
3.2.1. Psychoeducational and Psychotherapeutic Programs
3.2.2. Social and Expressive Programming to Improve Body Image
3.2.3. Exercise Programs
3.2.4. Cosmetic Interventions to Improve Body Image Related to Skin Concerns
3.2.5. Summary: Interventions to Address Body Image Concerns
3.3. Genitourinary Syndrome Impacting Sexual Health in Women with and Surviving Cancer
3.3.1. Non-Hormonal Topical Agents for Genitourinary Syndrome
3.3.2. Hormonal Topical Agents for Genitourinary Syndrome
3.3.3. Fractional CO2 Laser Therapy for Genitourinary Syndrome
3.3.4. Educational Programs for Genitourinary Syndrome in Women with Cancer
3.3.5. Summary: Interventions to Address GSM
3.4. Pelvic Floor Dysfunction in Women after Cancer
Pelvic Floor Rehabilitation and Dilator Use for Female Cancer Survivors
3.5. Vasomotor Symptoms of Menopause in Women with and Surviving Cancer
3.5.1. Pharmaceutical Agents for Vasomotor Symptoms of Menopause
3.5.2. Psychoeducational and Psychotherapeutic Programs to Address Vasomotor Symptoms of Menopause after Cancer
3.5.3. Educational Programs to Address Vasomotor Symptoms of Menopause after Cancer
3.5.4. Exercise Programs to Address Vasomotor Symptoms of Menopause after Cancer
3.5.5. Complementary and Alternative Medicine Practices to Address Vasomotor Symptoms of Menopause after Cancer
3.5.6. Summary: Interventions to Address Vasomotor Symptoms of Menopause
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, E.S.; Nekhlyudov, L.; Bober, S.L. The primary health care physician and the cancer patient: Tips and strategies for managing sexual health. Transl. Androl. Urol. 2015, 4, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Sopfe, J.; Gupta, A.; Appiah, L.C.; Chow, E.J.; Peterson, P.N. Sexual Dysfunction in Adolescent and Young Adult Survivors of Childhood Cancer: Presentation, Risk Factors, and Evaluation of an Underdiagnosed Late Effect: A Narrative Review. J. Adolesc. Young Adult Oncol. 2020, 9, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R. Sexual quality of life in men and women after cancer. Climacteric 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Chiodini, P.; Bellastella, G.; Giugliano, D.; Esposito, K. Sexual dysfunction in women with cancer: A systematic review with meta-analysis of studies using the Female Sexual Function Index. Endocrine 2016, 54, 329–341. [Google Scholar] [CrossRef]
- Thomas, H.N.; Thurston, R.C. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: A narrative review. Maturitas 2016, 87, 49–60. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013.
- Ye, S.; Yang, J.; Cao, D.; Lang, J.; Shen, K. A systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int. J. Gynecol. Cancer 2014, 24, 1146–1157. [Google Scholar] [CrossRef]
- Carter, J.; Lacchetti, C.; Andersen, B.L.; Barton, D.L.; Bolte, S.; Damast, S.; Diefenbach, M.A.; DuHamel, K.; Florendo, J.; Ganz, P.A.; et al. Interventions to Address Sexual Problems in People with Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J. Clin. Oncol. 2018, 36, 492–511. [Google Scholar] [CrossRef]
- Hendren, S.K.; O’Connor, B.I.; Liu, M.; Asano, T.; Cohen, Z.; Swallow, C.J.; Macrae, H.M.; Gryfe, R.; McLeod, R.S. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann. Surg. 2005, 242, 212–223. [Google Scholar] [CrossRef]
- Frederick, N.N.; Revette, A.; Michaud, A.; Bober, S.L. A qualitative study of sexual and reproductive health communication with adolescent and young adult oncology patients. Pediatr. Blood Cancer 2019, e27673. [Google Scholar] [CrossRef]
- Park, E.R.; Bober, S.L.; Campbell, E.G.; Recklitis, C.J.; Kutner, J.S.; Diller, L. General internist communication about sexual function with cancer survivors. J. Gen. Intern. Med. 2009, 24 (Suppl. S2), S407–S411. [Google Scholar] [CrossRef][Green Version]
- White, I.D. Sexual Difficulties after Pelvic Radiotherapy: Improving Clinical Management. Clin. Oncol. R. Coll. Radiol. 2015, 27, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Frederick, N.N.; Campbell, K.; Kenney, L.B.; Moss, K.; Speckhart, A.; Bober, S.L. Barriers and facilitators to sexual and reproductive health communication between pediatric oncology clinicians and adolescent and young adult patients: The clinician perspective. Pediatr. Blood Cancer 2018, 65, e27087. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Zwaal, C.; Elterman, D.; McPherson, K.; Wolfman, W.; Katz, A.; Matthew, A. Interventions to Address Sexual Problems in People with Cancer. Curr. Oncol. 2017, 24, 192–200. [Google Scholar] [CrossRef]
- Rees, M.; Angioli, R.; Coleman, R.L.; Glasspool, R.M.; Plotti, F.; Simoncini, T.; Terranova, C. European Menopause and Andropause Society (EMAS) and International Gynecologic Cancer Society (IGCS) position statement on managing the menopause after gynecological cancer: Focus on menopausal symptoms and osteoporosis. Int. J. Gynecol. Cancer 2020, 30, 428–433. [Google Scholar] [CrossRef]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef]
- Candy, B.; Jones, L.; Vickerstaff, V.; Tookman, A.; King, M. Interventions for sexual dysfunction following treatments for cancer in women. Cochrane Database Syst. Rev. 2016, 2, CD005540. [Google Scholar] [CrossRef]
- Arthur, E.K.; Wills, C.E.; Menon, U. A Systematic Review of Interventions for Sexual Well-Being in Women with Gynecologic, Anal, or Rectal Cancer. Oncol. Nurs. Forum. 2018, 45, 469–482. [Google Scholar] [CrossRef]
- Flynn, P.; Kew, F.; Kisely, S.R. Interventions for psychosexual dysfunction in women treated for gynaecological malignancy. Cochrane Database Syst. Rev. 2009, CD004708. [Google Scholar] [CrossRef]
- Matthew, A.G.; Yang, Z.G. Online interventions for sexual health in cancer. Curr. Opin. Support. Palliat. Care 2020, 14, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Skrabal Ross, X.; Gunn, K.M.; Olver, I.; Willems, R.A.; Lechner, L.; Mesters, I.; Bolman, C.A.W. Online psychosocial interventions for posttreatment cancer survivors: An international evidence review and update. Curr. Opin. Support. Palliat. Care 2020, 14, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.; McDivitt, K.; Ryan, M.; Tomlinson, J.; Brotto, L.A. Feasibility of a Sexual Health Clinic Within Cancer Care: A Pilot Study Using Qualitative Methods. Cancer Nurs. 2016, 39, E32–E42. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Stabile, C.; Seidel, B.; Baser, R.E.; Goldfarb, S.; Goldfrank, D.J. Vaginal and sexual health treatment strategies within a female sexual medicine program for cancer patients and survivors. J. Cancer Surviv. 2017, 11, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Seaborne, L.A.; Peterson, M.; Kushner, D.M.; Sobecki, J.; Rash, J.K. Development, Implementation, and Patient Perspectives of the Women’s Integrative Sexual Health Program: A Program Designed to Address the Sexual Side Effects of Cancer Treatment. J. Adv. Pract. Oncol. 2021, 12, 32–38. [Google Scholar] [CrossRef]
- Greven, K.M.; Case, L.D.; Nycum, L.R.; Zekan, P.J.; Hurd, D.D.; Balcueva, E.P.; Mills, G.M.; Zon, R.; Flynn, P.J.; Biggs, D.; et al. Effect of ArginMax on sexual functioning and quality of life among female cancer survivors: Results of the WFU CCOP Research Base Protocol 97106. J. Community Support. Oncol. 2015, 13, 87–94. [Google Scholar] [CrossRef]
- Schover, L.R.; Strollo, S.; Stein, K.; Fallon, E.; Smith, T. Effectiveness trial of an online self-help intervention for sexual problems after cancer. J. Sex. Marital. Ther. 2020, 46, 576–588. [Google Scholar] [CrossRef]
- Barton, D.L.; Wender, D.B.; Sloan, J.A.; Dalton, R.J.; Balcueva, E.P.; Atherton, P.J.; Bernath, A.M., Jr.; DeKrey, W.L.; Larson, T.; Bearden, J.D., 3rd; et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central Cancer Treatment Group protocol N02C3. J. Natl. Cancer Inst. 2007, 99, 672–679. [Google Scholar] [CrossRef][Green Version]
- Canada, A.L.; Schover, L.R.; Li, Y. A pilot intervention to enhance psychosexual development in adolescents and young adults with cancer. Pediatr. Blood Cancer 2007, 49, 824–828. [Google Scholar] [CrossRef]
- Penttinen, H.; Utriainen, M.; Kellokumpu-Lehtinen, P.L.; Raitanen, J.; Sievänen, H.; Nikander, R.; Blomqvist, C.; Huovinen, R.; Vehmanen, L.; Saarto, T. Effectiveness of a 12-month Exercise Intervention on Physical Activity and Quality of Life of Breast Cancer Survivors; Five-year Results of the BREX-study. In Vivo 2019, 33, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Roine, E.; Sintonen, H.; Kellokumpu-Lehtinen, P.L.; Penttinen, H.; Utriainen, M.; Vehmanen, L.; Huovinen, R.; Kautiainen, H.; Nikander, R.; Blomqvist, C.; et al. Health-related Quality of Life of Breast Cancer Survivors Attending an Exercise Intervention Study: A Five-year Follow-up. In Vivo 2020, 34, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Bulsara, C.; Battaglini, C.; Hands, B.; Naumann, F.L. Breast and Prostate Cancer Survivor Responses to Group Exercise and Supportive Group Psychotherapy. J. Psychosoc. Oncol. 2015, 33, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Speck, R.M.; Gross, C.R.; Hormes, J.M.; Ahmed, R.L.; Lytle, L.A.; Hwang, W.T.; Schmitz, K.H. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res. Treat. 2010, 121, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.Y.; Kim, S.; Chang, S.B.; Oh, K.; Kang, H.S.; Kang, S.S. The effect of a sexual life reframing program on marital intimacy, body image, and sexual function among breast cancer survivors. Cancer Nurs. 2011, 34, 142–149. [Google Scholar] [CrossRef]
- Rowland, J.H.; Meyerowitz, B.E.; Crespi, C.M.; Leedham, B.; Desmond, K.; Belin, T.R.; Ganz, P.A. Addressing intimacy and partner communication after breast cancer: A randomized controlled group intervention. Breast Cancer Res. Treat. 2009, 118, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Marchand, E.; Williams, V.; Coscarelli, A.; Ganz, P.A. Development and pilot testing of a psychosocial intervention program for young breast cancer survivors. Patient. Educ. Couns. 2016, 99, 414–420. [Google Scholar] [CrossRef]
- Faghani, S.; Ghaffari, F. Effects of Sexual Rehabilitation Using the PLISSIT Model on Quality of Sexual Life and Sexual Functioning in Post-Mastectomy Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2016, 17, 4845–4851. [Google Scholar] [CrossRef]
- Esplen, M.J.; Wong, J.; Warner, E.; Toner, B. Restoring Body Image After Cancer (ReBIC): Results of a Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 749–756. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, N.G.; Knobf, T.M.; de Oliveira, M.R.; Salvetti, M.G.; Oriá, M.O.B.; Fialho, A.V.M. A Pilot Intervention Study to Improve Sexuality Outcomes in Breast Cancer Survivors. Asia Pac. J. Oncol. Nurs. 2020, 7, 161–166. [Google Scholar] [CrossRef]
- Fatehi, S.; Maasoumi, R.; Atashsokhan, G.; Hamidzadeh, A.; Janbabaei, G.; Mirrezaie, S.M. The effects of psychosexual counseling on sexual quality of life and function in Iranian breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2019, 175, 171–179. [Google Scholar] [CrossRef]
- Cieslak, A.; Elkins, G.; Banerjee, T.; Marsack, J.; Hickman, K.; Johnson, A.; Henry, N.; Barton, D. Developing a Hypnotic Relaxation Intervention to Improve Body Image: A Feasibility Study. Oncol. Nurs. Forum. 2016, 43, E233–E241. [Google Scholar] [CrossRef]
- Schover, L.R.; Jenkins, R.; Sui, D.; Adams, J.H.; Marion, M.S.; Jackson, K.E. Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. J. Clin. Oncol. 2006, 24, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Rhodes, M.M.; Baum, G.; Adams, J.H.; Jenkins, R.; Lewis, P.; Jackson, K.E. Sisters Peer Counseling in Reproductive Issues After Treatment (SPIRIT): A peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer 2011, 117, 4983–4992. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.L.; Pais, S.; Miller, K.D.; Goulet, R.; Fifea, B.L. A brief intervention to minimize psychosexual morbidity in dyads coping with breast cancer. Oncol. Nurs. Forum. 2012, 39, 176–185. [Google Scholar] [CrossRef]
- Marcus, A.C.; Garrett, K.M.; Cella, D.; Wenzel, L.; Brady, M.J.; Fairclough, D.; Pate-Willig, M.; Barnes, D.; Emsbo, S.P.; Kluhsman, B.C.; et al. Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors? Psychooncology 2010, 19, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.B.; Smith, K.C.; Handorf, E.; Sorice, K.; Bober, S.L.; Bantug, E.T.; Schwartz, S.; Porter, L.S. A randomized pilot trial of a couple-based intervention addressing sexual concerns for breast cancer survivors. J. Psychosoc. Oncol. 2019, 37, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.B.; van Lankveld, J.; Oldenburg, H.S.A.; Hahn, D.E.E.; Kieffer, J.M.; Gerritsma, M.A.; Kuenen, M.A.; Bijker, N.; Borgstein, P.J.; Heuff, G.; et al. Efficacy of Internet-Based Cognitive Behavioral Therapy in Improving Sexual Functioning of Breast Cancer Survivors: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 1328–1340. [Google Scholar] [CrossRef]
- Hummel, S.B.; van Lankveld, J.; Oldenburg, H.S.A.; Hahn, D.E.E.; Kieffer, J.M.; Gerritsma, M.A.; Kuenen, M.A.; Bijker, N.; Borgstein, P.J.; Heuff, G.; et al. Internet-Based Cognitive Behavioral Therapy Realizes Long-Term Improvement in the Sexual Functioning and Body Image of Breast Cancer Survivors. J. Sex. Marital. Ther. 2018, 44, 485–496. [Google Scholar] [CrossRef]
- Schover, L.R.; Yuan, Y.; Fellman, B.M.; Odensky, E.; Lewis, P.E.; Martinetti, P. Efficacy trial of an Internet-based intervention for cancer-related female sexual dysfunction. J. Natl. Compr. Cancer Netw. 2013, 11, 1389–1397. [Google Scholar] [CrossRef]
- Afiyanti, Y.; Rachmawati, I.N.; Milanti, A. Evaluating Sexual Nursing Care Intervention for Reducing Sexual Dysfunction in Indonesian Cervical Cancer Survivors. Asia Pac. J. Oncol. Nurs. 2016, 3, 266–271. [Google Scholar] [CrossRef]
- Brotto, L.A.; Dunkley, C.R.; Breckon, E.; Carter, J.; Brown, C.; Daniluk, J.; Miller, D. Integrating Quantitative and Qualitative Methods to Evaluate an Online Psychoeducational Program for Sexual Difficulties in Colorectal and Gynecologic Cancer Survivors. J. Sex. Marital. Ther. 2017, 43, 645–662. [Google Scholar] [CrossRef]
- Armbruster, S.D.; Song, J.; Bradford, A.; Carmack, C.L.; Lu, K.H.; Basen-Engquist, K.M. Sexual health of endometrial cancer survivors before and after a physical activity intervention: A retrospective cohort analysis. Gynecol. Oncol. 2016, 143, 589–595. [Google Scholar] [CrossRef]
- Barbera, L.; Fitch, M.; Adams, L.; Doyle, C.; Dasgupta, T.; Blake, J. Improving care for women after gynecological cancer: The development of a sexuality clinic. Menopause 2011, 18, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Brotto, L.A.; Erskine, Y.; Carey, M.; Ehlen, T.; Finlayson, S.; Heywood, M.; Kwon, J.; McAlpine, J.; Stuart, G.; Thomson, S.; et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol. Oncol. 2012, 125, 320–325. [Google Scholar] [CrossRef]
- Chow, K.M.; Chan, C.W.H.; Choi, K.C.; Siu, K.Y.; Fung, H.K.S.; Sum, W.M. A theory-driven psycho-educational intervention programme for gynaecological cancer patients during treatment trajectory: A randomised controlled trial. Psychooncology 2020, 29, 437–443. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Fishman, S.R.; Vanderklish, J.; Dizon, D.S.; Pensak, N.; Traeger, L.; Greer, J.A.; Park, E.R.; Markovitz, N.; Waldman, L.; et al. Pilot study of a multimodal intervention to enhance sexual function in survivors of hematopoietic stem cell transplantation. Cancer 2018, 124, 2438–2446. [Google Scholar] [CrossRef]
- Bober, S.L.; Fine, E.; Recklitis, C.J. Sexual health and rehabilitation after ovarian suppression treatment (SHARE-OS): A clinical intervention for young breast cancer survivors. J. Cancer Surviv. 2020, 14, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Bober, S.L.; Recklitis, C.J.; Michaud, A.L.; Wright, A.A. Improvement in sexual function after ovarian cancer: Effects of sexual therapy and rehabilitation after treatment for ovarian cancer. Cancer 2018, 124, 176–182. [Google Scholar] [CrossRef]
- Hungr, C.; Recklitis, C.J.; Wright, A.A.; Bober, S.L. How does a single session group intervention improve sexual function in ovarian cancer survivors? A secondary analysis of effects of self-efficacy, knowledge and emotional distress. Psychol. Health Med. 2020, 25, 110–120. [Google Scholar] [CrossRef] [PubMed]
- DuHamel, K.; Schuler, T.; Nelson, C.; Philip, E.; Temple, L.; Schover, L.; Baser, R.E.; Starr, T.D.; Cannon, K.; Jennings, S.; et al. The sexual health of female rectal and anal cancer survivors: Results of a pilot randomized psycho-educational intervention trial. J. Cancer Surviv. 2016, 10, 553–563. [Google Scholar] [CrossRef]
- Campo, R.A.; Bluth, K.; Santacroce, S.J.; Knapik, S.; Tan, J.; Gold, S.; Philips, K.; Gaylord, S.; Asher, G.N. A mindful self-compassion videoconference intervention for nationally recruited posttreatment young adult cancer survivors: Feasibility, acceptability, and psychosocial outcomes. Support. Care Cancer 2017, 25, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.S.; Lange, W.; Zebrack, B.; Moulton, S.; Kosslyn, S.M. An outdoor adventure program for young adults with cancer: Positive effects on body image and psychosocial functioning. J. Psychosoc. Oncol. 2014, 32, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.A.; Przezdziecki, A.; Alcorso, J.; Kilby, C.J.; Elder, E.; Boyages, J.; Koelmeyer, L.; Mackie, H. Reducing Body Image-Related Distress in Women with Breast Cancer Using a Structured Online Writing Exercise: Results From the My Changed Body Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Saita, E.; Acquati, C. Evaluating the Framed Portrait Experience as an Intervention to Enhance Self-Efficacy and Self-Esteem in a Sample of Adolescent and Young Adult Cancer Survivors: Results of a Pilot Study. J. Adolesc. Young Adult Oncol. 2020, 9, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Dalenc, F.; Ribet, V.; Rossi, A.B.; Guyonnaud, J.; Bernard-Marty, C.; de Lafontan, B.; Salas, S.; Ranc Royo, A.L.; Sarda, C.; Levasseur, N.; et al. Efficacy of a global supportive skin care programme with hydrotherapy after non-metastatic breast cancer treatment: A randomised, controlled study. Eur. J. Cancer Care Engl. 2018, 27. [Google Scholar] [CrossRef]
- Stan, D.L.; Rausch, S.M.; Sundt, K.; Cheville, A.L.; Youdas, J.W.; Krause, D.A.; Boughey, J.C.; Walsh, M.F.; Cha, S.S.; Pruthi, S. Pilates for breast cancer survivors. Clin. J. Oncol. Nurs. 2012, 16, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Smith, H.; Diedrichs, P.C.; Harcourt, D. A pilot study of a body image intervention for breast cancer survivors. Body Image 2018, 27, 21–31. [Google Scholar] [CrossRef]
- Hamzehgardeshi, Z.; Moosazadeh, M.; Elyasi, F.; Janbabai, G.; Rezaei, M.; Yeganeh, Z.; Rashidi Alashti, M. Effect of Midwifery-Based Counseling Support Program on Body Image of Breast Cancer Women Survivors. Asian Pac. J. Cancer Prev. 2017, 18, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, L.; Wong, J.; Rennie, H.; McLeod, D.; Leung, Y.; Warner, E.; Esplen, M.J. Feasibility and acceptability of i-Restoring Body Image after Cancer (i-ReBIC): A pilot trial for female cancer survivors. Psychooncology 2020, 29, 639–646. [Google Scholar] [CrossRef]
- Chen, S.C.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T.; Wu, S.C.; Lai, Y.H. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: A randomized controlled trial. Psychooncology 2017, 26, 1376–1383. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Maurer, S.; Park, Y.; Marsh, C.H.; McElligott, J.T.; Day, T.A.; Hornig, J.D.; Sterba, K.R. Evaluation of a novel telemedicine-based intervention to manage body image disturbance in head and neck cancer survivors. Psychooncology 2020. [Google Scholar] [CrossRef]
- Bepko, C.; Krestan, J.A. Too Good for Her Own Good: Breaking Free from the Burden of Female Responsibility, 1st ed.; Harper & Row: New York, NY, USA, 1990; p. 256. [Google Scholar]
- Cherven, B.; Sampson, A.; Bober, S.L.; Bingen, K.; Frederick, N.; Freyer, D.R.; Quinn, G.P. Sexual health among adolescent and young adult cancer survivors: A scoping review from the Children’s Oncology Group Adolescent and Young Adult Oncology Discipline Committee. CA Cancer J. Clin. 2020. [Google Scholar] [CrossRef]
- Carpentier, M.Y.; Fortenberry, J.D. Romantic and sexual relationships, body image, and fertility in adolescent and young adult testicular cancer survivors: A review of the literature. J. Adolesc. Health 2010, 47, 115–125. [Google Scholar] [CrossRef]
- Lehmann, V.; Hagedoorn, M.; Gerhardt, C.A.; Fults, M.; Olshefski, R.S.; Sanderman, R.; Tuinman, M.A. Body issues, sexual satisfaction, and relationship status satisfaction in long-term childhood cancer survivors and healthy controls. Psychooncology 2016, 25, 210–216. [Google Scholar] [CrossRef]
- Becorpi, A.; Campisciano, G.; Zanotta, N.; Tredici, Z.; Guaschino, S.; Petraglia, F.; Pieralli, A.; Sisti, G.; De Seta, F.; Comar, M. Fractional CO(2) laser for genitourinary syndrome of menopause in breast cancer survivors: Clinical, immunological, and microbiological aspects. Lasers Med. Sci. 2018, 33, 1047–1054. [Google Scholar] [CrossRef]
- Irene Su, H.; Stark, S.; Kwan, B.; Boles, S.; Chingos, D.; Ehren, J.; Gorman, J.R.; Krychman, M.; Romero, S.A.D.; Mao, J.J.; et al. Efficacy of a web-based women’s health survivorship care plan for young breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2019, 176, 579–589. [Google Scholar] [CrossRef]
- Mothes, A.R.; Runnebaum, M.; Runnebaum, I.B. Ablative dual-phase Erbium:YAG laser treatment of atrophy-related vaginal symptoms in post-menopausal breast cancer survivors omitting hormonal treatment. J. Cancer Res. Clin. Oncol. 2018, 144, 955–960. [Google Scholar] [CrossRef]
- Gittens, P.; Mullen, G. The effects of fractional microablative CO(2) laser therapy on sexual function in postmenopausal women and women with a history of breast cancer treated with endocrine therapy. J. Cosmet. Laser Ther. 2019, 21, 127–131. [Google Scholar] [CrossRef]
- Angioli, R.; Stefano, S.; Filippini, M.; Pieralli, A.; Montera, R.; Plotti, F.; Gatti, A.; Bartolone, M.; Luvero, D. Effectiveness of CO(2) laser on urogenital syndrome in women with a previous gynecological neoplasia: A multicentric study. Int. J. Gynecol. Cancer 2020, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, A.; Fallani, M.G.; Becorpi, A.; Bianchi, C.; Corioni, S.; Longinotti, M.; Tredici, Z.; Guaschino, S. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch. Gynecol. Obstet. 2016, 294, 841–846. [Google Scholar] [CrossRef]
- Quick, A.M.; Zvinovski, F.; Hudson, C.; Hundley, A.; Evans, C.; Suresh, A.; Stephens, J.A.; Arthur, E.; Ramaswamy, B.; Reinbolt, R.E.; et al. Fractional CO2 laser therapy for genitourinary syndrome of menopause for breast cancer survivors. Support. Care Cancer 2020, 28, 3669–3677. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; Werkoff, G.; Sawan, D.; Sidahmed-Mezi, M.; Bosc, R.; La Padula, S.; Kalsoum, S.; Ouidir, N.; Meningaud, J.P.; Belkacemi, Y. Carbon dioxide laser treatment for vulvovaginal atrophy in women treated for breast cancer: Preliminary results of the feasibility EPIONE trial. Ann. Chir. Plast. Esthet. 2020, 65, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Pagano, T.; De Rosa, P.; Vallone, R.; Schettini, F.; Arpino, G.; Giuliano, M.; Lauria, R.; De Santo, I.; Conforti, A.; Gallo, A.; et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments: A retrospective study. Menopause 2018, 25, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Biglia, N.; Peano, E.; Sgandurra, P.; Moggio, G.; Panuccio, E.; Migliardi, M.; Ravarino, N.; Ponzone, R.; Sismondi, P. Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: A preliminary study. Gynecol. Endocrinol. 2010, 26, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, S.; Mögele, M.; Lintermans, A.; Bellen, G.; Prasauskas, V.; Ortmann, O.; Grob, P.; Neven, P.; Donders, G. Vaginal estriol-lactobacilli combination and quality of life in endocrine-treated breast cancer. Climacteric 2015, 18, 252–259. [Google Scholar] [CrossRef]
- Barton, D.L.; Sloan, J.A.; Shuster, L.T.; Gill, P.; Griffin, P.; Flynn, K.; Terstriep, S.A.; Rana, F.N.; Dockter, T.; Atherton, P.J.; et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support. Care Cancer 2018, 26, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, M.F.; Lim, J.Y.; Caughey, A.B. A Practical Solution for Dyspareunia in Breast Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3394–3400. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Belkacemi, Y.; Darmon, F.; Bastuji-Garin, S.; Werkoff, G.; Bosc, R.; Niddam, J.; Hermeziu, O.; La Padula, S.; et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: A phase 2 pilot study. Menopause 2018, 25, 1124–1130. [Google Scholar] [CrossRef]
- Hickey, M.; Marino, J.L.; Braat, S.; Wong, S. A randomized, double-blind, crossover trial comparing a silicone- versus water-based lubricant for sexual discomfort after breast cancer. Breast Cancer Res. Treat. 2016, 158, 79–90. [Google Scholar] [CrossRef]
- Juraskova, I.; Jarvis, S.; Mok, K.; Peate, M.; Meiser, B.; Cheah, B.C.; Mireskandari, S.; Friedlander, M. The acceptability, feasibility, and efficacy (phase I/II study) of the OVERcome (Olive Oil, Vaginal Exercise, and MoisturizeR) intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J. Sex. Med. 2013, 10, 2549–2558. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.; Lee, M.; Hahn, S.; Jeon, M.J. Effect of a pH-Balanced Vaginal Gel on Dyspareunia and Sexual Function in Breast Cancer Survivors Who Were Premenopausal at Diagnosis: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 129, 870–876. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: A randomized controlled trial. Obstet. Gynecol. 2011, 117, 922–927. [Google Scholar] [CrossRef]
- Carter, J.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Stabile, C.; Canty, J.; Saban, S.; Goldfarb, S.; Dickler, M.N.; et al. A single-arm, prospective trial investigating the effectiveness of a non-hormonal vaginal moisturizer containing hyaluronic acid in postmenopausal cancer survivors. Support. Care Cancer 2021, 29, 311–322. [Google Scholar] [CrossRef]
- De Rosa, N.; Lavitola, G.; Giampaolino, P.; Morra, I.; Nappi, C.; Bifulco, G. Impact of Ospemifene on Quality of Life and Sexual Function in Young Survivors of Cervical Cancer: A Prospective Study. Biomed. Res. Int. 2017, 2017, 7513610. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Goldfarb, S.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Saban, S.; Stabile, C.; Canty, J.; Gardner, G.J.; et al. A single-arm clinical trial investigating the effectiveness of a non-hormonal, hyaluronic acid-based vaginal moisturizer in endometrial cancer survivors. Gynecol. Oncol. 2020, 158, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Law, E.; Kelvin, J.F.; Thom, B.; Riedel, E.; Tom, A.; Carter, J.; Alektiar, K.M.; Goodman, K.A. Prospective study of vaginal dilator use adherence and efficacy following radiotherapy. Radiother. Oncol. 2015, 116, 149–155. [Google Scholar] [CrossRef]
- Yang, E.J.; Lim, J.Y.; Rah, U.W.; Kim, Y.B. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: A randomized controlled trial. Gynecol. Oncol. 2012, 125, 705–711. [Google Scholar] [CrossRef]
- Cyr, M.P.; Dumoulin, C.; Bessette, P.; Pina, A.; Gotlieb, W.H.; Lapointe-Milot, K.; Mayrand, M.H.; Morin, M. Feasibility, acceptability and effects of multimodal pelvic floor physical therapy for gynecological cancer survivors suffering from painful sexual intercourse: A multicenter prospective interventional study. Gynecol. Oncol. 2020. [Google Scholar] [CrossRef]
- Otte, J.L.; Carpenter, J.S.; Zhong, X.; Johnstone, P.A. Feasibility study of acupuncture for reducing sleep disturbances and hot flashes in postmenopausal breast cancer survivors. Clin. Nurse. Spec. 2011, 25, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Herman, P.; Heron, K.; Olsen, S.; Vaughters, L. Homeopathy for menopausal symptoms in breast cancer survivors: A preliminary randomized controlled trial. J. Altern. Complement. Med. 2005, 11, 21–27. [Google Scholar] [CrossRef]
- Al-Akoum, M.; Maunsell, E.; Verreault, R.; Provencher, L.; Otis, H.; Dodin, S. Effects of Hypericum perforatum (St. John’s wort) on hot flashes and quality of life in perimenopausal women: A randomized pilot trial. Menopause 2009, 16, 307–314. [Google Scholar] [CrossRef]
- MacGregor, C.A.; Canney, P.A.; Patterson, G.; McDonald, R.; Paul, J. A randomised double-blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur. J. Cancer 2005, 41, 708–714. [Google Scholar] [CrossRef]
- Cramer, H.; Rabsilber, S.; Lauche, R.; Kümmel, S.; Dobos, G. Yoga and meditation for menopausal symptoms in breast cancer survivors-A randomized controlled trial. Cancer 2015, 121, 2175–2184. [Google Scholar] [CrossRef]
- Carson, J.W.; Carson, K.M.; Porter, L.S.; Keefe, F.J.; Seewaldt, V.L. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: Results from a randomized trial. Support. Care Cancer 2009, 17, 1301–1309. [Google Scholar] [CrossRef]
- Kimmick, G.G.; Lovato, J.; McQuellon, R.; Robinson, E.; Muss, H.B. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006, 12, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, G.R.; Pinczowski, H.; Zanellato, R.; Tateyama, L.; Schindler, F.; Fonseca, F.; Del Giglio, A. Bupropion for control of hot flashes in breast cancer survivors: A prospective, double-blind, randomized, crossover, pilot phase II trial. J. Pain Symptom Manag. 2013, 45, 969–979. [Google Scholar] [CrossRef]
- Lipov, E.G.; Lipov, S.; Joshi, J.R.; Santucci, V.D.; Slavin, K.V.; Beck Vigue, S.G. Stellate ganglion block may relieve hot flashes by interrupting the sympathetic nervous system. Med. Hypotheses 2007, 69, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Smith, M.J.; Hellier, J.; Balabanovic, J.A.; Hamed, H.; Grunfeld, E.A.; Hunter, M.S. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): A randomised controlled trial. Lancet Oncol. 2012, 13, 309–318. [Google Scholar] [CrossRef]
- Elkins, G.; Marcus, J.; Stearns, V.; Hasan Rajab, M. Pilot evaluation of hypnosis for the treatment of hot flashes in breast cancer survivors. Psychooncology 2007, 16, 487–492. [Google Scholar] [CrossRef]
- Atema, V.; van Leeuwen, M.; Kieffer, J.M.; Oldenburg, H.S.A.; van Beurden, M.; Gerritsma, M.A.; Kuenen, M.A.; Plaisier, P.W.; Lopes Cardozo, A.M.F.; van Riet, Y.E.A.; et al. Efficacy of Internet-Based Cognitive Behavioral Therapy for Treatment-Induced Menopausal Symptoms in Breast Cancer Survivors: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Atema, V.; van Leeuwen, M.; Kieffer, J.M.; Oldenburg, H.S.A.; van Beurden, M.; Hunter, M.S.; Aaronson, N.K. Internet-based cognitive behavioral therapy aimed at alleviating treatment-induced menopausal symptoms in breast cancer survivors: Moderators and mediators of treatment effects. Maturitas 2020, 131, 8–13. [Google Scholar] [CrossRef]
- Atema, V.; van Leeuwen, M.; Oldenburg, H.S.A.; van Beurden, M.; Hunter, M.S.; Aaronson, N.K. An Internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: Results of a pilot study. Menopause 2017, 24, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, J.G.E.; Atema, V.; Mewes, J.C.; van Leeuwen, M.; Oldenburg, H.S.A.; van Beurden, M.; Hunter, M.S.; van Harten, W.H.; Aaronson, N.K.; Retèl, V.P. Cost-utility, cost-effectiveness, and budget impact of Internet-based cognitive behavioral therapy for breast cancer survivors with treatment-induced menopausal symptoms. Breast Cancer Res. Treat. 2019, 178, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.S.; Burns, D.S.; Wu, J.; Otte, J.L.; Schneider, B.; Ryker, K.; Tallman, E.; Yu, M. Paced respiration for vasomotor and other menopausal symptoms: A randomized, controlled trial. J. Gen. Intern. Med. 2013, 28, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Im, E.O.; Kim, S.; Lee, C.; Chee, E.; Mao, J.J.; Chee, W. Decreasing menopausal symptoms of Asian American breast cancer survivors through a technology-based information and coaching/support program. Menopause 2019, 26, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Capriglione, S.; Plotti, F.; Montera, R.; Luvero, D.; Lopez, S.; Scaletta, G.; Aloisi, A.; Serra, G.B.; Angioli, R. Role of paroxetine in the management of hot flashes in gynecological cancer survivors: Results of the first randomized single-center controlled trial. Gynecol. Oncol. 2016, 143, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Krouwel, E.M.; Nicolai, M.P.; van der Wielen, G.J.; Putter, H.; Krol, A.D.; Pelger, R.C.; Incrocci, L.; Elzevier, H.W. Sexual Concerns after (Pelvic) Radiotherapy: Is There Any Role for the Radiation Oncologist? J. Sex. Med. 2015, 12, 1927–1939. [Google Scholar] [CrossRef]
- Frederick, N.N.; Recklitis, C.J.; Blackmon, J.E.; Bober, S. Sexual Dysfunction in Young Adult Survivors of Childhood Cancer. Pediatr. Blood Cancer 2016, 63, 1622–1628. [Google Scholar] [CrossRef]
- Bjornard, K.L.; Howell, C.R.; Klosky, J.L.; Chemaitilly, W.; Srivastava, D.K.; Brinkman, T.M.; Green, D.M.; Willard, V.W.; Jacola, L.M.; Krasin, M.J.; et al. Psychosexual Functioning of Female Childhood Cancer Survivors: A Report from the St. Jude Lifetime Cohort Study. J. Sex. Med. 2020. [Google Scholar] [CrossRef]
- Sopfe, J.; Marsh, R.; Appiah, L.C.; Klosky, J.L.; Peterson, P.N.; DorseyHolliman, B. Evaluating sexual function in adolescent and young adult childhood cancer survivors. J. Clin. Oncol. 2020, 38, e24180. [Google Scholar] [CrossRef]
| Patient Populations | Intervention Type | Reference Number(s) |
|---|---|---|
| General sexual function concerns | ||
| All adult cancers | Multimodal clinic | [22,23,24] |
| Complementary/alternative medicine | [25] | |
| Psychoeducation (technology-based) | [26] | |
| Testosterone replacement | [27] | |
| AYA patients | Psychoeducation (In-person, individual) | [28] |
| Breast cancer | Exercise | [29,30,31,32] |
| Psychoeducation/biomedical program (In-person, group) | [33] | |
| Psychoeducation (In-person, group) | [34,35,36,37,38] | |
| Psychoeducation (In-person, individual) | [39,40] | |
| Psychoeducation (technology-based) | [41,42,43,44,45,46,47,48] | |
| Cervical cancer | Psychoeducation/biomedical program (In-person, group) | [49] |
| Colorectal cancer | Psychoeducation (technology-based) | [50] |
| Endometrial cancer | Exercise | [51] |
| Gynecologic cancers (all) | Multimodal clinic | [52] |
| Psychoeducation (In-person, individual) | [40,50,53,54] | |
| Psychoeducation (technology-based) | [48] | |
| HSCT recipients | Multimodal clinic | [55] |
| Ovarian cancer | Psychoeducation/biomedical program (In-person, group) | [56,57] |
| Psychoeducation (In-person, group) | [58] | |
| Rectal and anal cancer | Psychoeducation (In-person, individual) | [59] |
| Body image concerns | ||
| AYA patients | Psychoeducation (In-person, individual) | [28] |
| Psychoeducation (technology-based) | [60] | |
| Social/expressive programming | [61,62,63] | |
| Breast cancer | Cosmetic programming | [64] |
| Exercise | [29,32,65] | |
| Psychoeducation/biomedical program (In-person, group) | [33] | |
| Psychoeducation (In-person, group) | [37,66,67] | |
| Psychoeducation (In-person, individual) | [40] | |
| Psychoeducation (technology-based) | [47,68] | |
| Gynecologic cancers (all) | Psychoeducation (In-person, individual) | [40] |
| Psychoeducation (technology-based) | [68] | |
| Head/neck cancers | Cosmetic programming | [69] |
| Psychoeducation (technology-based) | [70] | |
| Patient Populations | Intervention Type | Reference Number(s) |
|---|---|---|
| Genitourinary syndrome | ||
| All adult cancers | Non-hormonal topical agent | [23] |
| Breast cancer | Education | [76] |
| Er:YAG laser therapy | [77] | |
| Fractional CO2 laser therapy | [75,78,79,80,81,82,83] | |
| Hormonal topical agents | [84,85,86] | |
| Non-hormonal topical agent | [87,88,89,90,91,92,93] | |
| Cervical cancer | Hormonal topical agents | [94] |
| Endometrial cancer | Non-hormonal topical agent | [93,95] |
| Gynecologic cancers (all) | Fractional CO2 laser therapy | [79] |
| Hormonal topical agents | [86] | |
| Pelvic floor dysfunction | ||
| Breast cancer | Pelvic floor rehabilitation | [90] |
| Colorectal cancer | Dilator therapy | [96] |
| Gynecologic cancers (all) | Dilator therapy | [96] |
| Pelvic floor rehabilitation | [97,98] | |
| Vasomotor symptoms of menopause | ||
| Breast cancer | Complementary/alternative medicine | [99,100,101,102] |
| Education | [76] | |
| Exercise | [103,104] | |
| Pharmacologic agents | [105,106,107] | |
| Psychoeducation (In-person, group) | [108] | |
| Psychoeducation (In-person, individual) | [109] | |
| Psychoeducation (technology-based) | [110,111,112,113,114,115] | |
| Gynecologic cancers (all) | Pharmacologic agents | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopfe, J.; Pettigrew, J.; Afghahi, A.; Appiah, L.C.; Coons, H.L. Interventions to Improve Sexual Health in Women Living with and Surviving Cancer: Review and Recommendations. Cancers 2021, 13, 3153. https://doi.org/10.3390/cancers13133153
Sopfe J, Pettigrew J, Afghahi A, Appiah LC, Coons HL. Interventions to Improve Sexual Health in Women Living with and Surviving Cancer: Review and Recommendations. Cancers. 2021; 13(13):3153. https://doi.org/10.3390/cancers13133153
Chicago/Turabian StyleSopfe, Jenna, Jessica Pettigrew, Anosheh Afghahi, Leslie C. Appiah, and Helen L. Coons. 2021. "Interventions to Improve Sexual Health in Women Living with and Surviving Cancer: Review and Recommendations" Cancers 13, no. 13: 3153. https://doi.org/10.3390/cancers13133153
APA StyleSopfe, J., Pettigrew, J., Afghahi, A., Appiah, L. C., & Coons, H. L. (2021). Interventions to Improve Sexual Health in Women Living with and Surviving Cancer: Review and Recommendations. Cancers, 13(13), 3153. https://doi.org/10.3390/cancers13133153





