Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Treatment Plan
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Correlation between Clinicopathological Parameters and the Expression of Galectin-1
3.3. Correlation between Clinicopathological Parameters and Pathological Complete Response
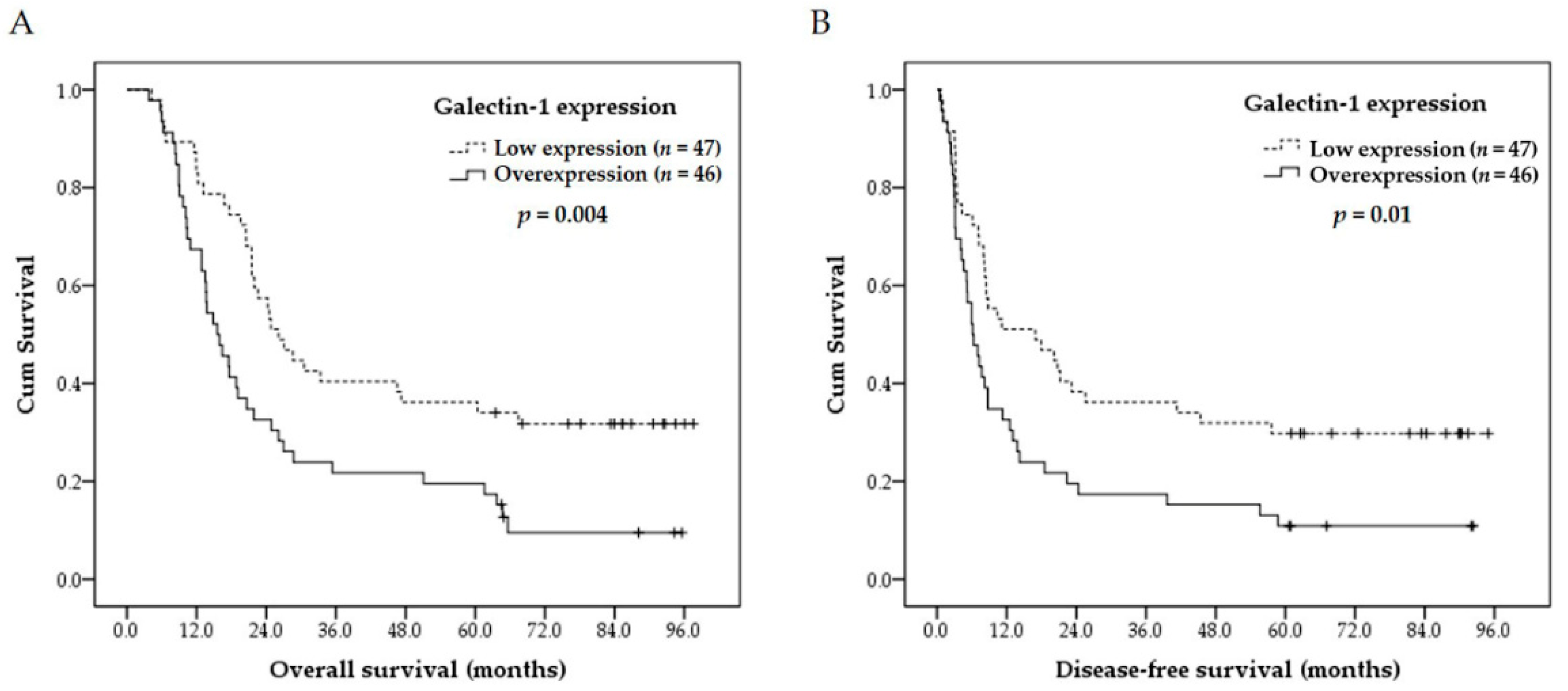
3.4. Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Esophageal squamous cell carcinoma | ESCC |
| Concurrent chemoradiotherapy | CCRT |
| Pathological complete response | pCR |
| Immunohistochemistry | IHC |
| American Joint Committee on Cancer | AJCC |
| Computed tomography | CT |
| Endoscopic ultrasound | EUS |
References
- Li, S.H.; Chen, C.H.; Lu, H.I.; Huang, W.T.; Tien, W.Y.; Lan, Y.C.; Lee, C.C.; Chen, Y.H.; Huang, H.Y.; Chang, A.Y.; et al. Phosphorylated p70S6K expression is an independent prognosticator for patients with esophageal squamous cell carcinoma. Surgery 2015, 157, 570–580. [Google Scholar] [CrossRef]
- Lu, H.I.; Li, S.H.; Huang, W.T.; Rau, K.M.; Fang, F.M.; Wang, Y.M.; Lin, W.C.; Tien, W.Y. A comparative study of isolated and metachronous oesophageal squamous cell carcinoma with antecedent upper aerodigestive tract cancer. Eur. J. Cardiothorac. Surg. 2013, 44, 860–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedenne, L.; Michel, P.; Bouche, O.; Milan, C.; Mariette, C.; Conroy, T.; Pezet, D.; Roullet, B.; Seitz, J.F.; Herr, J.P.; et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 2007, 25, 1160–1168. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Walz, M.K.; Seeber, S.; Klump, B.; Budach, W.; Teichmann, R.; Schmitt, M.; et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 2005, 23, 2310–2317. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.C.; Farma, J.; Scott, W.J.; Freedman, G.; Weiner, L.; Cheng, J.D.; Wang, H.; Goldberg, M. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J. Clin. Oncol. 2005, 23, 4330–4337. [Google Scholar] [CrossRef]
- Li, S.H.; Huang, E.Y.; Lu, H.I.; Huang, W.T.; Yen, C.C.; Huang, W.C.; Chen, C.H. Phosphorylated mammalian target of rapamycin expression is associated with the response to chemoradiotherapy in patients with esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2012, 144, 1352–1359. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Rau, K.M.; Lu, H.I.; Wang, Y.M.; Tien, W.Y.; Liang, J.L.; Lin, W.C. Pre-treatment maximal oesophageal wall thickness is independently associated with response to chemoradiotherapy in patients with T3-4 oesophageal squamous cell carcinoma. Eur. J. Cardiothorac. Surg. 2012, 42, 958–964. [Google Scholar] [CrossRef] [Green Version]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.; Cummings, R.D.; Drickamer, K.; Feizi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Scott, K.; Weinberg, C. Galectin-1: A bifunctional regulator of cellular proliferation. Glycoconj. J. 2004, 19, 467–477. [Google Scholar] [CrossRef]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Brandt, B.; Abou-Eladab, E.F.; Tiedge, M.; Walzel, H. Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis. 2010, 1, e23. [Google Scholar] [CrossRef] [Green Version]
- Croci, D.O.; Salatino, M.; Rubinstein, N.; Cerliani, J.P.; Cavallin, L.E.; Leung, H.J.; Ouyang, J.; Ilarregui, J.M.; Toscano, M.A.; Domaica, C.I.; et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 2012, 209, 1985–2000. [Google Scholar] [CrossRef]
- Jung, T.Y.; Jung, S.; Ryu, H.H.; Jeong, Y.I.; Jin, Y.H.; Jin, S.G.; Kim, I.Y.; Kang, S.S.; Kim, H.S. Role of galectin-1 in migration and invasion of human glioblastoma multiforme cell lines. J. Neurosurg. 2008, 109, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.Y.; Chen, T.T.; Xia, L.; Guo, M.; Xu, Y.; Yue, F.; Jiang, Y.; Chen, G.Q.; Zhao, K.W. Hypoxia inducible factor-1 mediates expression of galectin-1: The potential role in migration/invasion of colorectal cancer cells. Carcinogenesis 2010, 31, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- White, N.M.; Masui, O.; Newsted, D.; Scorilas, A.; Romaschin, A.D.; Bjarnason, G.A.; Siu, K.W.; Yousef, G.M. Galectin-1 has potential prognostic significance and is implicated in clear cell renal cell carcinoma progression through the HIF/mTOR signaling axis. Br. J. Cancer 2014, 110, 1250–1259. [Google Scholar] [CrossRef]
- Kuo, P.; Le, Q.T. Galectin-1 links tumor hypoxia and radiotherapy. Glycobiology 2014, 24, 921–925. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Hisano, H.; Nishikawa, Y.; Nagasaki, Y. Targeting and Treatment of Tumor Hypoxia by Newly Designed Prodrug Possessing High Permeability in Solid Tumors. Mol. Pharm. 2016, 13, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Chanchien, C.C.; Lin, H.; Wang, C.C.; Wang, C.J.; Huang, C.C. Galectin-1 is an independent prognostic factor for local recurrence and survival after definitive radiation therapy for patients with squamous cell carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 975–982. [Google Scholar] [CrossRef]
- Su, Y.C.; Davuluri, G.V.; Chen, C.H.; Shiau, D.C.; Chen, C.C.; Chen, C.L.; Lin, Y.S.; Chang, C.P. Galectin-1-induced autophagy facilitates cisplatin resistance of hepatocellular carcinoma. PLoS ONE 2016, 11, e0148408. [Google Scholar] [CrossRef]
- Messaoudi, K.; Clavreul, A.; Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today 2015, 20, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.Y.; Yen, S.L.; Huang, C.C.; Huang, E.Y. Galectin-1 is a poor prognostic factor in patients with glioblastoma multiforme after radiotherapy. BMC Cancer 2018, 18, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, P.; Shi, B.; Zhou, M.; Jiang, H.; Zhang, H.; Pan, X.; Gao, H.; Sun, H.; Li, Z. Galectin-1 overexpression promotes progression and chemoresistance to cisplatin in epithelial ovarian cancer. Cell Death Dis. 2014, 5, e991. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.Y.; Tang, S.J.; Sun, G.H.; Chou, T.Y.; Yeh, T.S.; Yu, S.L.; Sun, K.H. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin. Cancer Res. 2012, 18, 4037–4047. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.; Bratman, S.V.; Shultz, D.B.; von Eyben, R.; Chan, C.; Wang, Z.; Say, C.; Gupta, A.; Loo, B.W., Jr.; Giaccia, A.J.; et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin. Cancer Res. 2014, 20, 5558–5569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edge, S.B.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) Esophagus and esophagogastric junction. In AJCC Cancer Satging Manual, 7th ed.; Springer: New York, NY, USA, 2010; pp. 103–111. [Google Scholar]
- Saussez, S.; Decaestecker, C.; Lorfevre, F.; Cucu, D.R.; Mortuaire, G.; Chevalier, D.; Wacreniez, A.; Kaltner, H.; Andre, S.; Toubeau, G.; et al. High level of galectin-1 expression is a negative prognostic predictor of recurrence in laryngeal squamous cell carcinomas. Int. J. Oncol. 2007, 30, 1109–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Chen, P.; Liao, R.; Li, Y.W.; Yi, Y.; Wang, J.X.; Sun, T.W.; Zhou, J.; Shi, Y.H.; Yang, X.R.; et al. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J. Gastroenterol. Hepatol. 2012, 27, 1312–1319. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Ravi, N.; Hollywood, D.; Kennedy, M.J.; Rowley, S.; Ryan, A.; Hughes, N.; Carey, M.; Byrne, P. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J. Thorac. Cardiovasc. Surg. 2006, 132, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Huang, E.Y.; Chen, Y.F.; Chen, Y.M.; Lin, I.H.; Wang, C.C.; Su, W.H.; Chuang, P.C.; Yang, K.D. A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis. 2012, 3, e251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.F.; Wu, J.; Luo, J.H.; Li, K.S.; Wang, F.; Huang, W.; Wu, Y.; Gao, S.P.; Zhang, X.M.; Zhang, P.N. SNHG22 overexpression indicates poor prognosis and induces chemotherapy resistance via the miR-2467/Gal-1 signaling pathway in epithelial ovarian carcinoma. Aging 2019, 11, 8204–8216. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W. Knockdown of galectin-1 facilitated cisplatin sensitivity by inhibiting autophagy in neuroblastoma cells. Chem. Biol. Interact. 2019, 297, 50–56. [Google Scholar] [CrossRef] [PubMed]
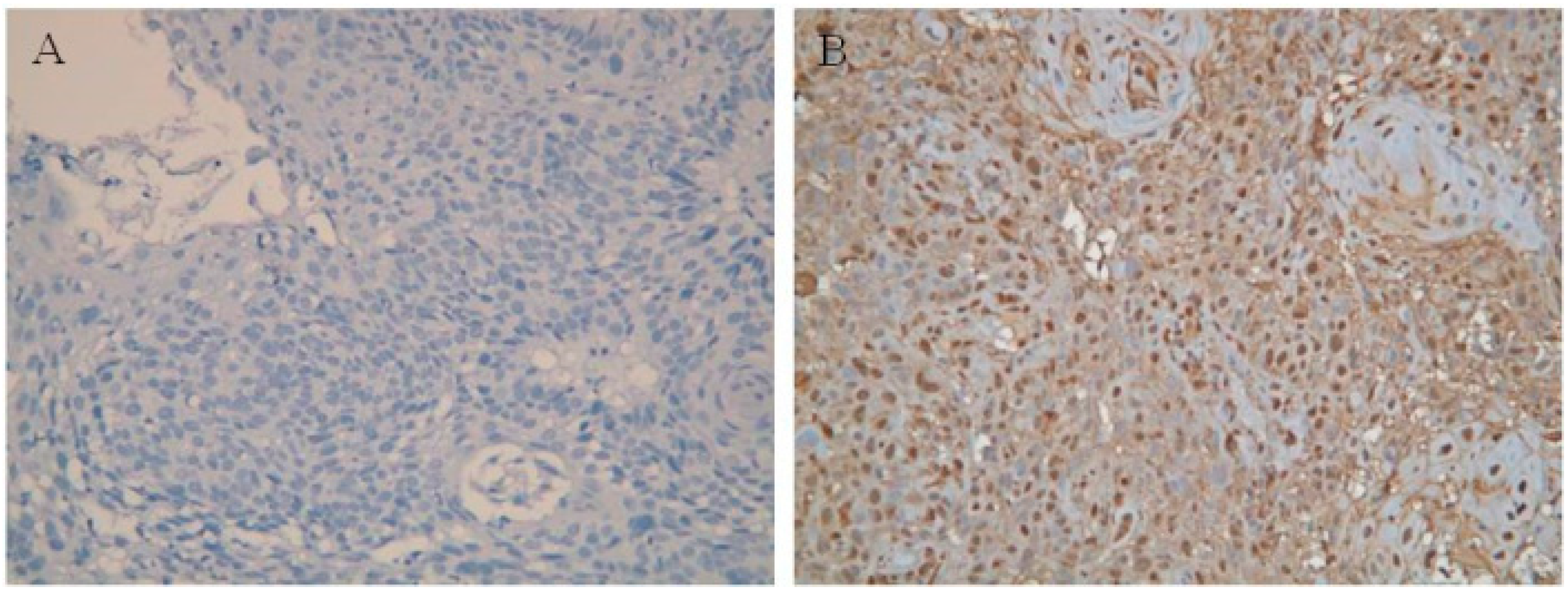
| Parameters | No. of Cases (Percentage) |
|---|---|
| Age (years) (mean: 53.2, median: 52, range: 37–77) | |
| <50 | 35 (38%) |
| 50 ≤ Age < 60 | 35 (38%) |
| 60 ≤ Age < 70 | 19 (20%) |
| 70 ≤ Age | 4 (4%) |
| Sex | |
| Male | 91 (98%) |
| Female | 2 (2%) |
| Clinical T classification | |
| T2 | 9 (10%) |
| T3 | 41 (44%) |
| T4 | 43 (46%) |
| Clinical N classification | |
| N0 | 23 (25%) |
| N1 | 30 (32%) |
| N2 | 29 (31%) |
| N3 | 11 (11%) |
| Clinical 7th AJCC stage | |
| II | 23 (25%) |
| III | 70 (75%) |
| Tumor grade | |
| 1 | 16 (17%) |
| 2 | 54 (58%) |
| 3 | 23 (25%) |
| Primary tumor location | |
| Upper | 13 (14%) |
| Middle | 46 (49%) |
| Lower | 34 (37%) |
| Galectin-1 expression | |
| Low expression | 47 (51%) |
| Overexpression | 46 (49%) |
| Surgical margin | |
| Negative | 77 (83%) |
| Positive | 16 (17%) |
| pCR | |
| Absent | 70 (75%) |
| Present | 23 (25%) |
| Parameters | Category | Galectin-1 Expression | ||
|---|---|---|---|---|
| Low Expression | Overexpression | p Value | ||
| Age | <52y/o | 28 | 22 | 0.35 |
| ≥52y/o | 29 | 24 | ||
| Clinical T classification | T2/3 | 29 | 21 | 0.12 |
| T4 | 18 | 25 | ||
| Clinical N classification | N0 | 14 | 9 | 0.25 |
| N1/2/3 | 33 | 37 | ||
| Clinical N classification | N0/1 | 29 | 24 | 0.35 |
| N2/3 | 18 | 22 | ||
| Clinical 7th AJCC stage | II | 14 | 9 | 0.25 |
| III | 33 | 37 | ||
| Tumor grade | 1/2 | 35 | 35 | 0.86 |
| 3 | 12 | 11 | ||
| Primary tumor location | Upper/Middle | 27 | 32 | 0.23 |
| Lower | 20 | 14 | ||
| Parameters | Category | Pathological Complete Response | ||
|---|---|---|---|---|
| Present | Absent | p Value | ||
| Age | <52y/o | 7 | 33 | 0.16 |
| ≥52y/o | 16 | 37 | ||
| Clinical T classification | T2/3 | 15 | 35 | 0.20 |
| T4 | 8 | 35 | ||
| Clinical N classification | N0 | 8 | 15 | 0.20 |
| N1/2/3 | 15 | 55 | ||
| Clinical N classification | N0/1 | 16 | 37 | 0.16 |
| N2/3 | 7 | 33 | ||
| Clinical 7th AJCC stage | II | 9 | 14 | 0.065 |
| III | 14 | 56 | ||
| Tumor grade | 1/2 | 18 | 52 | 0.70 |
| 3 | 5 | 18 | ||
| Primary tumor location | Upper/Middle | 12 | 47 | 0.20 |
| Lower | 11 | 23 | ||
| Galectin-1 | Low expression | 17 | 30 | 0.01 * |
| Overexpression | 6 | 40 | ||
| Factors | No. of Patients | Overall Survival (OS) | Disease-Free Survival (DFS) | ||
|---|---|---|---|---|---|
| 5-year OS Rate (%) | p Value | 5-year DFS Rate (%) | p Value | ||
| Age | |||||
| <52y/o | 40 | 25% | 0.93 | 20% | 0.99 |
| ≥52y/o | 53 | 30% | 21% | ||
| Clinical T classification | |||||
| T2/3 | 50 | 34% | 0.13 | 24% | 0.078 |
| T4 | 43 | 21% | 16% | ||
| Clinical N classification | |||||
| N0 | 23 | 44% | 0.22 | 30% | 0.12 |
| N1/2/3 | 70 | 23% | 17% | ||
| Clinical N classification | |||||
| N0/1 | 53 | 36% | 0.093 | 25% | 0.038 * |
| N2/3 | 40 | 18% | 15% | ||
| Clinical 7th AJCC stage | |||||
| II | 23 | 52% | 0.036 * | 35% | 0.018 * |
| III | 70 | 20% | 16% | ||
| Tumor grade | |||||
| 1/2 | 70 | 29% | 0.49 | 21% | 0.75 |
| 3 | 23 | 26% | 17% | ||
| Primary tumor location | |||||
| Upper/Middle | 59 | 29% | 0.83 | 20% | 0.67 |
| Lower | 34 | 27% | 21% | ||
| Surgical margin | |||||
| Negative | 77 | 34% | 0.001 * | 25% | 0.001 * |
| Positive | 16 | 0% | 0% | ||
| Galectin-1 | |||||
| Low expression | 47 | 36% | 0.004 * | 30% | 0.01 * |
| Overexpression | 46 | 20% | 11% | ||
| Factors | Overall Survival | Disease-free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Galectin-1 overexpression | 2.098 (1.310–3.359) | 0.002 * | 1.839 (1.157–2.923) | 0.01 * |
| Positive surgical margin | 2.778 (1.555–4.950) | 0.001 * | 2.577 (1.462–4.545) | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-H.; Chen, Y.-H.; Lu, H.-I.; Lo, C.-M.; Huang, C.-C.; Wang, Y.-M.; Huang, E.-Y. Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers 2021, 13, 3147. https://doi.org/10.3390/cancers13133147
Li S-H, Chen Y-H, Lu H-I, Lo C-M, Huang C-C, Wang Y-M, Huang E-Y. Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers. 2021; 13(13):3147. https://doi.org/10.3390/cancers13133147
Chicago/Turabian StyleLi, Shau-Hsuan, Yen-Hao Chen, Hung-I Lu, Chien-Ming Lo, Chao-Cheng Huang, Yu-Ming Wang, and Eng-Yen Huang. 2021. "Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma" Cancers 13, no. 13: 3147. https://doi.org/10.3390/cancers13133147
APA StyleLi, S.-H., Chen, Y.-H., Lu, H.-I., Lo, C.-M., Huang, C.-C., Wang, Y.-M., & Huang, E.-Y. (2021). Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma. Cancers, 13(13), 3147. https://doi.org/10.3390/cancers13133147







