PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Procedure
2.3. Image Interpretation and Calculation of Quantitative PET Parameters
2.4. Lu-177-PSMA-617 RLT
2.5. Assessment of Early PSA Response
2.6. Statistical Analysis
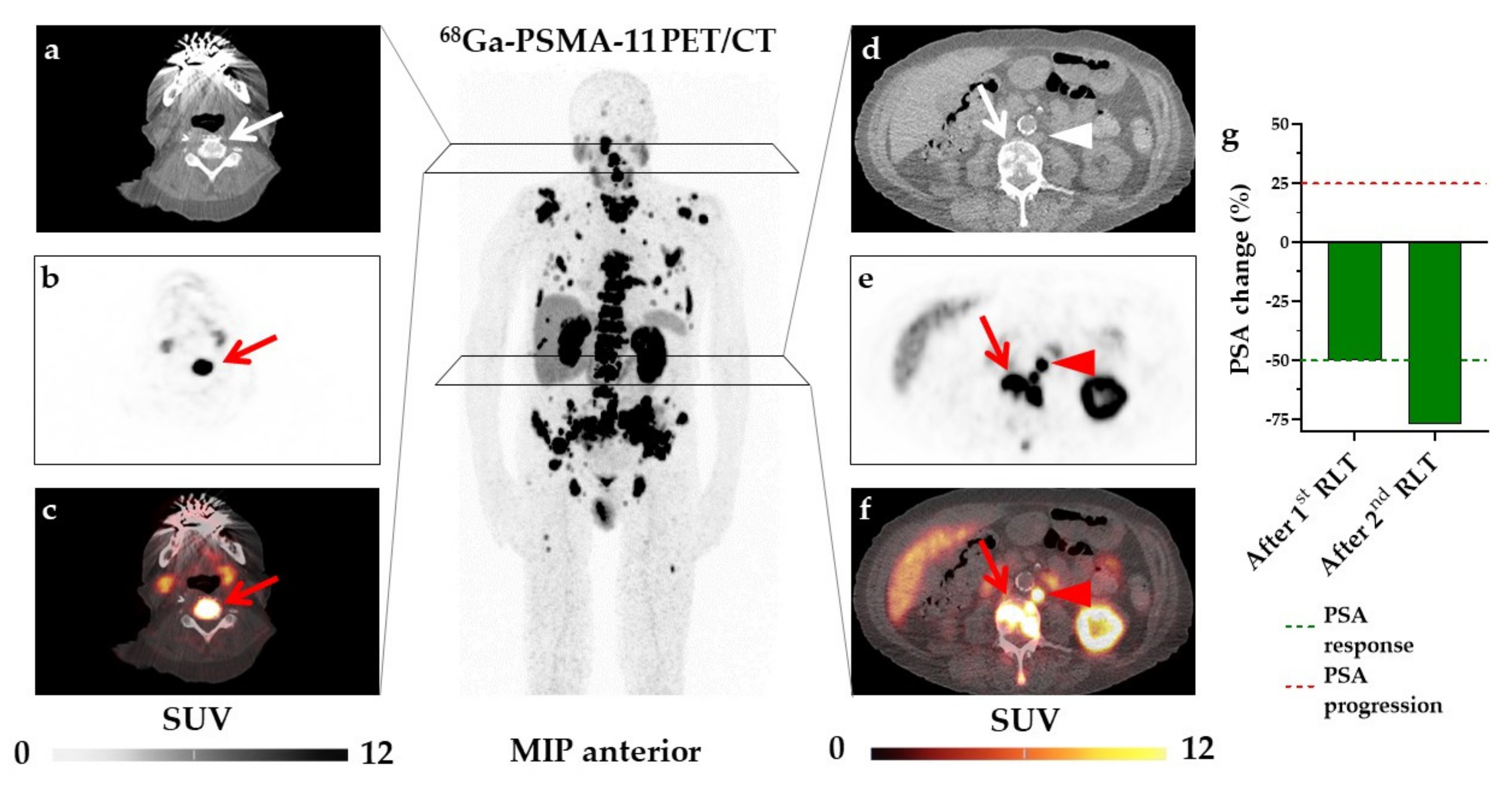
3. Results
3.1. SUVmax and PSMA-TV Are Independent Parameters for Baseline 68Ga-PSMA-11 PET/CT
3.2. SUVmax, but Not PSMA-TV or TL-PSMA, Is Associated with PSA Change under RLT
3.3. SUVmax and Age Are Independent Predictors of Early PSA Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual Comparison of (18)F-PSMA-1007 and (18)F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of (18)F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Uemura, M.; Soeda, F.; Naka, S.; Ujike, T.; Hatano, K.; Sasaki, H.; Kamiya, T.; Shimosegawa, E.; Kato, H.; et al. High detection rate in [(18)F]PSMA-1007 PET: Interim results focusing on biochemical recurrence in prostate cancer patients. Ann. Nucl. Med. 2021, 35, 523–528. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Kirchner, J.; Buddensieck, C.; Antke, C.; Ullrich, T.; Schimmoller, L.; Boos, J.; Schleich, C.; Schaarschmidt, B.M.; Buchbender, C.; et al. Prospective comparison of whole-body MRI and (68)Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1542–1550. [Google Scholar] [CrossRef]
- Rowe, S.P.; Macura, K.J.; Mena, E.; Blackford, A.L.; Nadal, R.; Antonarakis, E.S.; Eisenberger, M.; Carducci, M.; Fan, H.; Dannals, R.F.; et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol. 2016, 18, 411–419. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Schuchardt, C.; Singh, A.; Wirtz, M.; Wiessalla, S.; Schottelius, M.; Mueller, D.; Klette, I.; Wester, H.J. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J. Nucl. Med. 2016, 57, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the Vision of Radioligand Therapy for Prostate Cancer Becoming a Reality? An Overview of the Phase III VISION Trial and Its Importance for the Future of Theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Harsini, S.; Saidi, B.; Ahmadzadehfar, H.; Herrmann, K.; Briganti, A.; Walz, J.; Beheshti, M. Factors predicting biochemical response and survival benefits following radioligand therapy with [(177)Lu]Lu-PSMA in metastatic castrate-resistant prostate cancer: A review. Eur. J. Nucl. Med. Mol. Imaging 2021, 1–14. [Google Scholar] [CrossRef]
- Seifert, R.; Seitzer, K.; Herrmann, K.; Kessel, K.; Schafers, M.; Kleesiek, J.; Weckesser, M.; Boegemann, M.; Rahbar, K. Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving (177)Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7812–7820. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schafers, M.; et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [(177)Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Wang, H.; Weber, M.; Rathke, H.; Esfandiari, R.; Armstrong, W.; Kratochwil, C.; Tauber, R.; Delpassand, E.; et al. Predictive factors and prediction nomograms for LuPSMA radioligand therapy in patients with metastatic castration-resistant prostate cancer: An international multicentre retrospective study. J. Nucl. Med. 2020, 61, 593. [Google Scholar]
- Derlin, T.; Werner, R.A.; Lafos, M.; Henkenberens, C.; von Klot, C.A.J.; Sommerlath Sohns, J.M.; Ross, T.L.; Bengel, F.M. Neuroendocrine Differentiation and Response to PSMA-Targeted Radioligand Therapy in Advanced Metastatic Castration-Resistant Prostate Cancer: A Single-Center Retrospective Study. J. Nucl. Med. 2020, 61, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial Experience with Volumetric (68)Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lutzen, U.; Prasad, V.; Heinzel, A.; et al. [177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer]. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Di Carli, M.F. Methods and limitations of assessing new noninvasive tests: Part II: Outcomes-based validation and reliability assessment of noninvasive testing. Circulation 2008, 117, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.I. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef]
- Rathke, H.; Holland-Letz, T.; Mier, W.; Flechsig, P.; Mavriopoulou, E.; Rohrich, M.; Kopka, K.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; et al. Response Prediction of (177)Lu-PSMA-617 Radioligand Therapy Using Prostate-Specific Antigen, Chromogranin A, and Lactate Dehydrogenase. J. Nucl. Med. 2020, 61, 689–695. [Google Scholar] [CrossRef]
- Derlin, T.; Sommerlath Sohns, J.M.; Schmuck, S.; Henkenberens, C.; von Klot, C.A.J.; Ross, T.L.; Bengel, F.M. Influence of short-term dexamethasone on the efficacy of (177) Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Prostate 2020, 80, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Gadot, M.; Davidson, T.; Aharon, M.; Atenafu, E.G.; Malki, A.; Levartovsky, M.; Saad, A.; Domachevsky, L.; Berger, R.; Leibowitz, R. Clinical Variables Associated with PSA Response to Lutetium-177-PSMA ([177Lu]-PSMA-617) Radionuclide Treatment in Men with Metastatic Castration-Resistant Prostate Cancer. Cancers 2020, 12, 1078. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Heck, M.M.; Rauscher, I.; Tauber, R.; Cala, L.; Franz, C.; D’Alessandria, C.; Retz, M.; Weber, W.A.; Eiber, M. Early Prostate-Specific Antigen Changes and Clinical Outcome After (177)Lu-PSMA Radionuclide Treatment in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Michalski, K.; Mix, M.; Meyer, P.T.; Ruf, J. Determination of whole-body tumour burden on [68Ga]PSMA-11 PET/CT for response assessment of [177Lu]PSMA-617 radioligand therapy: A retrospective analysis of serum PSA level and imaging derived parameters before and after two cycles of therapy. Nuklearmedizin 2019, 58, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Michalski, K.; Ruf, J.; Goetz, C.; Seitz, A.K.; Buck, A.K.; Lapa, C.; Hartrampf, P.E. Prognostic implications of dual tracer PET/CT: PSMA ligand and [(18)F]FDG PET/CT in patients undergoing [(177)Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2024–2030. [Google Scholar] [CrossRef]
- Perez, P.M.; Hope, T.A.; Behr, S.C.; van Zante, A.; Small, E.J.; Flavell, R.R. Intertumoral Heterogeneity of 18F-FDG and 68Ga-PSMA Uptake in Prostate Cancer Pulmonary Metastases. Clin. Nucl. Med. 2019, 44, e28–e32. [Google Scholar] [CrossRef]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.H.; Khalil, M.I.; Alobuia, W.M.; Su, J.; Davis, R. Incidence of metastasis and prostate-specific antigen levels at diagnosis in Gleason 3+4 versus 4+3 prostate cancer. Urol. Ann. 2018, 10, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Kim, Y.I.; Lee, J.L.; Wibmer, A.G.; Schoder, H.; Ryu, J.S.; Vargas, H.A. Concordance between Response Assessment Using Prostate-Specific Membrane Antigen PET and Serum Prostate-Specific Antigen Levels after Systemic Treatment in Patients with Metastatic Castration Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 663. [Google Scholar] [CrossRef]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Lawhn-Heath, C.; Salavati, A.; Behr, S.C.; Rowe, S.P.; Calais, J.; Fendler, W.P.; Eiber, M.; Emmett, L.; Hofman, M.S.; Hope, T.A. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology 2021, 299, 248–260. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. PSMA-RADS Version 1.0: A Step Towards Standardizing the Interpretation and Reporting of PSMA-targeted PET Imaging Studies. Eur. Urol. 2018, 73, 485–487. [Google Scholar] [CrossRef]



| Variable | Responders (n = 34) | Non-Responders (n = 37) | p-Value |
|---|---|---|---|
| Age (years, mean ± SD) | 75.24 ± 6.6 | 69.22 ± 6.58 | <0.001 * |
| Time period between initial diagnosis and 1st RLT (years, mean ± SD) | 9.82 ± 5.19 | 6.62 ± 3.79 | 0.004 |
| Gleason score | 8 (7–9) | 8 (7–9) | 0.986 |
| Previous treatments (%) | |||
| Radical prostatectomy | 74 | 54 | 0.09 |
| Primary radiation therapy | 9 | 8 | 0.915 |
| Salvage radiation therapy | 59 | 62 | 0.778 |
| Androgen deprivation therapy | 100 | 100 | 1 |
| Novel hormonal agents # | 79 | 92 | 0.142 |
| Previous chemotherapy | 74 | 92 | 0.045 * |
| Median standard laboratory values at baseline (IQR in parentheses) | |||
| RBC (×106/μL) | 4.1 (3.8–4.4) | 3.9 (3.4–4.3) | 0.114 |
| WBC (×103/μL) | 4.9 (3.4–6.7) | 4.5 (3.5–5.7) | 0.789 |
| Platelets (×103/μL) | 224 (193–268) | 220 (196–274) | 0.797 |
| AST (U/L) | 26 (23–35) | 28 (22–53) | 0.244 |
| ALT (U/L) | 17 (12–22) | 15 (12–22) | 0.604 |
| LDH (U/L) | 264 (233–326) | 307 (221–472) | 0.181 |
| AP (U/L) | 117 (74–223) | 160 (74–337) | 0.346 |
| PSA (μg/L) | 134 (35–382) | 239 (48–483) | 0.766 |
| Site of tumor lesions (%) | |||
| Osseous | 79 | 84 | 0.64 |
| Lymph nodes | 76 | 84 | 0.446 |
| Hepatic | 6 | 30 | 0.009 * |
| Prostate bed | 18 | 30 | 0.236 |
| Other | 29 | 32 | 0.787 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Clinical parameters at baseline | |||
| RBC (×106/μL) | 2.53 | 0.95 to 6.75 | 0.09 |
| WBC (×103/μL) | 2.34 | 0.9 to 6.14 | 0.1 |
| Platelets (×103/μL) | 1.29 | 0.5 to 3.36 | 0.63 |
| AST (U/L) | 0.63 | 0.24 to 1.61 | 0.35 |
| ALT (U/L) | 0.82 | 0.31 to 2.22 | 0.8 |
| LDH (U/L) | 0.61 | 0.22 to 1.67 | 0.45 |
| AP (U/L) | 0.74 | 0.29 to 1.91 | 0.63 |
| PSA (μg/L) | 0.47 | 0.17 to 1.27 | 0.15 |
| Age (years) | 8.11 | 2.54 to 25.87 | <0.001 * |
| Time period between initial diagnosis and 1st RLT (years) | 7.33 | 2.13 to 25.27 | 0.001 * |
| Previous chemotherapy | 0.25 | 0.06 to 1 | 0.06 |
| PET-derived parameters at baseline | |||
| Hepatic metastases | 0.15 | 0.03 to 0.73 | 0.01 * |
| PSMA-TV (cm3) | 0.43 | 0.16 to 1.11 | 0.1 |
| TL-PSMA (cm3) | 5.6 | 1.43 to 21.89 | 0.01 * |
| SUVmax | 8.35 | 2.74 to 25.45 | <0.001 * |
| Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age | 8.05 | 2.15 to 30.09 | 0.002 * |
| Hepatic metastases | 0.22 | 0.03 to 1.57 | 0.132 |
| SUVmax | 7.94 | 2.25 to 28.05 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widjaja, L.; Werner, R.A.; Ross, T.L.; Bengel, F.M.; Derlin, T. PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy. Cancers 2021, 13, 2938. https://doi.org/10.3390/cancers13122938
Widjaja L, Werner RA, Ross TL, Bengel FM, Derlin T. PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy. Cancers. 2021; 13(12):2938. https://doi.org/10.3390/cancers13122938
Chicago/Turabian StyleWidjaja, Liam, Rudolf A. Werner, Tobias L. Ross, Frank M. Bengel, and Thorsten Derlin. 2021. "PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy" Cancers 13, no. 12: 2938. https://doi.org/10.3390/cancers13122938
APA StyleWidjaja, L., Werner, R. A., Ross, T. L., Bengel, F. M., & Derlin, T. (2021). PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy. Cancers, 13(12), 2938. https://doi.org/10.3390/cancers13122938







