Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies
Abstract
Simple Summary
Abstract
1. Introduction
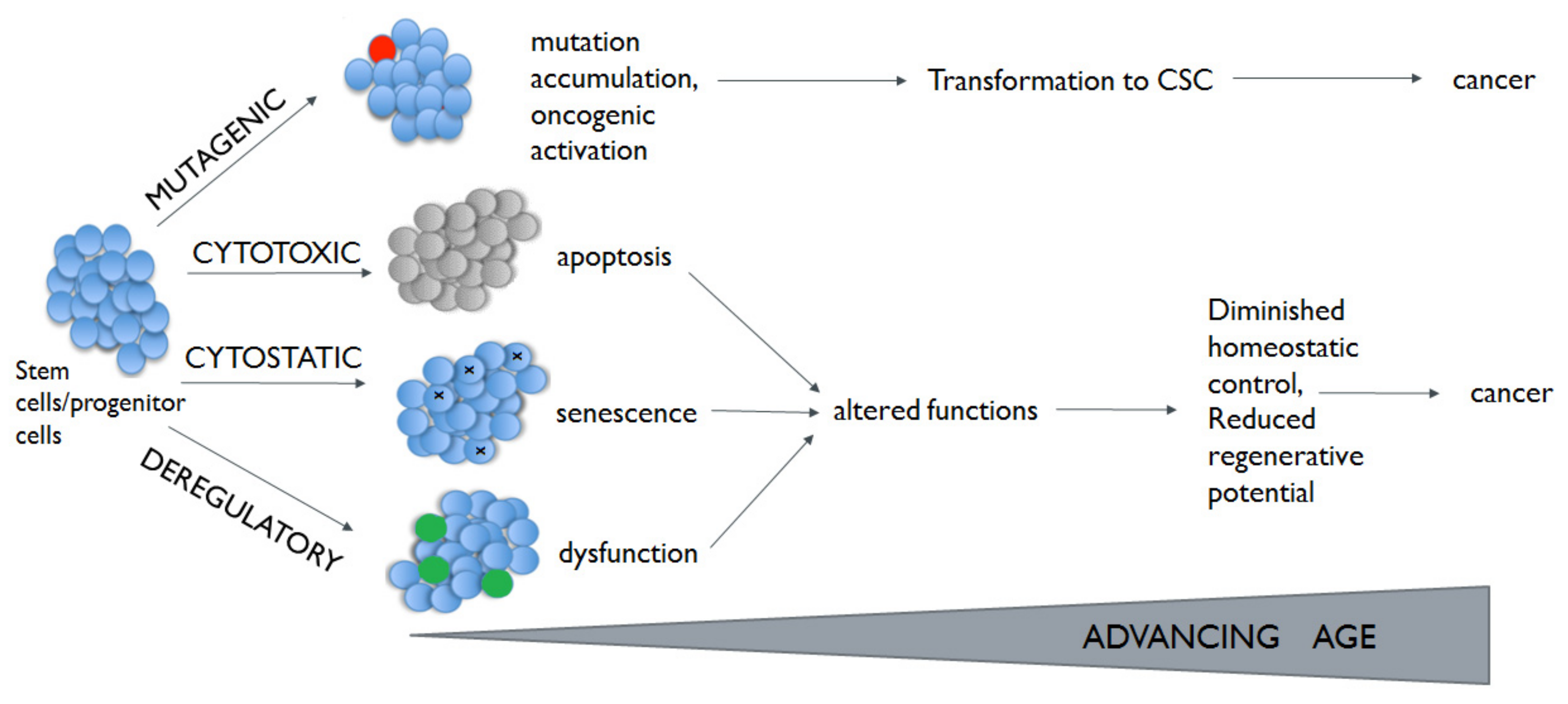
2. Lung Cancer and Aging
3. Lung Cancer Stem Cells
- EpCAM—transmembrane glycoprotein expressed in most human carcinomas; identified as a marker for carcinoma; can be attributed to its high expression on rapidly proliferating tumors of epithelial origin [52].
- CD133—a marker frequently used for identification of stem cells in both cancer and normal tissues. The process of CD133 transcription is regulated by five promoters, and promoter 5–P5 seems to play crucial role by CD133 expression in CSCs [53]. Some research has characterized CD133+ cells in NSCLCs [7,52]. For example, Eramo et al. showed that CD133 was present in a variable, but small number of NSCLCs, usually limited to <1% of cells [53]. CD133+ cells were capable in approximately 30% of cases to form tumor spheres in vitro when grown in serum-free medium; CD133+ cells derived from tumor spheres are capable to induce tumors when inoculated into immunodeficient mice with histological features similar to those of the original tumor [53]. Moreover, CD133+ positive cells display resistance to chemotherapy as a result of expressing high levels of ATP-binding cassette G2 [54].
- CD44—a transmembrane glycoprotein that binds hyaluronic acid, an abundant polysaccharide in stem cells. CD44 is responsible for various signaling functions (cell differentiation, survival, apoptosis, migration and proliferation). Some current studies revealed that CD44 plays a crucial role in CSC function such as self-renewal, resistance to apoptosis and niche preparation [7,55]. It has been shown that the mutations of p53 may be linked with up-regulation of CD44, leading to the promotion of CD44+ cells [56]. CD44+ cells demonstrate the ability to form spheroid bodies in vitro [57]. Additionally, cells with CD44 + phenotype are capable of forming a tumor mass in vivo in immunodeficient mice [51,58].
- CD90—a glycosylphosphatidylinositol-anchored glycoprotein is expressed mainly in white blood cells and is involved in cell–matrix and cell–cell interactions. Though, CD90 has been described as a marker for different types of CSCs, the potential role of CD90 as a marker for lung CSCs has not yet been fully described [7,49]. It has been reported that CSCs with co-expression of CD44 and CD90 could be detected in primary lung cell lines [56]. Up to date, the mutations that activate the CD90 expression are unknown. Studies performed on mice model suggest that the DNA methylation has a role in promoting CD90 expression. Serial xenotransplantation of EpCAM+ CD90+ cells in immunodeficient mice revealed a rapid growth of EpCAM+ cells in the subcutaneous lesion and a highly metastatic capacity of CD90+ cells in the lung [51].
- CXCR4—a chemokine receptor present on the surface of hematopoietic stem cells involved in trapping of these cells in the stem cell niches [59]. The CXCR4/CXCL12 pathway is responsible for tumor metastasis, progression, induction of angiogenesis, and resistance to apoptosis. Moreover, CXCR4 is presented on circulating tumor cells released from tumors into the peripheral blood, which induces their spread to CXCL12-positive distant sites [60]. The expression of CXCR4 is regulated by the Nuclear Respiratory Factor—NRF. NRF mutation may lead to the higher expression of CXCR4 [61]. CXCR4+ cells isolated from NSCLC lines were able to form the tumor spheres in vitro, had self-renewal capacity, demonstrated radiation resistance in vitro [62].
4. Cancer Stem Cells and Tumor Microenvironment
5. Liquid Biopsy
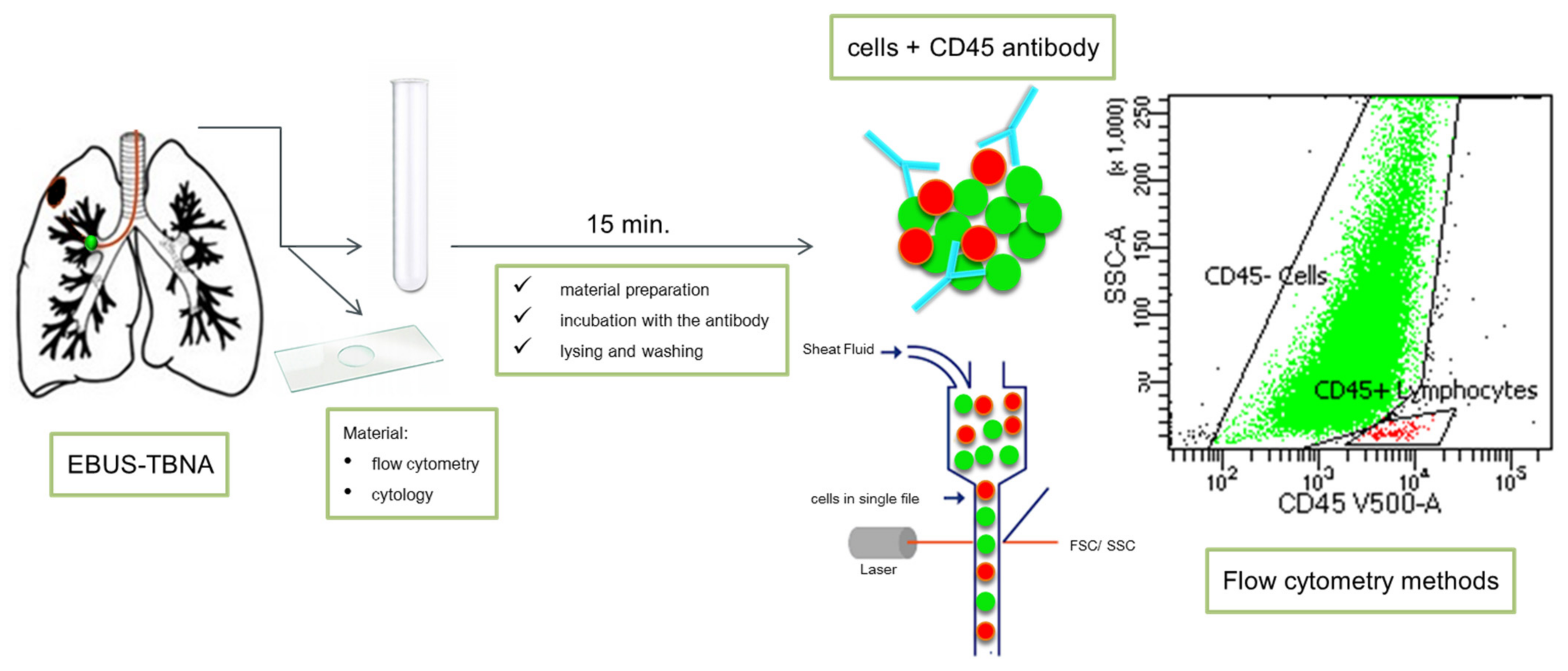
6. Endobronchial Ultrasound-Guided Trans-Bronchial Needle Aspiration (EBUS-TBNA)
7. Bronchoalveolar Lavage (BAL)
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.F.; Goggins, W.B.; Tse, S. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Wenmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 Europeancohorts: Prospective analyses from the European Study of cohorts for air pollution effects [ESCAPE]. Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. WHO panel. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Inamura, K. Lung cancer: Understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung cancers: Molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J. New frontiers for molecular pathology. Front. Med. 2019, 4, 284. [Google Scholar] [CrossRef]
- Levy, M.A.; Lovly, C.M.; Pao, W. Translating genomic information into clinical medicine: Lung cancer as a paradigm. Genome Res. 2012, 22, 2101–2108. [Google Scholar] [CrossRef]
- Choi, W.I.; Jeong, J.; Lee, C.W. Association between EGFR mutation and ageing, history of pneumonia and gastroesophageal reflux disease among patients with advanced lung cancer. Eur. J. Cancer 2019, 122, 101–108. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, J.K.; Kang, H.J.; Lee, T.S.; Kim, H.R.; Kim, C.H.; Koh, J.S.; Baek, H.J.; Lee, J.C.; Na, I.I. Association between age at diagnosis and the presence of EGFR mutations in female patients with resected non-small cell lung cancer. J. Thorac. Oncol. 2010, 12, 1949–1952. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe-Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny-Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora, J.E.; et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 38, 2020–2031. [Google Scholar] [CrossRef]
- Jenkins, S.; Yang, J.C.; Ramalingam, S.S.; Yu, K.; Patel, S.; Weston, S.; Hodge, R.; Cantarini, M.; Janne, P.A.; Mitsudomi, T.; et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 1061–1070. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A statement paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with ate-zolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef]
- Nakagawa, K.; Garon, E.B.; Seto, T.; Nishio, M.; Ponce-Aix, S.; Paz-Ares, L.; Chiu, C.H.; Park, K.; Novello, S.; Nadal, E. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, dou-ble-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1655–1669. [Google Scholar] [CrossRef]
- Peters, S.; Ramalingam, S.S.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; Sakai, H. Nivolumab + low-dose ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non–small cell lung cancer: CheckMate-227 part 1 final analysis. Ann. Oncol. 2019, 30 (Suppl. S5), v851–v934. [Google Scholar] [CrossRef]
- Camidge, R.; Kim, H.R.; Ahn, M.; Yang, J.C.; Han, J.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Garcia-Campelo, M.R.; Kim, D. Brigatinib vs. crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: Updated results from the phase III ALTA-1L trial. Ann. Oncol. 2019, 30 (Suppl. S9). [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Riely, G.J.; Bang, Y.J.; Kim, D.W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated re-sults, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Kim, H.R.; Lee, J.S.; Lee, K.H.; Lee, Y.G.; Min, Y.J.; Cho, E.K.; Lee, S.S.; Kim, B.S.; Choi, M.Y.; et al. Open-label, multicenter, phase II study of ceritinib in patients with non–small-cell lung cancer harboring ROS1 rearrangement. J. Clin. Oncol. 2017, 35, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.; Wirth, L.; Besse, B.; Gautschi, O.; Tan, S.W.D.; Loong, H.; Bauer, T.; Kim, Y.J.; Horiike, A.; et al. PL02.08 registrational results of LI-BRETTO-001: A phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J. Thorac. Oncol. 2019, 14, S6–S7. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Revisiting the antagonistic pleiotropy theory of ageing: TOR-driven program and quasi-program. Cell Cycle 2010, 9, 3151–3156. [Google Scholar] [CrossRef]
- Lezzerini, M.; Smith, R.L.; Budovskaya, Y. Developmental drift as a mechanism for ageing: Lessons from nematodes. Biogerontology 2013, 14, 693–701. [Google Scholar] [CrossRef]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–2554. [Google Scholar] [CrossRef]
- Yu, B.; Chang, J.; Liu, Y.; Li, J.; Kevork, K.; Al-Hezaimi, K.; Graves, D.T.; Park, N.H.; Wang, C.Y. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB. Nat. Med. 2014, 20, 1009–1017, Erratum in: 2015, 21, 1101. [Google Scholar] [CrossRef]
- Castilho, R.M.; Squarize, C.H.; Chodosh, L.A.; Williams, B.O.; Gutkind, J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009, 5, 279–289. [Google Scholar] [CrossRef]
- Kirkwood, T.B. Evolution of ageing. Nature 1977, 270, 301–304. [Google Scholar] [CrossRef]
- Santos Franco, S.; Raveh-Amit, H.; Kobolák, J.; Alqahtani, M.H.; Mobasheri, A.; Dinnyes, A. The crossroads between cancer stem cells and aging. BMC Cancer 2015, 15 (Suppl. S1). [Google Scholar] [CrossRef]
- Lee, G.; Hall, R.R., III; Ahmed, A.U. Cancer stem cells: Cellular plasticity, niche, and its clinical relevance. J. Stem. Cell Res. Ther. 2016, 6, 363. [Google Scholar] [CrossRef]
- Ascolani, G.; Liò, P. Modeling breast cancer progression to bone: How driver mutation order and metabolism matter. BMC Med. Genom. 2019, 12 (Suppl. S6). [Google Scholar] [CrossRef]
- Hwang, J.H.; Yoon, J.; Cho, Y.H.; Cha, P.H.; Park, J.C.; Choi, K.Y. A mutant KRAS-induced factor REG4 promotes cancer stem cell properties via Wnt/β-catenin signaling. Int. J. Cancer 2020, 146, 2877–2890. [Google Scholar] [CrossRef]
- Klevebring, D.; Rosin, G.; Ma, R.; Lindberg, J.; Czene, K.; Kere, J.; Fredriksson, I.; Bergh, J.; Hartman, J. Sequencing of breast cancer stem cell populations indicates a dynamic conversion between differentiation states in vivo. Breast Cancer Res. 2014, 16. [Google Scholar] [CrossRef]
- Prado, K.; Zhang, K.X.; Pellegrini, M.; Chin, A.I. Sequencing of cancer cell subpopulations identifies micrometastases in a bladder cancer patient. Oncotarget 2017, 8, 45619–45625. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Hung, R.J.; Rafnar, T.; Christiani, D.C.; Field, J.K.; Bickeböller, H.; Risch, A.; McKay, J.D.; Wang, Y.; Dai, J.; et al. Transdisciplinary research in cancer of the lung (TRICL) research team. Influence of common genetic variation on lung cancer risk: Meta-analysis of 14 900 cases and 29 485 controls. Hum. Mol. Genet. 2012, 21, 4980–4995. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- Frías, C.; García-Aranda, C.; De Juan, C.; Morán, A.; Ortega, P.; Gómez, A.; Hernando, F.; López-Asenjo, J.A.; Torres, A.J.; Benito, M.; et al. Telomere shortening is associated with poor prognosis and telomerase activity correlates with DNA repair impairment in non-small cell lung cancer. Lung Cancer 2008, 60, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Jakopovic, M.; Thomas, A.; Balasubramaniam, S.; Schrump, D.; Giaccone, G.; Bates, S.E. Targeting the epigenome in lung cancer: Expanding approaches to epigenetic therapy. Front. Oncol. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.A.; Lam, D.C.; O’Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5). [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Cox, T.R. The role of the ECM in lung cancer dormancy and outgrowth. Front. Oncol. 2020, 10, 1766. [Google Scholar] [CrossRef]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef]
- Hardavella, G.; George, R.; Sethi, T. Lung cancer stem cells-characteristics, phenotype. Transl. Lung Cancer Res. 2016, 5, 272–279. [Google Scholar] [CrossRef]
- Barr, M.P.; Gray, S.G.; Hoffmann, A.C.; Hilger, R.A.; Thomale, J.; O’Flaherty, J.D.; Fennell, D.A.; Richard, D.; O’Leary, J.J.; O’Byrne, K.J. Generation and characterisation of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PLoS ONE 2013, 8, e54193, Erratum in: 2020, 21, e0233739. [Google Scholar] [CrossRef]
- Zhang, W.C.; Shyh-Chang, N.; Yang, H.; Rai, A.; Umashankar, S.; Ma, S.; Soh, B.S.; Sun, L.L.; Tai, B.C.; Nga, M.E. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012, 20, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; de-Maya-Girones, J.D.; Calabuig-Fariñas, S.; Lucas, R.; Martínez, A.; Pardo-Sánchez, J.M.; Alonso, S.; Blasco, A.; Guijarro, R.; Martorell, M. Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Ohnishi, S.; Maehara, O.; Nakagawa, K.; Kameya, A.; Otaki, K.; Fujita, H.; Higashi, R.; Takagi, K.; Asaka, M.; Sakamoto, N.; et al. hypoxia-inducible factors activate CD133 promoter through ETS family transcription factors. PLoS ONE 2013, 8, e66255. [Google Scholar] [CrossRef]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; DeMaria, R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008, 15, 504–514. [Google Scholar] [CrossRef]
- Alamgeer, M.; Peacock, C.D.; Matsui, W.; Ganju, V.; Watkins, D.N. Cancer stem cells in lung cancer: Evidence and controversies. Respirology 2013, 18, 757–764. [Google Scholar] [CrossRef]
- Hou, Y.C.; Chao, Y.J.; Hsieh, M.H.; Tung, H.L.; Wang, H.C.; Shan, Y.S. Low CD8+ T cell infiltration and high PD-L1 expression are associated with level of CD44+/CD133+ cancer stem cells and predict an unfavorable prognosis in pancreatic cancer. Cancers 2019, 11, 541. [Google Scholar] [CrossRef]
- Godar, S.; Ince, T.A.; Bell, G.W.; Feldser, D.; Donaher, J.L.; Bergh, J.; Liu, A.; Miu, K.; Watnick, R.S.; Reinhardt, F.; et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008, 134, 62–73. [Google Scholar] [CrossRef]
- Leung, E.L.; Fiscus, R.R.; Tung, J.W.; Tin, V.P.; Cheng, L.C.; Sihoe, A.D.; Fink, L.M.; Ma, Y.; Wong, M.P. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS ONE 2010, 5, e14062. [Google Scholar] [CrossRef]
- Eckert, F.; Schilbach, K.; Klumpp, L.; Bardoscia, L.; Sezgin, E.C.; Schwab, M.; Zips, D.; Huber, S.M. Potential role of CXCR4 targeting in the context of radiotherapy and immunotherapy of cancer. Front. Immunol. 2018, 21, 3018. [Google Scholar] [CrossRef]
- Trautmann, F.; Cojoc, M.; Kurth, I.; Melin, N.; Bouchez, L.C.; Dubrovska, A.; Peitzsch, C. CXCR4 as biomarker for radioresistant cancer stem cells. Int. J. Radiat. Biol. 2014, 90, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Gzil, A.; Zarębska, I.; Bursiewicz, W.; Antosik, P.; Grzanka, D.; Szylberg, Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol. Biol. Rep. 2019, 46, 6629–6645. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-J.; Rho, J.-K.; Kim, Y.-M.; Jung, J.E.; Jin, Y.B.; Ko, Y.-G.; Lee, J.C.; Lee, S.-J.; Park, M.-J. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 2013, 32, 209–221. [Google Scholar] [CrossRef]
- Raniszewska, A.; Polubiec-Kownacka, M.; Rutkowska, E.; Domagała-Kulawik, J. PD-L1 expression on lung cancer stem cells in metastatic lymph nodes aspirates. Stem Cell Rev. 2019, 15, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Raniszewska, A.; Vroman, H.; Dumoulin, D.; Cornelissen, R.; Aerts, J.G.J.V.; Domagała-Kulawik, J. PD-L1+ lung cancer stem cells modify the metastatic lymph-node immunomicroenvironment in nsclc patients. Cancer Immunol. Immunother. 2021, 70, 453–461. [Google Scholar] [CrossRef]
- Raniszewska, A.; Kwiecień, I.; Sokołowski, R.; Rutkowska, E.; Domagała-Kulawik, J. Immunomodulatory molecules on lung cancer stem cells from lymph nodes aspirates. Cancers 2020, 12, 838. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Sotgia, F.; Lisanti, M.P. Cancer stem cells (CSCs): Metabolic strategies for their identification and eradication. Biochem. J. 2018, 475, 1611–1634. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Chung, M.-I.; Fioret, B.; Gao, X.; Katsura, H.; Hogan, B.L.M. Lung organoids: Current uses and future promise. Development 2017, 144, 986–997. [Google Scholar] [CrossRef]
- Matano, M.; Date, S.; Shimokawa, M.; Takano, A.; Fujii, M.; Ohta, Y.; Watanabe, T.; Kanai, T.; Sato, T. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 2015, 21, 256–262. [Google Scholar] [CrossRef]
- Nagle, P.W.; Plukker, J.T.M.; Muijs, C.T.; van Luijk, P.; Coppes, R.P. Patient-derived tumor organoids for prediction of cancer treatment response. Semin. Cancer Biol. 2018, 53, 258–264. [Google Scholar] [CrossRef]
- Shimono, Y.; Mukohyama, J.; Isobe, T.; Johnston, D.M.; Dalerba, P.; Suzuki, A. Organoid culture of human cancer stem cells. In Organoids, Methods in Molecular Biology; Turksen, K., Ed.; Humana: New York, NY, USA, 2016; Volume 1576. [Google Scholar]
- Morrison, R.; Schleicher, S.M.; Sun, Y.; Niermann, K.J.; Kim, S.; Spratt, D.E. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J. Oncol. 2011, 2011. [Google Scholar] [CrossRef]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2010, 8, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Driscoll, B. Lung cancer stem cells: Progress and prospects. Cancer Lett. 2013, 338, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Sanchez-Rivera, F.J.; Cetinbas, N.M.; Wu, K.; Joshi, N.S.; Helenius, K.; Park, Y.; Azimi, R.; Kerper, N.R.; Wesselhoeft, R.A.; et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Bocchetta, M. Notch signaling in lung cancer. Exp. Rev. Anticancer Ther. 2011, 11, 533–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Guo, H.; Zhang, Y.; He, Y.; Lee, S.H.; Song, X.; Li, X.; Guo, Y.; Zhao, Y.; et al. NOTCH1 signaling regulates self-renewal and platinum chemoresistance of cancer stem-like cells in human non-small cell lung cancer. Cancer Res. 2017, 77, 3082–3091. [Google Scholar] [CrossRef]
- Hassan, K.A.; Wang, L.; Korkaya, H.; Chen, G.; Maillard, I.; Etherton-Beer, C.; Kalemkerian, G.P.; Wicha, M.S. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 1972–1980. [Google Scholar] [CrossRef]
- Giroux-Leprieur, E.; Costantini, A.; Ding, V.W.; He, B. Hedgehog signaling in lung cancer: From oncogenesis to cancer treatment resistance. Int. J. Mol. Sci. 2018, 19, 2835. [Google Scholar] [CrossRef]
- Cochrane, C.R.; Szczepny, A.; Watkins, D.N.; Cain, J.E. Hedgehog signaling in the maintenance of cancer stem cells. Cancers 2015, 7, 1554–1585. [Google Scholar] [CrossRef]
- Ogino, S.; Galon, J.; Fuchs, C.S.; Dranoff, G. Cancer immunology--analysis of host and tumorfactors for personalized medicine. Nat. Rev. Clin. Oncol. 2011, 8, 711–719. [Google Scholar] [CrossRef]
- Attili, I.; Tarantino, P.; Passaro, A.; Stati, V.; Curigliano, G.; de Marinis, F. Strategies to overcome resistance to immune checkpoint blockade in lung cancer. Lung Cancer 2021, 3, 151–160. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 7, 59. [Google Scholar] [CrossRef]
- Zhang, D.G.; Tang, K.; Rycaj, K. Cancer stem cells: Regulation programs, immunological properties and immunotherapy. Semin. Cancer Biol. 2018, 52, 94–106. [Google Scholar] [CrossRef]
- Maccalli, C.; Parmiani, G.; Ferrone, S. Immunomodulating and immunoresistance properties of cancer-initiating cells: Implications for the clinical success of immunotherapy. Immunol. Investig. 2017, 46, 221–238. [Google Scholar] [CrossRef]
- Maccalli, C.; Volonte, A.; Cimminiello, C.; Parmiani, G. Immunology of cancer stem cells in solid tumours. A review. Eur. J. Cancer 2014, 50, 649–655. [Google Scholar] [CrossRef]
- Park, T.S.; Donnenberg, V.S.; Donnenberg, A.D.; Zambidis, E.T.; Zimmerlin, L. Dynamic interactions between cancer stem cells and their stromal partners. Curr. Pathobiol. Rep. 2014, 2, 41–52. [Google Scholar] [CrossRef]
- Quian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Zhang, C.H.; Guo, F.L.; Xu, G.L.; Jia, W.D.; Ge, Y.S. STAT3 activation mediates epithelial-to-mesenchymal transition in human hepatocellular carcinoma cells. Hepatogastroenterology 2014, 61, 1082–1089. [Google Scholar]
- Miranda-Lorenzo, I.; Dorado, J.; Lonardo, E.; Alcala, S.; Serrano, A.G.; Clausell-Tormos, J.; Cioffi, M.; Megias, D.; Zagorac, S.; Balic, A.; et al. Intracellular autofluorescence: A biomarker for epithelial cancer stem cells. Nat. Methods 2014, 11, 1161–1169. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Rezalotfi, A.; Ahmadian, E.; Aazami, H.; Solgi, G.; Ebrahimi, M. Gastric cancer stem cells effect on Th17/treg balance; A bench to beside perspective. Front. Oncol. 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.Q. Adoptive t-cell therapy of prostate cancer targeting the cancer stem cell antigen epcam. BMC Immunol. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.; Ebrahimi, M.; Hadjati, J.; Memarnejadian, A.; Moazzeni, S.M. Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett. 2016, 374, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tao, H.; Chang, A.E.; Hu, Y.; Shu, G.; Chen, Q.; Egenti, M.; Owen, J.; Moyer, J.S.; Prince, M.E.; et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology 2015, 4, e990767. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Kochin, V.; Kanaseki, T.; Hongo, A.; Tokita, S.; Kikuchi, Y.; Takaya, A.; Hirohashi, Y.; Tsukahara, T.; Terui, T.; et al. The antigen asb4 on cancer stem cells serves as a target for ctl immunotherapy of colorectal cancer. Cancer Immunol. Res. 2018, 6, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Sato-Dahlman, M.; Miura, Y.; Huang, J.L.; Hajeri, P.; Jacobsen, K.; Davydova, J.; Yamamoto, M. Cd133-targeted oncolytic adenovirus demonstrates anti-tumor effect in colorectal cancer. Oncotarget 2017, 8, 76044–76056. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Komorowski, M.P.; Seshadri, M.; Rokita, H.; McGray, A.J.; Opyrchal, M.; Odunsi, K.O.; Kozbor, D. Cxcl12/cxcr4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J. Immunol. 2014, 193, 5327–5337. [Google Scholar] [CrossRef]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.; Lauer, U.M.; Herold-Mende, C.; et al. Specific elimination of cd133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.G.; Minev, B.R.; Szalay, A.A. Oncolytic vaccinia virus glv-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J. Transl. Med. 2012, 10, 167. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Li, J.; Mo, L.; Zhao, H.; Zhu, Y.; Hu, Z.; Gao, J.; Tan, W. Pd-1 blockade enhances the antitumor efficacy of gm-csf surface-modified bladder cancer stem cells vaccine. Int. J. Cancer 2018, 142, 2106–2117. [Google Scholar] [CrossRef]
- Aires, A.; Ocampo, S.M.; Simoes, B.M.; Josefa Rodriguez, M.; Cadenas, J.F.; Couleaud, P.; Spence, K.; Latorre, A.; Miranda, R.; Somoza, A.; et al. Multifunctionalized iron oxide nanoparticles for selective drug delivery to CD44-positive cancer cells. Nanotechnology 2016, 27, 65103. [Google Scholar] [CrossRef]
- Tian, F.; Mysliwietz, J.; Ellwart, J.; Gamarra, F.; Huber, R.M.; Bergner, A. Effects of the Hedgehog pathway inhibitor GDC-0449 on lung cancer cell lines are mediated by side populations. Clin. Exp. Med. 2012, 12, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, C.; Fang, C.; Seeruttun, S.R.; Lv, L.; Wang, W. A new strategy using ALDHhigh-CD8+T cells to inhibit tumorigenesis. PLoS ONE 2014, 9, e103193. [Google Scholar] [CrossRef]
- Dietel, M.; Bubendorf, L.; Dingemans, A.M.; Dooms, C.; Elmberger, G.; García, R.C.; Kerr, K.M.; Lim, E.; López-Ríos, F.; Thunnissen, E.; et al. Diagnostic procedures for non-small-cell lung cancer (NSCLC): Recommendations of the European Expert Group. Thorax 2016, 71, 177–184. [Google Scholar] [CrossRef]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer 2021, 22, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Pradines, A.; Favre, G.; Mazieres, J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur. Respir. Rev. 2020, 12, 190052. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 9, 613–628. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, H.Y.; Li, K.C.; Kuo, M.L.; Yang, J.C.; Chan, W.K.; Ho, B.C.; Chang, G.C.; Shih, J.Y.; Yu, S.L.; et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012, 30, 1–9. [Google Scholar] [CrossRef]
- Haber, D.A.; Velculescu, V.E. Blood-based analyses of cancer: Circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014, 4, 650–661. [Google Scholar] [CrossRef]
- Hanssen, A.; Loges, S.; Pantel, K.; Wikman, H. Detection of circulating tumor cells in non-small cell lung cancer. Front. Oncol. 2015, 5, 207. [Google Scholar] [CrossRef]
- Skirecki, T.; Hoser, G.; Kawiak, J.; Dziedzic, D.; Domagała-Kulawik, J. Flow cytometric analysis of CD133- and EpCAM-positive cells in the peripheral blood of patients with lung cancer. Arch. Immunol. Ther. Exp. 2014, 62, 67–75. [Google Scholar] [CrossRef]
- Nanou, A.; Miller, M.C.; Zeune, L.L.; de Wit, S.; Punt, C.J.A.; Groen, H.J.M.; Hayes, D.F.; de Bono, J.S.; Terstappen, L.W.M.M. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br. J. Cancer 2020, 122, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, T.; Rigau, M.; Chiva, C.; Montes, M.; Garcia-Grau, I.; Garcia, M.; Diaz, S.; Celma, A.; Bijnsdorp, I.; Campos, A.; et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget 2017, 8, 4960–4976. [Google Scholar] [CrossRef] [PubMed]
- Nanou, A.; Coumans, F.A.; Van Dalum, G.; Zeune, L.L.; Dolling, D.; Onstenk, W.; Crespo, M.; Fontes, M.S.; Rescigno, P.; Fowler, G.; et al. Circulating tumor cells, tumor-derived extracellular vesicles and plasma cytokeratins in castration-resistant prostate cancer patients. Oncotarget 2018, 9, 19283–19293. [Google Scholar] [CrossRef]
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A.; Asghari, R.; Salimi, L.; Kalashani, S.A.; Feghhi, M.; Etemadi, T.; Akbariazar, E.; Mahmoudi, M.; et al. Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019, 17, 1–17. [Google Scholar] [CrossRef]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional cell movement through tissues is controlled by exosome secretion. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Chen, J.; Fei, X.; Wang, J.; Cai, Z. Tumor-derived extracellular vesicles: Regulators of tumor microenvironment and the enlightenment in tumor therapy. Pharmacol. Res. 2020, 159. [Google Scholar] [CrossRef]
- Rolfo, C.; Castiglia, M.; Hong, D.; Alessandro, R.; Mertens, I.; Baggerman, G.; Zwaenepoel, K.; Gil-Bazo, I.; Passiglia, F.; Carreca, A.P.; et al. Liquid biopsies in lung cancer: The new ambrosia of researchers. Biochim. Biophys. Acta 2014, 1846, 539–546, Erratum in: 2015, 1855, 17. [Google Scholar] [CrossRef]
- Navani, N.; Nankivell, M.; Lawrence, D.R.; Lock, S.; Makker, H.; Baldwin, D.R.; Stephens, R.J.; Parmar, M.K.; Spiro, S.G.; Morris, S.; et al. Lung-BOOST trial investigators. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: An open-label, pragmatic, randomised controlled trial. Lancet Respir. Med. 2015, 4, 282–289. [Google Scholar] [CrossRef]
- Murthi, M.; Donna, E.; Arias, S.; Villamizar, N.R.; Nguyen, D.M.; Holt, G.E.; Mirsaeidi, M.S. Diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in real life. Front. Med. 2020, 7, 118. [Google Scholar] [CrossRef]
- Szlubowski, A.; Kuzdzał, J.; Kołodziej, M.; Soja, J.; Pankowski, J.; Obrochta, A.; Kopiński, P.; Zieliński, M. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur. J. Cardiothorac. Surg. 2009, 2, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Tajarernmuang, P.; Ofiara, L.; Beaudoin, S.; Wang, H.; Benedetti, A.; Gonzalez, A.V. Real-world outcomes of patients with advanced non-small cell lung cancer treated with anti-PD1 therapy on the basis of PD-L1 Results in EBUS-TBNA vs. histological specimens. Chest 2021, 3. [Google Scholar] [CrossRef]
- Chcialowski, A.; Chorostowska-Wynimko, J.; Fal, A.; Pawlowicz, R.; Domagala-Kulawik, J. Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling, processing and analysis methods. Pneumonol. Alergol. Pol. 2011, 79, 75–89. [Google Scholar] [PubMed]
- Kwiecien, I.; Skirecki, T.; Polubiec-Kownacka, M.; Raniszewska, A.; Domagala-Kulawik, J. Immunophenotype of T cells expressing programmed death-1 and cytotoxic T cell antigen-4 in early lung cancer: Local vs. systemic immune response. Cancers 2019, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, I.; Stelmaszczyk-Emmel, A.; Polubiec-Kownacka, M.; Dziedzic, D.; Domagala-Kulawik, J. Elevated regulatory T cells, surface and intracellular CTLA-4 expression and interleukin-17 in the lung cancer microenvironment in humans. Cancer Immunol. Immunother. 2017, 66, 161–170. [Google Scholar] [CrossRef]
- Osinska, I.; Stelmaszczyk-Emmel, A.; Polubiec-Kownacka, M.; Dziedzic, D.; Domagala-Kulawik, J. CD4+/CD25high/FoxP3+/CD127− regulatory T cells in bronchoalveolar lavage fluid of lung cancer patients. Hum. Immunol. 2016, 77, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J. The relevance of bronchoalveolar lavage fluid analysis for lung cancer patients. Exp. Rev. Respir. Med. 2020, 14, 329–337. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J.; Raniszewska, A. How to evaluate the immune status of lung cancer patients before immunotherapy. Breathe 2017, 4, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes in cancer: Another mechanism of tumor-induced immune suppression. Adv. Exp. Med. Biol. 2017, 1036, 81–89. [Google Scholar] [CrossRef]

| Drug | Trial | Study Population | Study Intervention | Reference |
|---|---|---|---|---|
| EGFR mutation positive | ||||
| Osimertinib | FLAURA | Advanced untreated NSCLC, Central nervous system metastases allowed | Osimertinib vs. control (Gefitinib/Erlotinib) | [13] |
| Afatinib | LUX-Lung3, LUX-Lung 6 | Advanced untreated NSCLC | Afatinib vs. chemotherapy | [14] |
| Erlotinib | EURTAC | Advanced untreated NSCLC | Erlotinib vs. chemotherapy | [15] |
| Dacomitinib | ARCHER 1050 | Advanced untreated NSCLC | Dacomitinib vs. Gefitinib | [16] |
| Gefitinib | Advanced untreated patients; | Gefitinib vs. Carboplatin/Paclitaxel | [17] | |
| Erlotinib + Ramucirumab | RELAY | Advanced untreated NSCLC | Erlotinib + Ramicriumab versus Erlotinib | [18] |
| ALK rearrangement positive | ||||
| Alectinib | ALEX | Advanced untreated NSCLC; Central nervous system metastases included | Alectinib vs. Crizotinib | [19] |
| Brigatinib | ALTA-1L | Advanced untreated NSCLC; Central nervous system metastases included | Brigatinib vs. Crizotinib | [20] |
| Ceritinib | ASCEND-4 | Advanced untreated NSCLC; Central nervous system metastases included | Ceritinib vs. platinum + Pemetrexed | [21] |
| Crizotinib | PROFILE 1014 | Advanced untreated non-SQCLC | Crizotinib vs. platinum + pemetrexed | [20] |
| ROS1 rearrangement positive | ||||
| Crizotinib | Advanced NSCLC | Phase I trial; no comparator | [22] | |
| Ceritinib | Advanced NSCLC included central nervous system metastases | Phase II trial; no comparator | [23] | |
| Entrectinib | ALK-372–001, STARTRK-1, STARTRK-2 | Advanced NSCLC | Integrated analysis of three phase1/2 trials; no comparator | [24] |
| BRAF V600E mutation positive | ||||
| Dabrafenib/Trametinib | Advanced NSCLC; pretreated | Phase II; no comparator | [25] | |
| MET Exon 14 Skipping mutation | ||||
| Crizotinib | Advanced NSCLC | Phase II; no comparator | [24] | |
| Capmatinib | GEOMETRY mono-1 | Advanced NSCLC | Phase II; no comparator | [24] |
| NTRK Gene fusion positive | ||||
| Larotrectinib | Any TRK-positive cancers (3 Lung tumors) | Phase I/II; no comparator | [25] | |
| Entrectinib | STARTRK-1; STARTRK-2 | Advanced NSCLC; pretreated | Phase I; no comparator | [24] |
| RET Rearrangement positive | ||||
| Selpercatinib/LOXO-292 | LIBRETTO-001 | Any RET rearranged tumor includes central nervous system metastases | Phase I; no comparator | [26] |
| Cabozantinib | Advanced NSCLC | Phase II; no comparator | [27] | |
| Vandetanib | Advanced NSCLC | Phase II; no comparator | [23] | |
| Feature | Effect | Reference |
|---|---|---|
| Genomic instability | The major cause of neoplasia, cancer initiation, progression, and impact the overall prognosis of the affected lung cancer patient | [41] |
| Inhibition of telomerase activity | Chromosome destabilization causes cellular senescence and death; in lung cancer telomere dysfunction promotes progression, metastasis and was associated with poor prognosis | [42,43] |
| Epigenetic mechanisms: (DNA hypermethylation, altered chromatin remodeling and histone modifications) | Established during differentiation, stably inherited and maintained through multiple rounds of cell division; deregulation of miRNAs is associated with early recurrence of lung cancer lesions | [42,44] |
| Mitochondrial DNA alteration | Plays a pivotal role in tumorigenesis; evasion of apoptosis | [42,45] |
| Intercellular communication | establishes a distinct tumor microenvironment (TME) with various stromal cell types to support growth, angiogenesis and invasion; altered communication of tumor cells to immune cells enable immune surveillance | [46] |
| Extracellular matrix (ECM) dysregulation | ECM actively undergoes dynamic remodeling during all stages of cancer progression; crosstalk between tumor cells and immune cells within primary and secondary sites is fundamental to ECM remodeling that feeds back to regulate tumor cell dormancy and outgrowth | [47] |
| Stem cell exhaustion | Cancer and aging are two possible endpoints of stem cells exposed to mutagenic hits, which will cause cell cycle arrest, and apoptosis or senescence. Through the acquisition of mutation and genetic or epigenetic alterations, normal stem cells can become CSCs | [48,49] |
| Type of Immunotherapy | Condition | Study Intervention | Reference |
|---|---|---|---|
| DCs vaccination | SQCLC, melanoma | ALDHhigh CSC-pulsed DCs | [93] |
| DCs vaccination | Squamous cell cancer, melanoma | CSCs lysate-pulsed DCs | [94,95] |
| T-cell therapy | Colon cancer | CD8+ cytotoxic T-cells, specific for the CSCs antigen | [96] |
| T-cell therapy | Prostate cancer | CAR T-cells against EpCAM antigen | [97] |
| Virotherapy | Glioblastoma | Oncolytic adenovirus targeting CD133+ CSCs | [98] |
| Virotherapy | Ovarian cancer | Oncolytic vaccinia virus targeting ID8-T tumor model that harbors CSCs | [99] |
| Virotherapy | Hepatocellular carcinom | Oncolytic measles viruses: targeting CD133+ CSCs | [100] |
| Virotherapy | Breast cancer | Oncolytic vaccinia virus targeting ALDHhigh CSCs | [101] |
| Combined therapy | Bladder cancer | CSCs vaccine combinated with anti-PD-1 | [93] |
| Monoclonal antibody | Breast cancer | Anti-CD44 antibody | [102] |
| CSC-CAR T | Prostate | EpCAM-specific CAR T cell | [93] |
| Targeting signaling pathway | Lung cancer | Hedgedog pathway inhibitor | [103] |
| CSC-primed T cells | Lung cancer | CD8+ cytotoxic T-cells, ALDHhigh specific CSCs | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raniszewska, A.; Kwiecień, I.; Rutkowska, E.; Rzepecki, P.; Domagała-Kulawik, J. Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies. Cancers 2021, 13, 2996. https://doi.org/10.3390/cancers13122996
Raniszewska A, Kwiecień I, Rutkowska E, Rzepecki P, Domagała-Kulawik J. Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies. Cancers. 2021; 13(12):2996. https://doi.org/10.3390/cancers13122996
Chicago/Turabian StyleRaniszewska, Agata, Iwona Kwiecień, Elżbieta Rutkowska, Piotr Rzepecki, and Joanna Domagała-Kulawik. 2021. "Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies" Cancers 13, no. 12: 2996. https://doi.org/10.3390/cancers13122996
APA StyleRaniszewska, A., Kwiecień, I., Rutkowska, E., Rzepecki, P., & Domagała-Kulawik, J. (2021). Lung Cancer Stem Cells—Origin, Diagnostic Techniques and Perspective for Therapies. Cancers, 13(12), 2996. https://doi.org/10.3390/cancers13122996









