Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis
Abstract
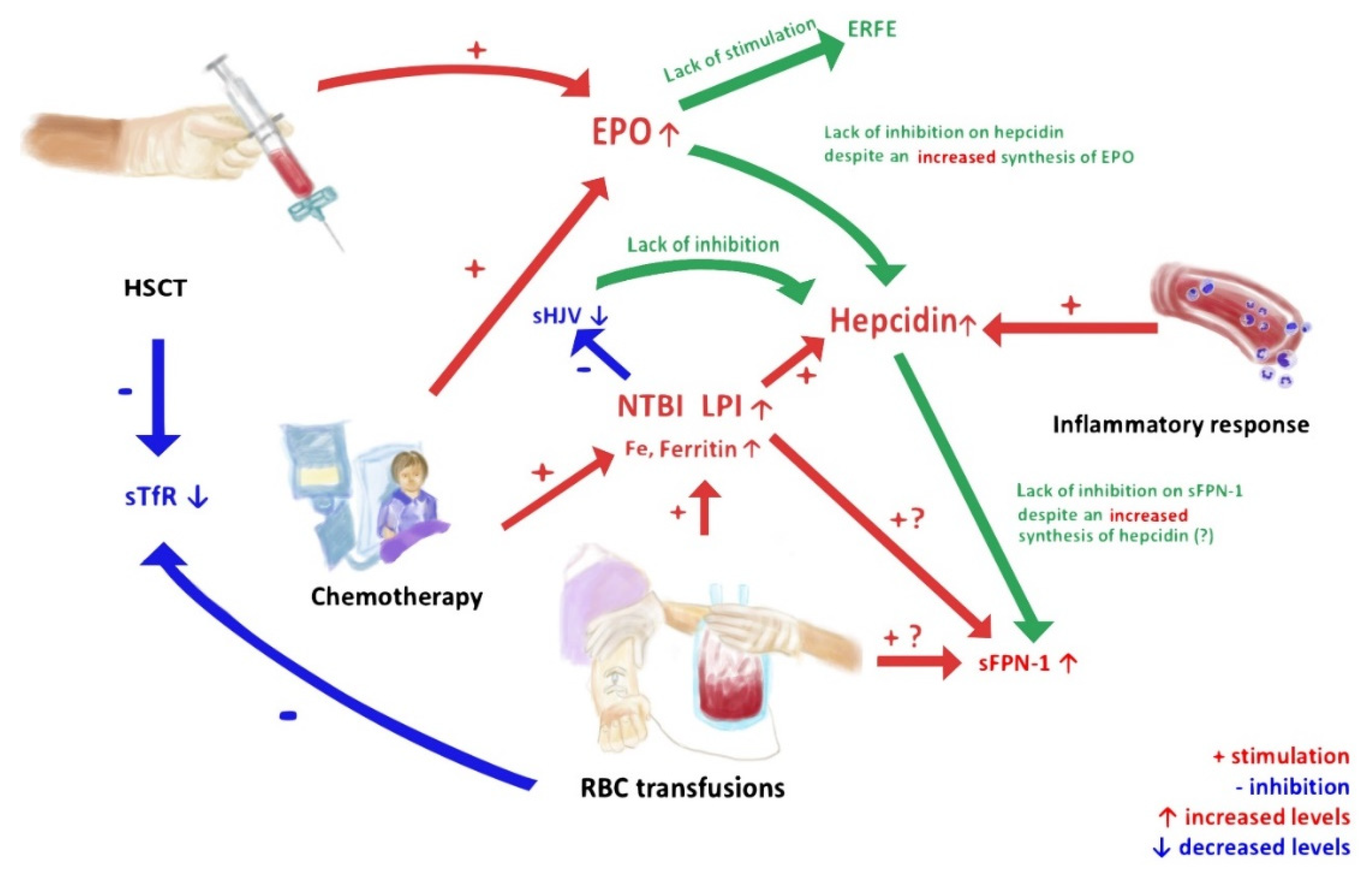
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. NTBI/LPI and Other Parameters Measuring Functional and Storage Iron Pools
3.2. Proteins Regulating Absorption of Iron and Its Release from the Tissue Stores
3.3. Proteins Regulating Erythropoietic Activity of Bone Marrow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coates, T.D. Iron overload in transfusion-dependent patients. Hematology 2019, 2019, 337–344. [Google Scholar] [CrossRef]
- Olcay, L.; Serteser, M.; Kolay, M.; Balci, H.F.; Yildirim, Ü.M.; Tekgündüz, S.A.; Hazirolan, T.; Terzi, Y.K. The Impact of Iron Overload in Acute Leukemia: Chronic Inflammation, But Not the Presence of Nontransferrin Bound Iron is a Determinant of Oxidative Stress. J. Pediatr. Hematol. 2017, 39, 425–439. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; Cutler, C.S.; Ho, V.T.; Koreth, J.; Alyea, E.P.; Soiffer, R.J.; Antin, J.H. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007, 109, 4586–4588. [Google Scholar] [CrossRef]
- Green, R.; Charlton, R.; Seftel, H.; Bothwell, T.; Mayet, F.; Adams, B.; Finch, C.; Layrisse, M. Body iron excretion in man: A collaborative study. Am. J. Med. 1968, 45, 336–353. [Google Scholar] [CrossRef]
- Belotti, A.; Duca, L.; Borin, L.; Realini, S.; Renso, R.; Parma, M.; Pioltelli, P.; Pogliani, E.; Cappellini, M.D. Non transferrin bound iron (NTBI) in acute leukemias throughout conventional intensive chemotherapy: Kinetics of its appearance and potential predictive role in infectious complications. Leuk. Res. 2015, 39, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Pullarkat, V. Iron Overload in Patients Undergoing Hematopoietic Stem Cell Transplantation. Adv. Hematol. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic. Biol. Med. 2014, 72, 23–40. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free. Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Sahlstedt, L.; Von Bonsdorff, L.; Ebeling, F.; Parkkinen, J.; Juvonen, E.; Ruutu, T. Non-transferrin-bound iron in haematological patients during chemotherapy and conditioning for autologous stem cell transplantation. Eur. J. Haematol. 2009, 83, 455–459. [Google Scholar] [CrossRef]
- Naoum, F.A.; Espósito, B.P.; Ruiz, L.P.; Ruiz, M.A.; Tanaka, P.Y.; Sobreira, J.T.; Cançado, R.D.; De Barros, J.C. Assessment of Labile Plasma Iron in Patients Who Undergo Hematopoietic Stem Cell Transplantation. Acta Haematol. 2014, 131, 222–226. [Google Scholar] [CrossRef]
- Pullarkat, V.; Blanchard, S.; Tegtmeier, B.; Dagis, A.; Patane, K.; Ito, J.; Forman, S.J. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008, 42, 799–805. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Breuer, W.; Zanninelli, G.; Cianciulli, P. LPI-labile plasma iron in iron overload. Best Pr. Res. Clin. Haematol. 2005, 18, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Koren, A.; Fink, D.; Admoni, O.; Tennenbaum-Rakover, Y.; Levin, C. Non-transferrin bound labile plasma iron and iron overload in Sickle Cell Disease: A comparative study between Sickle Cell Disease and β thalassemic patients. Eur. J. Haematol. 2010, 84, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Esposito, B.P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z.I. Labile plasma iron in iron overload: Redox activity and susceptibility to chelation. Blood 2003, 102, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pr. Res. Clin. Haematol. 2005, 18, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, Y.Z. New diagnostic tools for delineating iron status. Hematology 2019, 2019, 327–336. [Google Scholar] [CrossRef]
- Babitt, J.L.; Huang, F.W.; Xia, Y.; Sidis, Y.; Andrews, N.; Lin, H.Y. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Investig. 2007, 117, 1933–1939. [Google Scholar] [CrossRef]
- Zhang, A.-S. Control of Systemic Iron Homeostasis by the Hemojuvelin-Hepcidin Axis. Adv. Nutr. 2010, 1, 38–45. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef]
- Gupta, A.; Damania, R.C.; Talati, R.; O’Riordan, M.A.; Matloub, Y.H.; Ahuja, S.P. Increased Toxicity Among Adolescents and Young Adults Compared with Children Hospitalized with Acute Lymphoblastic Leukemia at Children’s Hospitals in the United States. J. Adolesc. Young Adult Oncol. 2021. [Google Scholar] [CrossRef]
- Safniyat, S.; Shakibazad, N.; Haghpanah, S.; Amoozegar, H.; Karimi, M.; Safaei, S.; Safniyat, S.; Mohammadi, H.; Zekavat, O.R. Parameters of tissue iron overload and cardiac function in patients with thalassemia major and intermedia. Acta Haematol. Pol. 2020, 51, 95–101. [Google Scholar] [CrossRef]
- Bennett, T.; Hayward, K.N.; Farris, R.W.D.; Ringold, S.; Wallace, C.A.; Brogan, T.V. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr. Crit. Care Med. 2011, 12, e233–e236. [Google Scholar] [CrossRef] [PubMed]
- Bazuave, G.N.; Buser, A.; Gerull, S.; Tichelli, A.; Stern, M. Prognostic impact of iron parameters in patients undergoing allo-SCT. Bone Marrow Transplant. 2011, 47, 60–64. [Google Scholar] [CrossRef]
- Łęcka, M.; Czyżewski, K.; Dębski, R.; Wysocki, M.; Styczyński, J. Impact of ferritin serum concentration on survival in children with acute leukemia: A long-term follow-up. Acta Haematol. Pol. 2021, 52, 54–60. [Google Scholar] [CrossRef]
- Sammarco, M.C.; Ditch, S.; Banerjee, A.; Grabczyk, E. Ferritin L and H Subunits Are Differentially Regulated on a Post-transcriptional Level. J. Biol. Chem. 2008, 283, 4578–4587. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, D.; Arosio, P. Biology of ferritin in mammals: An update on iron storage, oxidative damage and neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Plays, M.; Müller, S.; Rodriguez, R. Chemistry and biology of ferritin. Metallomics 2021, 13. [Google Scholar] [CrossRef]
| Characteristics | Total (%) (n = 85) | Group I (n = 18) | Group II (n = 21) | Group III (n = 25) | Group IV (n = 21) |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median (range) | 7 (0–19) | 8 (2–16) | 7 (0–17) | 5 (1–19) | 8 (1–19) |
| Years: <10 vs. >10 | 59 (69.5):26 (30.5) | 11 (61.1):7 (38.9) | 16 (76.2):5 (23.8) | 23 (92.0):2 (8.0) | 9 (42.8):12 (57.2) |
| Gender | |||||
| male/female (%) | 45 (52.9):40 (45.1) | 8 (44.4):10 (55.6) | 12 (57.2):9 (48.8) | 13 (52.0):12 (48.0) | 12 (57.2):9 (42.8) |
| Diagnosis | |||||
| ALL (%) | 48 (56.4) | 0 | 19 (90.4) | 23 (92.0) | 6 (28.6) |
| AML (%) | 12 (0.1) | 0 | 2 (9.5) | 2 (8.0) | 8 (38.1) |
| Other (%) | 25 (0.3) | 18 (100.0) | 0 | 0 | 7 (33.3) |
| HCT (%) | 21 (0.2) | 0 | 0 | 0 | 21 (100) |
| PRBC transfusions | |||||
| >5 units (%) | 0 | 2 (9.5) | 23 (92.0) | 21 (100) | |
| >10 units (%) | 0 | 1 (4.8) | 11 (44.0) | 19 (90.4) | |
| >20 units (%) | 0 | 0 | 2 (8.0) | 15 (71.4) |
| Kit | Manufacturer | Assay Range | Detection Limit | Intra-Assay Coefficient of Variation (CV, %) | Inter-Assay Coefficient of Variation (CV, %) |
|---|---|---|---|---|---|
| Intrinsic Hepcidin IDx™ ELISA Kit (ICE-007) | Intrinsic Life Sciences (La Jolla, CA, USA) | 5–250 ng/mL | 2.5 ng/mL | 2.4–4.0 | 2.6–3.8 |
| Intrinsic Erythroferrone IE™ ELISA Kit (ERF-001) | 0.16–10 ng/mL | 0.02 ng/mL | 4.7–6.7 | 7.0–14.9 | |
| ELISA Kit for Hemojuvelin (HJV) (CEB995Hu) | Cloud-Clone Corp. (Katy, TX, USA) | 12.35–1000 ng/mL | 4.93 ng/mL | <10 | <12 |
| Erythropoietin Human ELISA (RAF013R) | BioVendor-Laboratorni medicina a.s., (Brno, Czech Republic) | 1.6–100 mIU/mL | 0.14 mIU/mL | 6.2 | 4.3 |
| sTfR Human ELISA (soluble Transferrin Receptor) (RD194011100) | 0.05–2 µg/mL | 0.002 µg/mL | 6.8 | 6.3 | |
| Transferrin Human ELISA Kit (EHTF) | Thermo Fisher Scientific (Waltham, MA, USA) | 1.029–750 ng/mL | 1 ng/mL | <10 | <12 |
| Human SLC40A1/Ferroportin-1 (Sandwich ELISA) ELISA Kit (LS-F33705) | LifeSpan BioSciences, Inc. (Seattle, WA, USA) | 0.156–10 ng/mL | 0.094 ng/mL | <8 | <10 |
| Human FTH1/Ferritin Heavy Chain (Sandwich ELISA) ELISA Kit (LS-F26901) | 50–1000 pg/mL | 1 pg/mL | <9 | <10 | |
| Human FTL / Ferritin Light Chain (Sandwich ELISA) ELISA Kit (LS-F26902) | 100–2500 pg/mL | 1 pg/mL | <10 | <12 |
| Parameters | Controls (Group I) | Acute Leukemia de Novo (Group II) | Acute Leukemia after Intensive Chemotherapy (Group III) | After HCT (Group IV) | p-Value |
|---|---|---|---|---|---|
| PRBC transfusions (units) median (range) | 0 (0–0) | 1 (0–10) | 9 (2–35) | 23 (6–99) | I vs. II; p < 0.001 I vs. III; p < 0.001 I vs. IV; p < 0.001 II vs. III; p < 0.001 II vs. IV; p < 0.001 III vs. IV; p < 0.001 |
| NTBI positive (number) | 0/18 (0%) | 3/21 (14.3%) | 3/25 (12.0%) | 6/21 (28.6%) | I vs. II; p = 0.459 I vs. III; p = 0.058 I vs. IV; p = 0.012 II vs. III; p = 0.272 II vs. IV; p = 0.079 III vs. IV; p = 0.359 |
| LPI positive (number) | 2/18 (11.1%) | 5/21 (23.8%) | 6/25 (24.0%) | 10/21 (47.6%) | I vs. II; p = 0.820 I vs. III; p = 0.398 I vs. IV; p = 0.018 II vs. III; p = 0.666 II vs. IV; p = 0.063 III vs. IV; p = 0.062 |
| Serum iron (mg/dL) median (range) | 69.2 (20.10–97.40) | 125.05 (40.40–259.00) | 103.6 (10.00–236.70) | 128.40 (41.90–265.40) | I vs. II; p = 0.001 I vs. III; p = 0.007 I vs. IV; p = 0.001 II vs. III; p = 0.424 II vs. IV; p = 0.754 III vs. IV; p = 0.295 |
| Transferrin (ng/mL) median (range) | 32.11 (10.94–750.00) | 32.95 (7.59–750.00) | 38.00 (12.16–260.30) | 23.36 (6.57–94.54) | I vs. II; p = 0.481 I vs. III; p = 0.109 I vs. IV; p = 0.371 II vs. III; p = 0.316 II vs. IV; p = 0.076 III vs. IV; p = 0.011 |
| TIBC (µg/L) median (range) | 356.00 (292.00–404.00) | 276.50 (125.00–329.00) | 262.50 (183.00–328.00) | 234.00 (128.00–434.00) | I vs. II; p < 0.001 I vs. III; p < 0.001 I vs. IV; p < 0.001 II vs. III; p = 0.316 II vs. IV; p = 0.004 III vs. IV; p = 0.004 |
| Ferritin (µg/L) median (range) | 27.40 (11.00–73.30) | 238.50 (14.20–1660.00) | 739.00 (26.40–5278.00) | 3670.00 (51.10–12,000.00) | I vs. II; p < 0.001 I vs. III; p < 0.001 I vs. IV; p < 0.001 II vs. III; p = 0.069 II vs. IV; p < 0.001 III vs. IV; p < 0.001 |
| FTH1 (pg/mL) median (range) | 16.45 (0.54–70.05) | 24.45 (1.00–137.90) | 18.81 (0.50–132.00) | 22.76 (1.00–309.40) | I vs. II; p = 0.091 I vs. III; p = 0.481 I vs. IV; p = 0.528 II vs. III; p = 0.275 II vs. IV; p = 0.345 III vs. IV; p = 0.930 |
| FTL (pg/mL) median (range) | 94.65 (41.09–571.80) | 129.30 (44.45–363.00) | 113.30 (3.86–286.10) | 117.00 (20.40–301.70) | I vs. II; p = 0.547 I vs. III; p = 0.990 I vs. IV; p = 0.918 II vs. III; p = 0.372 II vs. IV; p = 0.372 III vs. IV; p = 0.991 |
| Hepcidin (ng/mL) median (range) | 30.61 (14.55–468.20) | 158.50 (21.69–738.60) | 106.60 (17.26–383.20) | 278.30 (22.15–1000.00) | I vs. II; p = 0.001 I vs. III; p = 0.013 I vs. IV; p < 0.001 II vs. III; p = 0.087 II vs. IV; p = 0.031 III vs. IV; p < 0.001 |
| sHJV (ng/mL) median (range) | 65.58 (49.02–91.47) | 52.77 (27.33–88.23) | 57.95 (24.78–136.80) | 40.62 (18.34–98.41) | I vs. II; p = 0.029 I vs. III; p = 0.025 I vs. IV; p < 0.001 II vs. III; p = 0.635 II vs. IV; p = 0.051 III vs. IV; p = 0.007 |
| FNP (pg/mL) median (range) | 76.46 (41.94–251.90) | 80.03 (29.12–924.70) | 74.20 (38.57–618.20) | 110.00 (37.69–1250.00) | I vs. II; p = 0.872 I vs. III; p = 0.990 I vs. IV; p = 0.212 II vs. III; p = 0.921 II vs. IV; p = 0.213 III vs. IV; p = 0.310 |
| Erythroferrone (ERFE) (ng/mL) median (range) | 0.83 (0.30–10.00) | 1.29 (0.23–10.00) | 0.69 (0.14–10.00) | 1.26 (0.13–10.00) | I vs. II; p = 0.290 I vs. III; p = 0.191 I vs. IV; p = 0.837 II vs. III; p = 0.024 II vs. IV; p = 0.279 III vs. IV; p = 0.494 |
| EPO (mIU/mL) median (range) | 5.26 (0.45–12.73) | 44.85 (1.81–100.00) | 12.73 (5.31–100.00) | 12.32 (3.10–100.00) | I vs. II; p < 0.001 I vs. III; p < 0.001 I vs. IV; p < 0.001 II vs. III; p = 0.004 II vs. IV; p < 0.001 III vs. IV; p = 0.372 |
| STfR (µg/mL) median (range) | 0.12 (0.07–0.29) | 0.11 (0.09–0.52) | 0.11 (0.08–0.71) | 0.09 (0.07–0.32) | I vs. II; p = 0.340 I vs. III; p = 0.918 I vs. IV; p = 0.020 II vs. III; p = 0.480 II vs. IV; p = 0.024 III vs. IV; p = 0.012 |
| CRP (mg/L) median (range) | 0.49 (0.16–2.34) | 6.68 (0.37–156.50) | 0.50 (0.16–36.37) | 3.51 (0.22–297.54) | I vs. II; p < 0.001 I vs. III; p = 0.739 I vs. IV; p < 0.001 II vs. III; p < 0.001 II vs. IV; p = 0.580 III vs. IV; p < 0.001 |
| PCT (ng/mL) median (range) | 0.02 (0.02–0.10) | 0.10 (0.01–2.82) | 0.05 (0.00–0.36) | 0.16 (0.02–11.47) | I vs. II; p < 0.001 I vs. III; p = 0.002 I vs. IV; p < 0.001 II vs. III; p = 0.005 II vs. IV; p = 0.669 III vs. IV; p = 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łęcka, M.; Słomka, A.; Albrecht, K.; Żekanowska, E.; Romiszewski, M.; Styczyński, J. Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis. Cancers 2021, 13, 3029. https://doi.org/10.3390/cancers13123029
Łęcka M, Słomka A, Albrecht K, Żekanowska E, Romiszewski M, Styczyński J. Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis. Cancers. 2021; 13(12):3029. https://doi.org/10.3390/cancers13123029
Chicago/Turabian StyleŁęcka, Monika, Artur Słomka, Katarzyna Albrecht, Ewa Żekanowska, Michał Romiszewski, and Jan Styczyński. 2021. "Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis" Cancers 13, no. 12: 3029. https://doi.org/10.3390/cancers13123029
APA StyleŁęcka, M., Słomka, A., Albrecht, K., Żekanowska, E., Romiszewski, M., & Styczyński, J. (2021). Unbalance in Iron Metabolism in Childhood Leukemia Converges with Treatment Intensity: Biochemical and Clinical Analysis. Cancers, 13(12), 3029. https://doi.org/10.3390/cancers13123029







