How Researchers, Clinicians and Patient Advocates Can Accelerate Lobular Breast Cancer Research
Abstract
:Simple Summary
Abstract
1. Historic View of Lobular Carcinoma Is Challenged by New Evidence
2. First International Lobular Breast Cancer Symposium Sparks New Patient Advocacy
- Patient advocates were hungry for quality information about their disease which was often omitted in breast cancer educational resources. This left patients without the tools to be informed partners in their self-care, lacking even basic information such as unusual sites where lobular tumors can spread and symptoms of recurrence to report to their treating physicians.
- Patient advocates expressed frustration and lack of confidence in standard of care imaging and therapies that were primarily designed and trialed on patients with IDC, but were not always the best fit or the efficacy was unknown in patients with ILC.
- Advocates were eager to identify and bridge gaps between research and patients and support studies and trials focused on ILC.
- Researchers and clinicians found that they needed critical input from advocates to identify the most important needs for patients.
- Researchers and clinicians needed the patient voice to advocate with funders, institutions and organizations to make the case for more ILC-focused research and to raise awareness of this disease in the broader breast cancer and research community.
3. Role of Patient Advocacy in Lobular Breast Cancer
4. Launching of the Lobular Breast Cancer Alliance and Global ILC-Focused Advocacy Efforts
- Lobular Breast Cancer UK (LBCUK) (Lobularbreastcancer.org.uk) is a patient-advocate-driven organization in the United Kingdom that has charitable status and a website. LBCUK’s goals are to drive education, research, policy change and ensure patient support is available for patients with ILC within outside organizations and support services. LBCUK will focus on informing and supporting patients to be their own best advocates, partnering with researchers, representing ILC advocacy at conferences and developing and driving funded lobular research programs. LBCUK has a Scientific Advisory Group involving researchers and clinicians from institutions across the UK working in partnership with the charity who provide scientific and medical advice and guidance and the opportunity to develop longer-term ILC-focused research programs.
- Lobular Ireland (LobularIreland.com) is a network of ILC advocates and breast cancer researchers from the Royal College of Surgeons, University College Dublin with a clinical advisor from Cancer Trials Ireland. Collectively, they advocate for more research into ILC and raise awareness about ILC. Lobular Ireland’s goals are to partner with researchers, build patient awareness and have ILC representation at key breast cancer conferences and seminars.
5. Elements of Successful Researcher and Patient Research Advocate Collaboration
- Timing and opportunity: As evidence mounted that ILC was a different disease, the research community realized there were unique aspects of ILC that deserved and required more research which would benefit from advocate participation. The new interest in research coincided with early communication and organization among patients with lobular breast cancer on early social media platforms, leading to strong advocate attendance at the 1st ILC Symposium and motivated engagement after the conference.
- Leadership: The prior professional experience of LBCA’s founding leader Leigh Pate in successful large-scale political and issue advocacy campaigns provided a strong foundation in organizing, strategic communications and management of volunteers and coalitions. Scientific Advisory Board founder and past-chair Steffi Oesterreich PhD leveraged her experience through various foundations and institutions, and passionately partnered with advocates to guide LBCA’s interactions within the breast cancer research community. This advocate/researcher partnership opened doors, lent professionalism and credibility to LBCA’s public content and efforts, and led to the engagement of a strong network of committed scientific advisors from diverse institutions as well as broad breast cancer organizational support.
- Partnerships: Importantly, LBCA’s leaders developed a strong working relationship and a level of trust that allowed them to pursue an ambitious agenda. LBCA’s early organizational structure was never dependent on one individual to carry the efforts, was consensus based and included a strong co-coordinator and advocate leader in Lori Petitti and a volunteer advocate steering committee with diverse backgrounds and skills.
- Collaborations: A founding tenet of lobular advocate leaders internationally is grounded in collaboration—the belief that patient advocates, clinicians and researchers must work together to overcome challenges in lobular research, create change and advance patient-centered research initiatives.
- Commitment to scientific review: LBCA developed educational content on lobular breast cancer, working with Scientific Advisors who lent their expertise, making LBCA a reliable resource within the breast cancer community and to our community partners.
- Dedicated communications platform: LBCA’s website and communications platforms, as well as a growing network of advocates worldwide, provide a center of information on ILC. This benefits not only patients looking for education and resources, but researchers who have a platform to share information with an audience specifically interested in ILC.
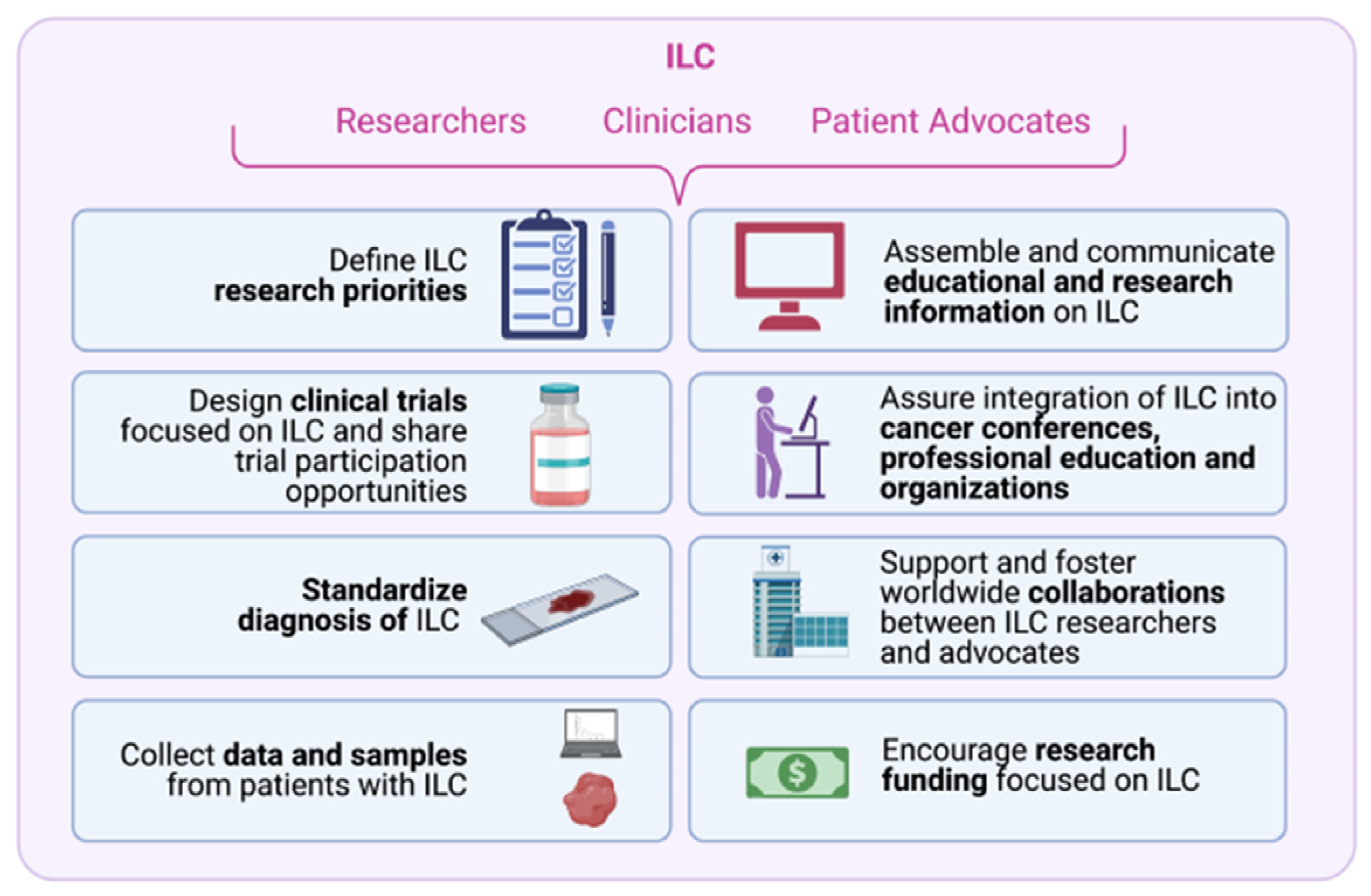
6. Challenges and Opportunities for Researchers, Clinicians and Patient Advocates to Accelerate ILC Research
6.1. Challenge #1: Define ILC Research Priorities
Opportunities
6.2. Challenge #2: Design Clinical Trials Focused on ILC and Share Trial Participation Opportunities
Opportunities
6.3. Challenge #3: Standardize Diagnosis of ILC
Opportunities
6.4. Challenge #4: Collect Data and Samples from Patients with ILC
Opportunities
6.5. Challenge #5: Assemble and Communicate Up-to-Date and Accurate Educational and Research Information on ILC
Opportunities
6.6. Challenge #6: Integrate ILC into Cancer Conferences, Professional Education and Cancer Organizations
Opportunities
6.7. Challenge #7: Establish a Collaborative, Coordinated Worldwide ILC Research Strategy
Opportunities
6.8. Challenge #8: Encourage Funding Focused on ILC Research
Opportunities
7. Discussion
How Researchers, Clinicians and Advocates Can Work Together to Move Lobular Research Forward in the Future
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R. SEER Cancer Statistics Review, 1975–2017 (Based on November 2019 SEER Data Sub-mission) National Cancer Institute. Based on November 2019 SEER data submission, posted to the SEER web site, April 2020. Available online: https://seer.cancer.gov/csr/1975_2017 (accessed on 5 May 2021).
- Ewing, J. Neoplastic Diseases: A Textbook on Tumors; W.B. Saunders: Philadelphia, PA. USA, 1919. [Google Scholar]
- Foote, F.W.; Stewart, F.W. Lobular Carcinoma In Situ: A Rare Form of Mammary Cancer. CA A Cancer J. Clin. 1982, 32, 234–237. [Google Scholar] [CrossRef]
- Martinez, V.; Azzopardi, J.G. Invasive lobular carcinoma of the breast: Incidence and variants. Histopathology 1979, 3, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.R.; Fisher, B. Lobular carcinoma of the breast: An overview. Ann. Surg. 1977, 185, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishiguro, J.; Kotani, H.; Hisada, T.; Ichikawa, M.; Gondo, N.; Yoshimura, A.; Kondo, N.; Hattori, M.; Sawaki, M.; et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 2016, 16, 248. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, M.J.; Opdahl, S.; Vatten, L.J.; Haugen, O.A.; Bofin, A.M. Invasive lobular breast cancer: The prognostic impact of histo-pathological grade, E-cadherin and molecular subtypes. Histopathology 2015, 66, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Pestalozzi, B.C.; Zahrieh, D.; Mallon, E.; Gusterson, B.A.; Price, K.N.; Gelber, R.D.; Holmberg, S.B.; Lindtner, J.; Snyder, R.; Thürlimann, B.; et al. Distinct Clinical and Prognostic Features of Infiltrating Lobular Carcinoma of the Breast: Combined Results of 15 International Breast Cancer Study Group Clinical Trials. J. Clin. Oncol. 2008, 26, 3006–3014. [Google Scholar] [CrossRef]
- Arpino, G.; Bardou, V.J.; Clark, G.M.; Elledge, R.M. Infiltrating lobular carcinoma of the breast: Tumor characteristics and clinical outcome. Breast Cancer Res. 2004, 6, R149–R156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso-Sousa, R.; Metzger-Filho, O. Differences between invasive lobular and invasive ductal carcinoma of the breast: Results and therapeutic implications. Ther. Adv. Med Oncol. 2016, 8, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christgen, M.; Steinemann, D.; Kühnle, E.; Länger, F.; Gluz, O.; Harbeck, N.; Kreipe, H. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol. Res. Pract. 2016, 212, 583–597. [Google Scholar] [CrossRef]
- Rakha, E.A.; Ellis, I. Lobular breast carcinoma and its variants. Semin. Diagn. Pathol. 2010, 27, 49–61. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive lobular carcinoma of the breast: The increasing importance of this special subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Kelly, E.D.; Abraham, J.; Kruse, M. Invasive lobular breast cancer: A review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin. Oncol. 2019, 46, 121–132. [Google Scholar] [CrossRef]
- Bajrami, I.; Marlow, R.; Van De Ven, M.; Brough, R.; Pemberton, H.N.; Frankum, J.; Song, F.; Rafiq, R.; Konde, A.; Krastev, D.; et al. E-Cadherin/ROS1 Inhibitor Synthetic Lethality in Breast Cancer. Cancer Discov. 2018, 8, 498–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christgen, M.; Bartels, S.; Radner, M.; Raap, M.; Rieger, L.; Christgen, H.; Gluz, O.; Nitz, U.; Harbeck, N.; Lehmann, U.; et al. ERBB2 mutation frequency in lobular breast cancer with pleomorphic histology or high-risk characteristics by molecular expression profiling. Genes Chromosom. Cancer 2019, 58, 175–185. [Google Scholar] [CrossRef]
- Nagle, A.M.; Levine, K.; Tasdemir, N.; Scott, J.A.; Burlbaugh, K.; Kehm, J.W.; Katz, T.A.; Boone, D.N.; Jacobsen, B.M.; Atkinson, J.M.; et al. Loss of E-cadherin Enhances IGF1–IGF1R Pathway Activation and Sensitizes Breast Cancers to Anti-IGF1R/InsR Inhibitors. Clin. Cancer Res. 2018, 24, 5165–5177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Rosa, J.M.; Caniego-Casas, T.; Leskela, S.; Cristobal, E.; González-Martínez, S.; Moreno-Moreno, E.; López-Miranda, E.; Holgado, E.; Pérez-Mies, B.; Garrido, P.; et al. High Frequency of ERBB2 Activating Mutations in Invasive Lobular Breast Carcinoma with Pleomorphic Features. Cancers 2019, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Teo, K.; Gomez-Cuadrado, L.; Tenhagen, M.; Byron, A.; Rätze, M.; van Amersfoort, M.; Renes, J.; Strengman, E.; Mandoli, A.; Singh, A.A.; et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci. Rep. 2018, 8, 15454. [Google Scholar] [CrossRef] [Green Version]
- Marmor, S.; Hui, J.Y.C.; Huang, J.L.; Kizy, S.; Beckwith, H.; Blaes, A.H.; Rueth, N.M.; Tuttle, T. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer 2017, 123, 3015–3021. [Google Scholar] [CrossRef]
- Truin, W.; Voogd, A.C.; Vreugdenhil, G.; Loo, M.V.D.H.-V.D.; Siesling, S.; Roumen, R.M. Effect of adjuvant chemotherapy in postmenopausal patients with invasive ductal versus lobular breast cancer. Ann. Oncol. 2012, 23, 2859–2865. [Google Scholar] [CrossRef]
- Metzger Filho, O.; Giobbie-Hurder, A.; Mallon, E.; Gusterson, B.; Viale, G.; Winer, E.P.; Thürlimann, B.; Gelber, R.D.; Colleoni, M.; Ejlertsen, B.; et al. Relative Effectiveness of Letrozole Compared With Tamoxifen for Patients With Lobular Carcinoma in the BIG 1-98. Trial. J. Clin. Oncol. 2015, 33, 2772–2779. [Google Scholar] [CrossRef]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16alpha-18F-Fluoro-17beta-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Inte-gration into Clinical Applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Jhaveri, K.; Chardarlapaty, S.; Hatzoglou, V.; Riedl, C.C.; Lewis, J.S.; Mauguen, A. Head-to-head evaluation of (18)F-FES and (18)F-FDG PET/CT in metastatic in-vasive lobular breast cancer. J. Nucl. Med. 2020, 62, 326–331. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Goldman, D.; Gonen, M.; Pham, H.; Castillo, R.; Lyashchenko, S.; Lewis, J.; Dang, C. Initial results of a prospective clinical trial of 18F-Fluciclovine PET/CT in newly diagnosed invasive ductal and invasive lobular breast cancers. J. Nucl. Med. 2016, 57, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, O.; Cardoso, F.; Poncet, C.; Desmedt, C.; Linn, S.; Wesseling, J.; Hilbers, F.; Aalders, K.; Delorenzi, M.; Delaloge, S.; et al. Clinical utility of MammaPrint testing in Invasive Lobular Carcinoma: Results from the MINDACT phase III trial. Eur. J. Cancer 2020, 138, S5–S6. [Google Scholar] [CrossRef]
- Sestak, I.; Filipits, M.; Buus, R.; Rudas, M.; Balic, M.; Knauer, M.; Kronenwett, R.; Fitzal, F.; Cuzick, J.; Gnant, M.; et al. Prognostic Value of EndoPredict in Women with Hormone Receptor–Positive, HER2-Negative Invasive Lobular Breast Cancer. Clin. Cancer Res. 2020, 26, 4682–4687. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Lal, S.; Kutasovic, J.R.; Wockner, L.; Robertson, A.; de Luca, X.M.; Kalita-de Croft, P.; Dalley, A.; Coorey., C.P.; Kuo, L.; et al. LobSig is a multigene predictor of outcome in invasive lobular carcinoma. NPJ Breast Cancer 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- ILC. Symposium-Info. 2016. Available online: https://hillmanresearch.upmc.edu/research/centers/womens-cancer-re-search/meeting-archive/ilcsymposium-info/ (accessed on 5 May 2021).
- Flowers, M. Invasive Lobular Breast Cancer: The Second Most Common Form of Invasive Breast CANCER is understudied. 2016. Available online: https://www.bcrf.org/blog/invasive-lobular-breast-cancer-second-most-common-form-invasive-breast-cancer-understudied (accessed on 5 May 2021).
- Pate, L.; Hillier, H.; McAuliffe, P.F.; Jankowitz, R.; Oesterreich, S. Advancing Lobular Breast Cancer Research, Screening, Treatment, and Follow-Up Care. 2017. Available online: https://lobularbreastcancer.org/wp-content/uploads/2017/09/White-Paper-updated-for-website.pdf (accessed on 5 May 2021).
- Petitti, L.; Axelrod, J.; Campbell-Kotler, M.; Hutcheson, L.; Levine, J.; MacDonald, S.; Mapes, D.; Migyanka, F.; Neilsen, B.; Viggiano, E.; et al. Survey of LBCA-Sponsored Website Users Confirms Value, Identifies Unmet Information Needs. Available online: https://lobularbreastcancer.org/wp-content/uploads/2019/12/LBCA_FINAL_SABCS-2019-Poster.pdf (accessed on 5 May 2021).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pate, L.; Desmedt, C.; Metzger, O.; Burgess Hutcheson, L.; Turner, C.; Freeney, S.; Oesterreich, S. How Researchers, Clinicians and Patient Advocates Can Accelerate Lobular Breast Cancer Research. Cancers 2021, 13, 3094. https://doi.org/10.3390/cancers13133094
Pate L, Desmedt C, Metzger O, Burgess Hutcheson L, Turner C, Freeney S, Oesterreich S. How Researchers, Clinicians and Patient Advocates Can Accelerate Lobular Breast Cancer Research. Cancers. 2021; 13(13):3094. https://doi.org/10.3390/cancers13133094
Chicago/Turabian StylePate, Leigh, Christine Desmedt, Otto Metzger, Laurie Burgess Hutcheson, Claire Turner, Siobhán Freeney, and Steffi Oesterreich. 2021. "How Researchers, Clinicians and Patient Advocates Can Accelerate Lobular Breast Cancer Research" Cancers 13, no. 13: 3094. https://doi.org/10.3390/cancers13133094
APA StylePate, L., Desmedt, C., Metzger, O., Burgess Hutcheson, L., Turner, C., Freeney, S., & Oesterreich, S. (2021). How Researchers, Clinicians and Patient Advocates Can Accelerate Lobular Breast Cancer Research. Cancers, 13(13), 3094. https://doi.org/10.3390/cancers13133094






