Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
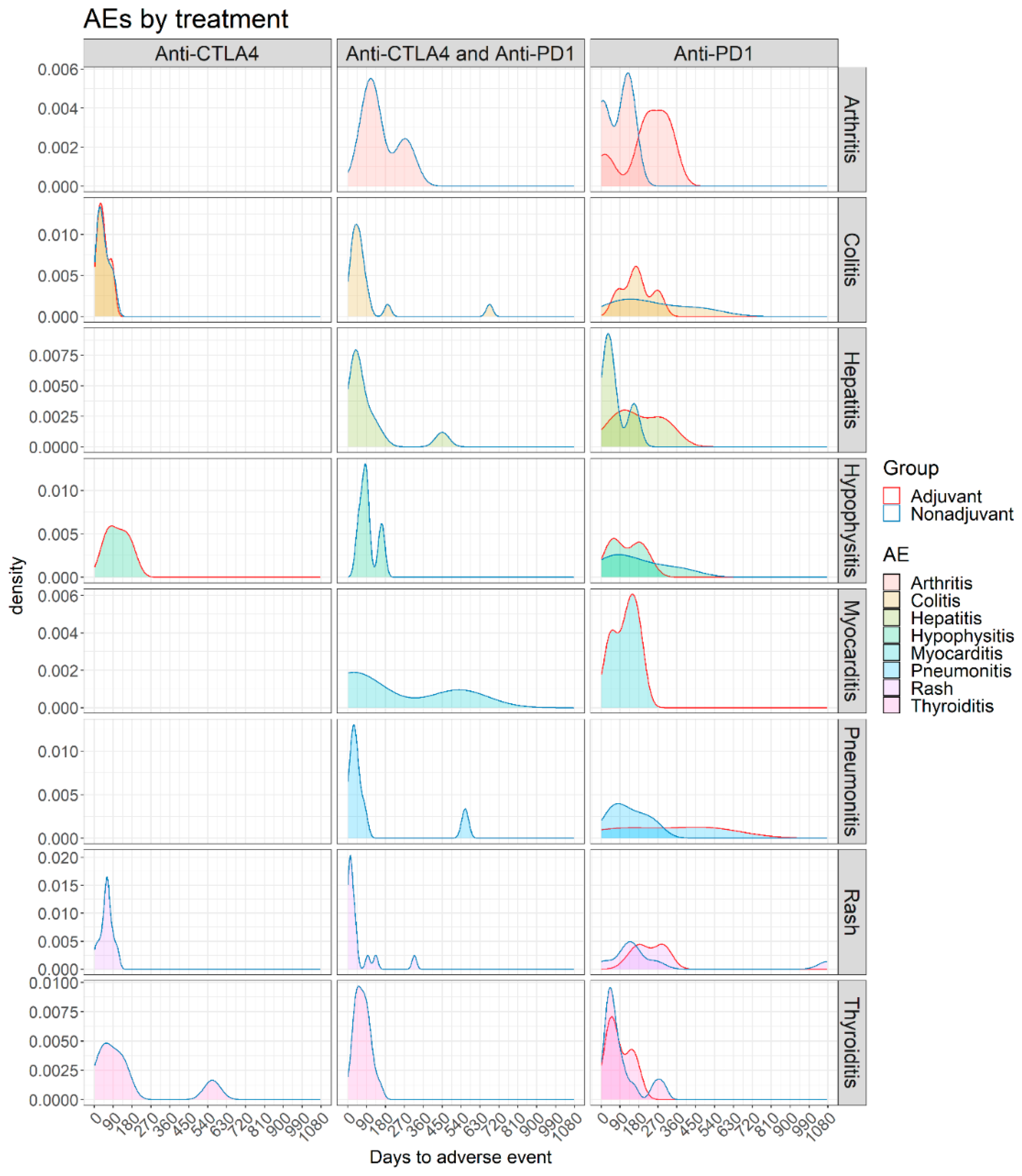
3.2. Adverse Events
3.3. Adverse Events in Non-Adjuvant/Unresectable Setting
3.4. Adverse Events in Adjuvant Setting
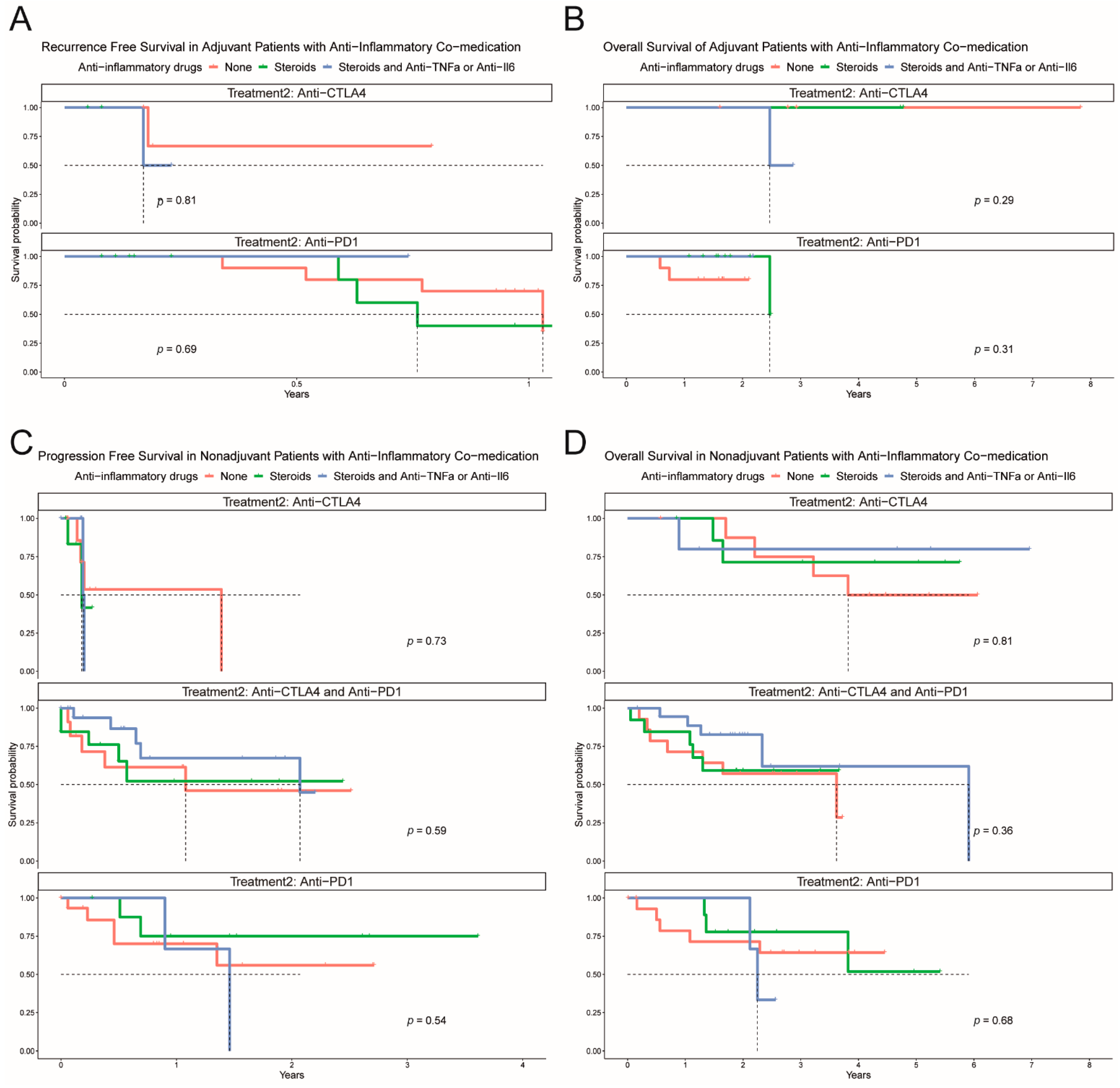
3.5. Immunomodulatory Agents
3.6. Patients with Underlying Autoimmune/Auto-Inflammatory Comorbidities
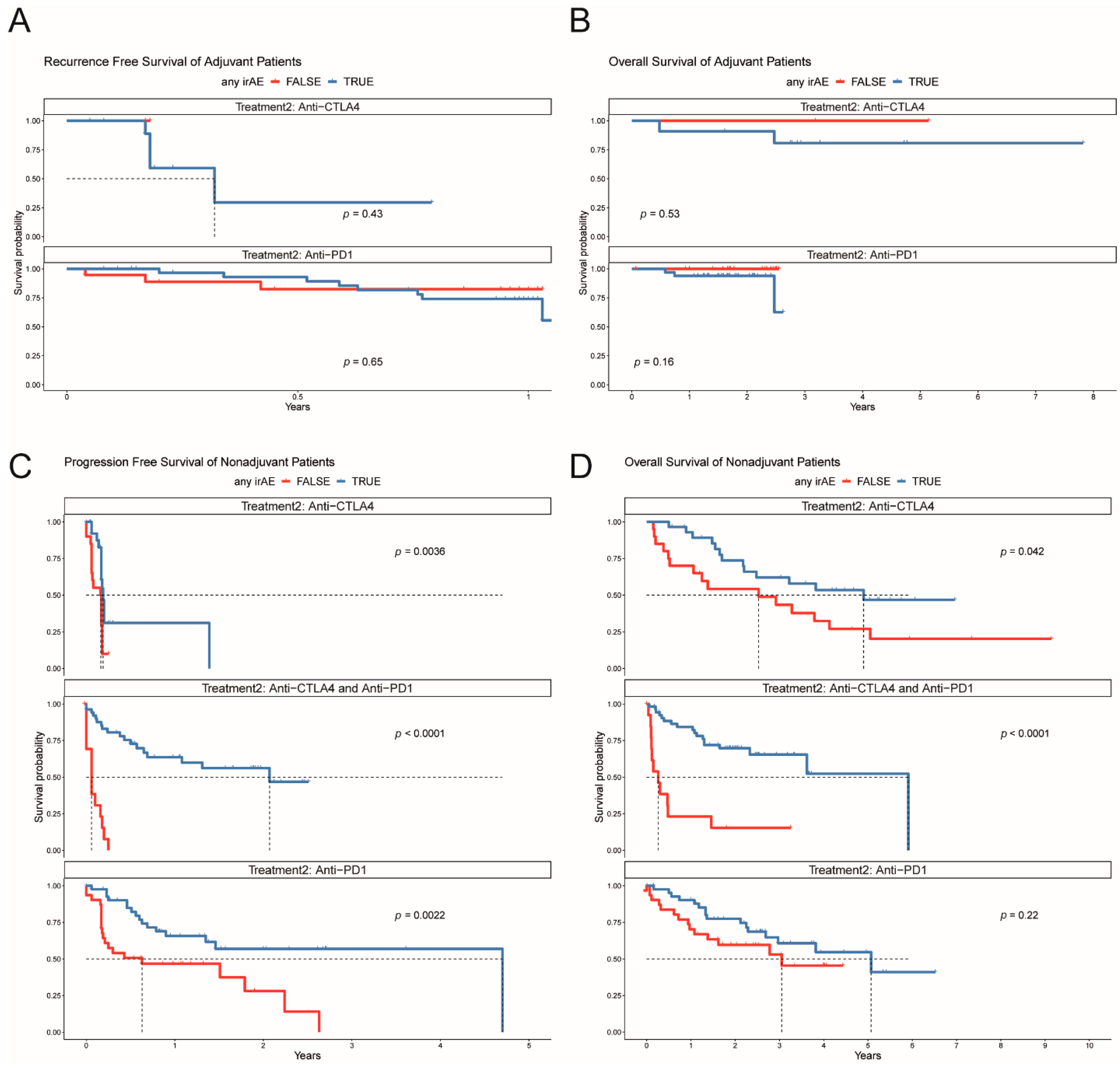
3.7. Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (checkmate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (keynote-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage iii melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant nivolumab versus ipilimumab in resected stage iii or iv melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, I.; Melero, I.; Ponz-Sarvise, M.; Castanon, E. Safety and tolerability of immune checkpoint inhibitors (pd-1 and pd-l1) in cancer. Drug Saf. 2019, 42, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Friedman, C.F.; Navid-Azarbaijani, P.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol. 2018, 4, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the society for immunotherapy of cancer (sitc) toxicity management working group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef]
- Stroud, C.R.G.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to pd-1 blockade. J. Oncol. Pharm. Pract. 2017, 25, 551–557. [Google Scholar] [CrossRef]
- Dimitriou, F.; Matter, A.V.; Mangana, J.; Urosevic-Maiwald, M.; Micaletto, S.; Braun, R.P.; French, L.E.; Dummer, R. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J. Immunother. (Hagerstown MD 1997) 2019, 42, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Larkin, J.M.G.; Schadendorf, D.; Wolchok, J.D.; Wagstaff, J.; Dummer, R.; Hogg, D.; Guidoboni, M.; Sosman, J.A.; Chmielowski, B.; et al. Management of gastrointestinal (gi) toxicity associated with nivolumab (nivo) plus ipilimumab (ipi) or ipi alone in phase ii and iii trials in advanced melanoma (mel). J. Clin. Oncol. 2017, 35, 9523. [Google Scholar] [CrossRef]
- Pan, E.Y.; Merl, M.Y.; Lin, K. The impact of corticosteroid use during anti-pd1 treatment. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2020, 26, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Genova, C.; De Giglio, A.; Bassanelli, M.; Dal Bello, M.G.; Metro, G.; Brambilla, M.; Baglivo, S.; Grossi, F.; Chiari, R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J. Cancer Res. Clin. Oncol. 2019, 145, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-related adverse events correlate with improved survival in patients undergoing anti-pd1 immunotherapy for metastatic melanoma. J. Cancer Res. Clin. Oncol. 2019, 145, 511–521. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association between immune-related adverse events and recurrence-free survival among patients with stage iii melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Robert, C.; Hwu, W.-J.; Hamid, O.; Ribas, A.; Weber, J.S.; Daud, A.I.; Hodi, F.S.; Wolchok, J.D.; Mitchell, T.C.; Hersey, P.; et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur. J. Cancer 2021, 144, 182–191. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (Ctcae). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 11 June 2021).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Dupont, R.; Bérard, E.; Puisset, F.; Comont, T.; Delord, J.P.; Guimbaud, R.; Meyer, N.; Mazieres, J.; Alric, L. The prognostic impact of immune-related adverse events during anti-pd1 treatment in melanoma and non-small-cell lung cancer: A real-life retrospective study. Oncoimmunology 2020, 9, 1682383. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Suo, A.; Chan, Y.; Beaulieu, C.; Kong, S.; Cheung, W.Y.; Monzon, J.G.; Smylie, M.; Walker, J.; Morris, D.; Cheng, T. Anti-pd1-induced immune-related adverse events and survival outcomes in advanced melanoma. Oncologist 2020, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, F.; Long, G.V.; Menzies, A.M. Novel adjuvant options for cutaneous melanoma. Ann. Oncol. 2021, 32, 854–865. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Teulings, H.E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage iii-iv melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Mangana, J.; Dimitriou, F.; Braun, R.; Ludwig, S.; Dummer, R.; Barysch, M.J. Single-center real-life experience with low-dose ipilimumab monotherapy in adjuvant setting for patients with stage iii melanoma. Melanoma Res. 2019, 29, 648–654. [Google Scholar] [CrossRef]
- Lebbé, C.; Meyer, N.; Mortier, L.; Marquez-Rodas, I.; Robert, C.; Rutkowski, P.; Menzies, A.M.; Eigentler, T.; Ascierto, P.A.; Smylie, M.; et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: Results from the phase iiib/iv checkmate 511 trial. J. Clin. Oncol. 2019, 37, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb Announces Update on Checkmate-915 for Opdivo (Nivolumab) Plus Yervoy (Ipilimumab) versus Opdivo alone in Patients with Resected High-Risk Melanoma and pd-l1 < 1%. Available online: https://news.bms.com/news/corporate-financial/2019/Bristol-Myers-Squibb-Announces-Update-on-CheckMate--915-for-Opdivo-nivolumab-Plus-Yervoy-ipilimumab-Versus-Opdivo-Alone-in-Patients-with-Resected-High-Risk-Melanoma-and-PD-L1-1/default.aspx (accessed on 11 June 2021).
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from pd-1/pd-l1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-pd-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.; Callaghan, S.; Martínez-Vila, C.; Bhave, P.; Reijers, I.; Gerard, C.L.; Aspelagh, S.; et al. 1138p delayed immune-related adverse events (iraes) on anti-pd1-based therapy. Ann. Oncol. 2020, 31, S761–S762. [Google Scholar] [CrossRef]


| Characteristic | Overall Population, n = 256 | Non-Adjuvant Setting, n = 191 | Adjuvant Setting, n = 65 | ||||
|---|---|---|---|---|---|---|---|
| Patients with No Toxicities, n = 65 | Patients with Toxicities, n = 126 | p-Value 1 | Patients with No Toxicities, n = 21 | Patients with Toxicities, n = 44 | p-Value 1 | ||
| Age at treatment start (range) | 63 (25–92) | 68 (35–89) | 61 (33–92) | 0.07 | 63 (31–83) | 55 (25–87) | 0.2 |
| AJCCv8 Stage at treatment start, n (%) | 0.8 | 0.8 | |||||
| II | 4 (1.6%) | - | - | - | 2 (9.5%) | 2 (4.5%) | |
| III | 65 (25%) | 4 (6.2%) | 10 (7.9%) | 16 (76%) | 35 (80%) | ||
| IV | 187 (73%) | 61 (94%) | 116 (92%) | 3 (14%) | 7 (16%) | ||
| Melanoma subtype, n (%) | 0.3 | >0.9 | |||||
| Cutaneous | 201 (79%) | 49 (75%) | 97 (77%) | 18 (86%) | 37 (84%) | ||
| Acral | 3 (1.2%) | 2 (3.1%) | - | - | 1 (2.3%) | ||
| Mucosal | 14 (5.5%) | 4 (6.2%) | 7 (5.6%) | 1 (4.8%) | 2 (4.5%) | ||
| Unknown primary | 24 (9.4%) | 7 (11%) | 11 (8.7%) | 2 (9.5%) | 4 (9.1%) | ||
| Uveal | 14 (5.5%) | 3 (4.6%) | 11 (8.7%) | - | - | ||
| Autoimmune disease in medical history, n (%) | 0.7 | 0.4 | |||||
| Chronic thyroiditis | 5 (2.0%) | 2 (3.1%) | 2 (1.6%) | 1 (4.8%) | - | ||
| Diabetes type 1 | 3 (1.2%) | 1 (1.5%) | 2 (1.6%) | - | - | ||
| Inflammatory bowel disease | 2 (0.8%) | - | 1 (0.8%) | - | 1 (2.3%) | ||
| Rheumatoid arthritis | 6 (2.3%) | - | 3 (2.4%) | - | 3 (6.8%) | ||
| Multiple Sclerosis | 1 (0.4%) | - | 1 (0.8%) | - | - | ||
| Bechterew’s disease | 1 (0.4%) | 1 (1.5%) | - | - | - | ||
| Sarcoidosis | 2 (0.8%) | - | 2 (1.6%) | - | - | ||
| Presence of brain metastases at treatment start, n (%) | 48 (19%) | 16 (25%) | 32 (25%) | >0.9 | - | - | |
| Type of treatment, n (%) | 0.01 | 0.2 | |||||
| Anti-PD1/Anti-CTLA4 | 68 (27%) | 14 (22%) | 54 (43%) | - | - | ||
| Anti-PD1 | 126 (49%) | 31 (48%) | 43 (34%) | 19 (90%) | 33 (75%) | ||
| Anti-CTLA4 | 62 (24%) | 20 (31%) | 29 (23%) | 2 (9.5%) | 11 (25%) | ||
| Reason for treatment discontinuation, n (%) | 0.01 | ||||||
| Toxicity | 35 (13.7%) | - | 25 (20%) | - | 10 (23%) | ||
| Disease progression/recurrence | 112 (43.8%) | 51 (79%) | 46 (71%) | 3 (14%) | 12 (27%) | ||
| Treatment completed | 40 (15.6%) | 1 (1.5%) | 4 (3.2%) | 16 (76%) | 19 (43.1%) | ||
| CR | 34 (13.3%) | 7 (11%) | 27 (21%) | - | - | ||
| Patient’s/Investigator’s choice | 22 (8.6%) | 5 (7.7%) | 12 (9.5%) | 2 (9.5%) | 3 (6.8%) | ||
| Best Overall Response (BOR), n (%) | <0.001 | ||||||
| CR | 95 (40%) | 8 (12%) | 44 (37%) | - | - | ||
| PR | 36 (15%) | 12 (19%) | 24 (20%) | - | - | ||
| SD | 12 (5.0%) | 2 (3.1%) | 10 (8.4%) | - | - | ||
| PD | 95 (40%) | 42 (66%) | 41 (34%) | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, F.; Staeger, R.; Ak, M.; Maissen, M.; Kudura, K.; Barysch, M.J.; Levesque, M.P.; Cheng, P.F.; Dummer, R.; Mangana, J. Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis. Cancers 2021, 13, 2931. https://doi.org/10.3390/cancers13122931
Dimitriou F, Staeger R, Ak M, Maissen M, Kudura K, Barysch MJ, Levesque MP, Cheng PF, Dummer R, Mangana J. Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis. Cancers. 2021; 13(12):2931. https://doi.org/10.3390/cancers13122931
Chicago/Turabian StyleDimitriou, Florentia, Ramon Staeger, Melike Ak, Matias Maissen, Ken Kudura, Marjam J. Barysch, Mitchell P. Levesque, Phil F. Cheng, Reinhard Dummer, and Joanna Mangana. 2021. "Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis" Cancers 13, no. 12: 2931. https://doi.org/10.3390/cancers13122931
APA StyleDimitriou, F., Staeger, R., Ak, M., Maissen, M., Kudura, K., Barysch, M. J., Levesque, M. P., Cheng, P. F., Dummer, R., & Mangana, J. (2021). Frequency, Treatment and Outcome of Immune-Related Toxicities in Patients with Immune-Checkpoint Inhibitors for Advanced Melanoma: Results from an Institutional Database Analysis. Cancers, 13(12), 2931. https://doi.org/10.3390/cancers13122931







