The Emerging Role of Amino Acids of the Brain Microenvironment in the Process of Metastasis Formation
Abstract
Simple Summary
Abstract
1. Introduction
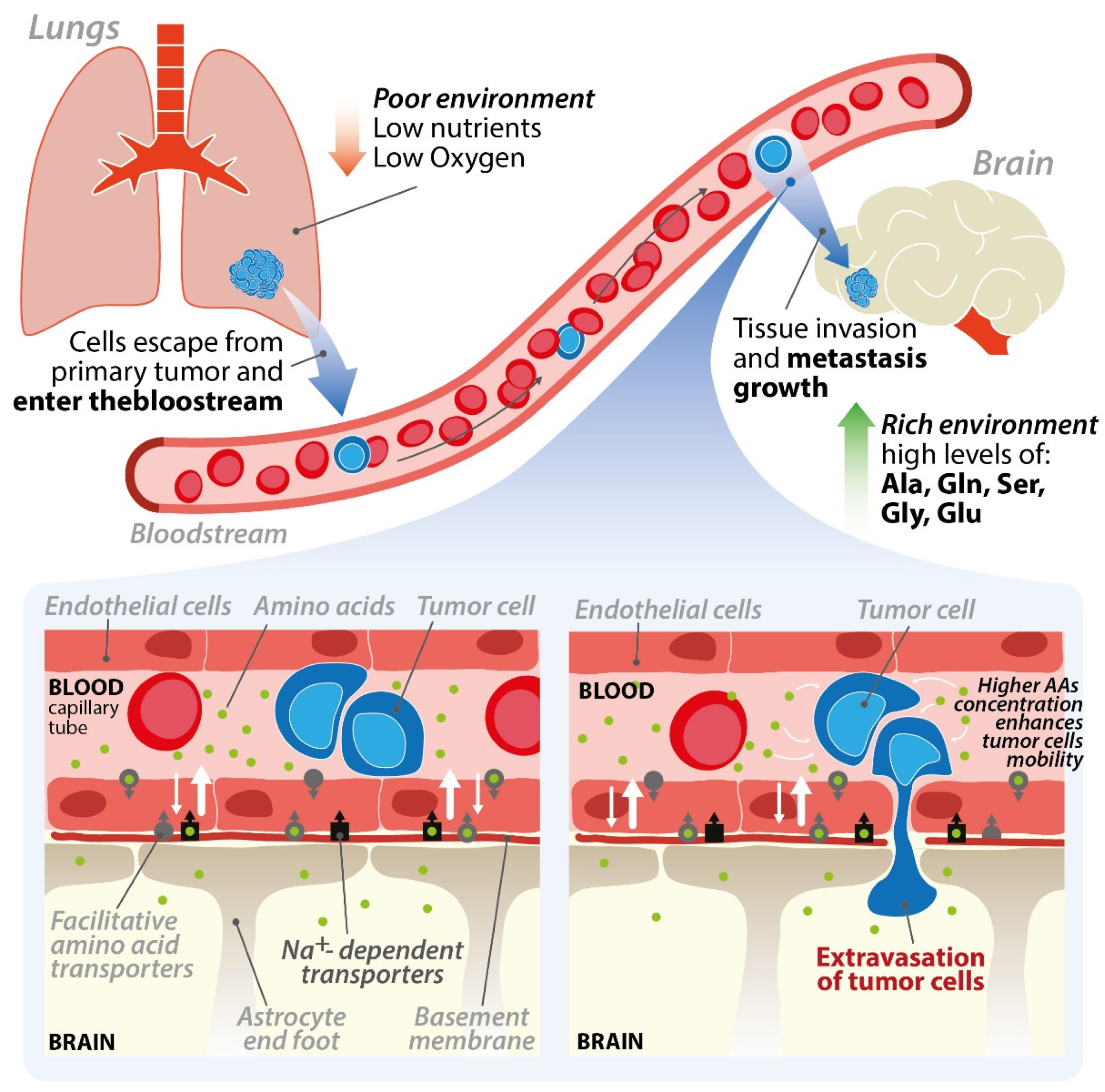
2. The Endothelial Cells of the Blood–Brain Barrier and the Brain Microenvironment
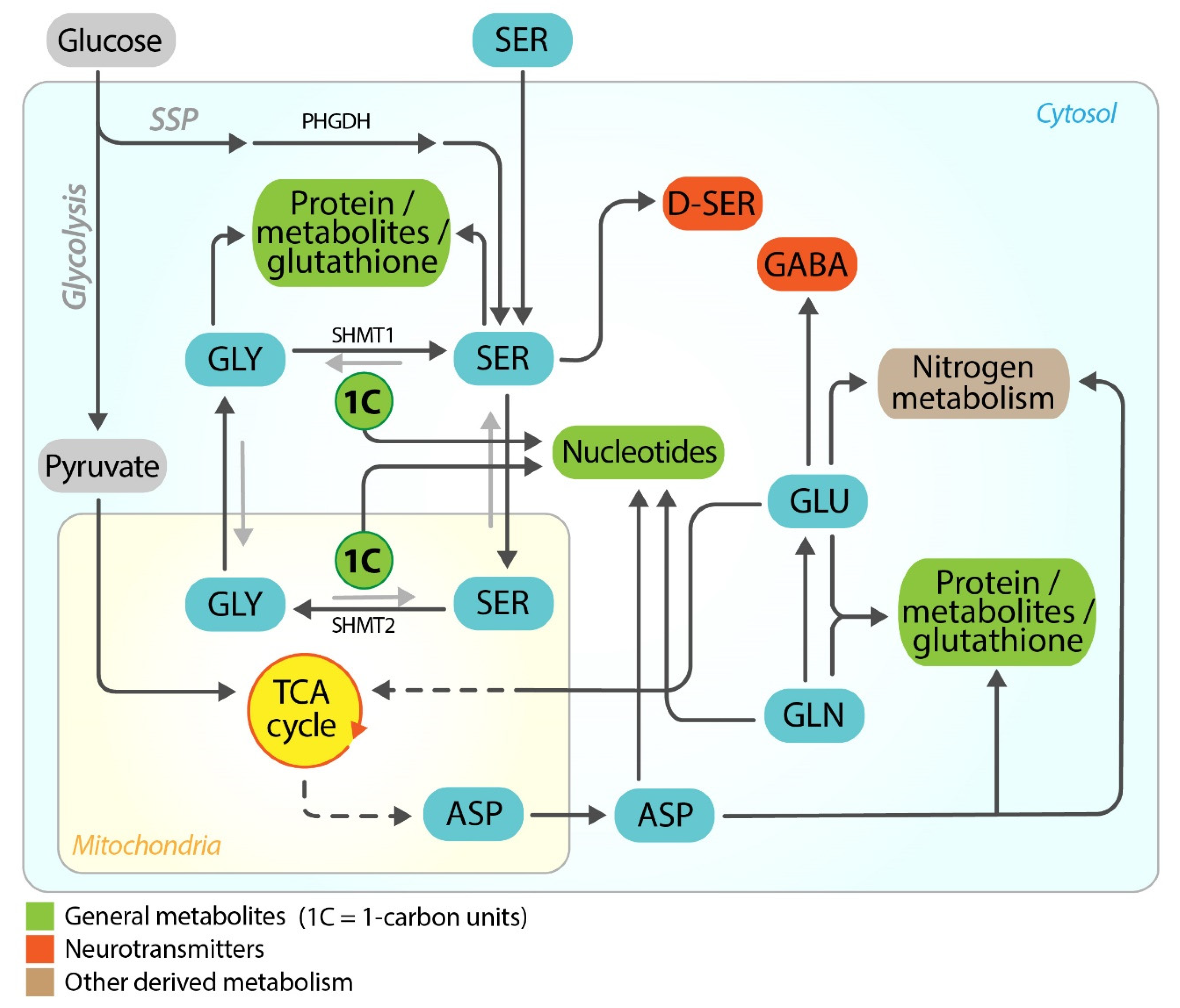
3. Serine/Glycine and Glutamate/Glutamine in Normal Brain
4. Serine/Glycine and Glutamate/Glutamine in Brain Metastasis
5. Importance of Amino Acids in Brain Parenchyma Invasion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CXCR-4 | C-X-C chemokine receptor type 4 |
| CCR7 | C-C chemokine receptor type 7 |
| CXCL12/SDF-1α | stromal cell-derived factor 1 |
| CCL21/6Ckine | C-C chemokine ligand 21 |
| BBB | blood–brain barrier |
| SSP | serine synthesis pathway |
| BEF | brain extracellular fluid |
| SGOC | serine and glycine one carbon metabolism |
| PHGDH | phosphoglycerate dehydrogenase |
| GlyR | strychnine-sensitive glycine receptor |
| GlyT1 | glycine transporter 1 |
| GlyT2 | glycine transporter 2 |
| NMDR | N-methyl-D-aspartate receptors |
| GABA | gamma-aminobutyric acid |
| SHMT2 | serine hydroxymethyltransferase 2 |
| MTHFD2 | methylenetetrahydrofolate dehydrogenase 2 |
| MTHFD1L | methylenetetrahydrofolate dehydrogenase 1L |
| PSAT | phosphoserine aminotransferase |
| PSP | phosphoserine phosphatase |
| GSH | glutathione |
| ROS | reactive oxygen species |
| PDAC | pancreatic ductal adenocarcinoma |
| mGluRs | metabotropic glutamate receptors |
| SLC1A4 | solute carrier 1A4 |
| SLC1A5 | solute carrier 1A5 |
| EAAT | excitatory amino acid transporter |
| SNAT3 | sodium neutral amino acid transporter 3 |
| THF | tetrahydrofolate |
| SHMT1 | serine hydroxymethyltransferase 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| TCA | tricarboxylic acid cycle |
References
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Lu, W.C.; Pardon, E.; Gumkowski, F.; Kaminska, G.; Kaminski, M. Specificity of adhesion between murine tumor cells and capillary endothelium: An in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987, 47, 1492–1496. [Google Scholar]
- Ewing, J. Neoplastic Diseases: A Treatise on Tumours. Br. J. Surg. 1928, 16, 174–175. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Ekici, K.; Temelli, O.; Dikilitas, M.; Dursun, I.H.; Kaplan, N.B.; Kekilli, E. Survival and prognostic factors in patients with brain metastasis: Single center experience. J. B.U.ON. 2016, 21, 958–963. [Google Scholar]
- Klotz, R.; Yu, M. Insights into brain metastasis: Recent advances in circulating tumor cell research. Cancer Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Djalilian, H.R.; Nussbaum, E.S.; Cho, K.H. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000, 17, 279–286. [Google Scholar] [CrossRef]
- Chow, B.W.; Gu, C. The Molecular Constituents of the Blood-Brain Barrier. Trends Neurosci. 2015, 38, 598–608. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Thrane, V.R.; Thrane, A.S.; Plog, B.A.; Thiyagarajan, M.; Iliff, J.J.; Deane, R.; Nagelhus, E.A.; Nedergaard, M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci. Rep. 2013, 3, 2582. [Google Scholar] [CrossRef]
- Natale, G.; Limanaqi, F.; Busceti, C.L.; Mastroiacovo, F.; Nicoletti, F.; Puglisi-Allegra, S.; Fornai, F. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. Front. Neurosci. 2021, 15, 639140. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Menter, D.G. Trophic factors and central nervous system metastasis. Cancer Metastasis Rev. 1995, 14, 303–321. [Google Scholar] [CrossRef]
- Drell, T.L., IV; Joseph, J.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 2003, 80, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; Niggemann, B.; Zanker, K.S.; Entschladen, F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by β-blockers. Cancer Res. 2001, 61, 2866–2869. [Google Scholar]
- Cutruzzolà, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose metabolism in the progression of prostate cancer. Front Physiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Paone, A.; Cutruzzolà, F.; Kobiela, T. Biophysical characterization of melanoma cell phenotype markers during metastatic progression. Eur. Biophys. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.K.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef]
- Lowery, F.J.; Yu, D. Brain metastasis: Unique challenges and open opportunities. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 49–57. [Google Scholar] [CrossRef]
- Basun, H.; Forssell, L.G.; Almkvist, O.; Cowburn, R.F.; Eklöf, R.; Winblad, B.; Wetterberg, L. Amino acid concentrations in cerebrospinal fluid and plasma in Alzheimer’s disease and healthy control subjects. J. Neural Transm. Park. Dis. Dement. Sect. 1990, 2, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Dolgodilina, E.; Imobersteg, S.; Laczko, E.; Welt, T.; Verrey, F.; Makrides, V. Brain interstitial fluid glutamine homeostasis is controlled by blood-brain barrier SLC7A5/LAT1 amino acid transporter. J. Cereb. Blood Flow Metab. 2016, 36, 1929–1941. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M.; et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef]
- Mathur, D.; Stratikopoulos, E.; Ozturk, S.; Steinbach, N.; Pegno, S.; Schoenfeld, S.; Yong, R.; Murty, V.V.; Asara, J.M.; Cantley, L.C.; et al. PTEN regulates glutamine flux to pyrimidine synthesis and sensitivity to dihydroorotate dehydrogenase inhibition. Cancer Discov. 2017, 7, 380–390. [Google Scholar] [CrossRef]
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, R. Transport of Amino Acids Across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Tabata, T.; Mitoma, J.; Yamada, K.; Yamasaki, M.; Makino, A.; Yamamoto, T.; Watanabe, M.; Kano, M.; Hirabayashi, Y. L-Serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc. Natl. Acad. Sci. USA 2000, 97, 11528–11533. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Yamada, K.; Furuya, S.; Mitoma, J.; Hirabayashi, Y.; Watanabe, M. 3-Phosphoglycerate dehydrogenase, a key enzyme for L-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J. Neurosci. 2001, 21, 7691–7704. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, J.T.; Ma, T.M.; Sason, H.; Rosenberg, D.; Ogo, T.; Furuya, S.; Snyder, S.H.; Wolosker, H. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J. Neurosci. 2013, 33, 12464–12469. [Google Scholar] [CrossRef]
- Yamashita, N.; Sakai, K.; Furuya, S.; Watanabe, M. Selective expression of L-serine synthetic enzyme 3PGDH in Schwann cells, perineuronal glia, and endoneurial fibroblasts along rat sciatic nerves and its upregulation after crush injury. Arch. Histol. Cytol. 2003, 66, 429–436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dickens, A.M.; Tovar-Y-Romo, L.B.; Yoo, S.W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M.; et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 2017, 10, eaai7696. [Google Scholar] [CrossRef] [PubMed]
- Neame, S.; Safory, H.; Radzishevsky, I.; Touitou, A.; Marchesani, F.; Marchetti, M.; Kellner, S.; Berlin, S.; Foltyn, V.N.; Engelender, S.; et al. The NMDA receptor activation by D-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc. Natl. Acad. Sci. USA 2019, 116, 20736–20742. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Radzishevsky, I. The serine shuttle between glia and neurons: Implications for neurotransmission and neurodegeneration. Biochem. Soc. Trans. 2013, 41, 1546–1550. [Google Scholar] [CrossRef]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.S.; Troncone, L.R.P. Glycine as a neurotransmitter in the forebrain: A short review. J. Neural Transm. 2009, 116, 1551–1560. [Google Scholar] [CrossRef]
- Hamberger, A.; Nyström, B.; Larsson, S.; Silfvenius, H.; Nordborg, C. Amino acids in the neuronal microenvironment of focal human epileptic lesions. Epilepsy Res. 1991, 9, 32–43. [Google Scholar] [CrossRef]
- Seidlitz, E.P.; Sharma, M.K.; Saikali, Z.; Ghert, M.; Singh, G. Cancer cell lines release glutamate into the extracellular environment. Clin. Exp. Metastasis 2009, 26, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Lin, J.H.C.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- Li, A.M.; Ducker, G.S.; Li, Y.; Seoane, J.A.; Xiao, Y.; Melemenidis, S.; Zhou, Y.; Liu, L.; Vanharanta, S.; Graves, E.E.; et al. Metabolic profiling reveals a dependency of human metastatic breast cancer on mitochondrial serine and one-carbon unit metabolism. Mol. Cancer Res. 2020, 18. [Google Scholar] [CrossRef]
- Kim, H.M.; Jung, W.H.; Koo, J.S. Site-specific metabolic phenotypes in metastatic breast cancer. J. Transl. Med. 2014, 12, 3428. [Google Scholar] [CrossRef]
- Blazquez, R.; Rietkötter, E.; Wenske, B.; Wlochowitz, D.; Sparrer, D.; Vollmer, E.; Müller, G.; Seegerer, J.; Sun, X.; Dettmer, K.; et al. LEF1 supports metastatic brain colonization by regulating glutathione metabolism and increasing ROS resistance in breast cancer. Int. J. Cancer 2020, 146, 3170–3183. [Google Scholar] [CrossRef]
- Chen, E.I.; Hewel, J.; Krueger, J.S.; Tiraby, C.; Weber, M.R.; Kralli, A.; Becker, K.; Yates, J.R.; Felding-Habermann, B. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007, 67, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Maher, E.A.; Marin-Valencia, I.; Bachoo, R.M.; Mashimo, T.; Raisanen, J.; Hatanpaa, K.J.; Jindal, A.; Jeffrey, F.M.; Choi, C.; Madden, C.; et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 2012, 25, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.K.; Jang, H.G.; Jha, A.K.; et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Kim, E.; Osorio-Vasquez, V.; Doll, S.; Bustraan, S.; Liang, R.J.; Luengo, A.; Davidson, S.M.; Ali, A.; Ferraro, G.B.; et al. Limited Environmental Serine and Glycine Confer Brain Metastasis Sensitivity to PHGDH Inhibition. Cancer Discov. 2020, 10, 1352–1373. [Google Scholar] [CrossRef]
- Banh, R.S.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.W.; Walters, B.; Kuljanin, M.; Gikandi, A.; Wang, H.; Mancias, J.D.; Schneider, R.J.; et al. Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer. Cell 2020, 183, 1202–1218.e25. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e7. [Google Scholar] [CrossRef]
- Sinha, S.; Fu, Y.Y.; Grimont, A.; Ketcham, M.; Lafaro, K.; Saglimbeni, J.A.; Askan, G.; Bailey, J.M.; Melchor, J.P.; Zhong, Y.; et al. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 2017, 77, 1868–1879. [Google Scholar] [CrossRef]
- Chen, J.; Lee, H.J.; Wu, X.; Huo, L.; Kim, S.J.; Xu, L.; Wang, Y.; He, J.; Bollu, L.R.; Gao, G.; et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015, 75, 554–565. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Q.; Bhutkar, A.; Galván, J.A.; Karamitopoulou, E.; Noordermeer, D.; Peng, M.W.; Piersigilli, A.; Perren, A.; Zlobec, I.; et al. GKAP Acts as a Genetic Modulator of NMDAR Signaling to Govern Invasive Tumor Growth. Cancer Cell 2018, 33, 736–751.e5. [Google Scholar] [CrossRef]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Wall, B.A.; Chen, S. The current management of brain metastasis in melanoma: A focus on riluzole. Expert Rev. Neurother. 2015, 15, 779–792. [Google Scholar] [CrossRef]
- Korangath, P.; Teo, W.W.; Sadik, H.; Han, L.; Mori, N.; Huijts, C.M.; Wildes, F.; Bharti, S.; Zhang, Z.; Santa-Maria, C.A.; et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin. Cancer Res. 2015, 21, 3263–3273. [Google Scholar] [CrossRef] [PubMed]
- Banda, M.; Speyer, C.L.; Semma, S.N.; Osuala, K.O.; Kounalakis, N.; Torres, K.E.T.; Barnard, N.J.; Kim, H.J.; Sloane, B.F.; Miller, F.R.; et al. Metabotropic glutamate receptor-1 contributes to progression in triple negative breast cancer. PLoS ONE 2014, 9, e81126. [Google Scholar] [CrossRef]
- Speyer, C.L.; Smith, J.S.; Banda, M.; DeVries, J.A.; Mekani, T.; Gorski, D.H. Metabotropic glutamate receptor-1: A potential therapeutic target for the treatment of breast cancer. Breast Cancer Res. Treat. 2012. [Google Scholar] [CrossRef]
- Speyer, C.L.; Hachem, A.H.; Assi, A.A.; Johnson, J.S.; DeVries, J.A.; Gorski, D.H. Metabotropic glutamate receptor-1 as a novel target for the antiangiogenic treatment of breast cancer. PLoS ONE 2014, 9, e88830. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, V.; Prasmickaite, L.; Vasiliauskaite, K.; Clancy, T.; Hovig, E. Melanoma brain colonization involves the emergence of a brain-adaptive phenotype. Oncoscience 2014. [Google Scholar] [CrossRef]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018. [Google Scholar] [CrossRef] [PubMed]
- Bouzidi, A.; Magnifico, M.C.; Paiardini, A.; Macone, A.; Boumis, G.; Giardina, G.; Rinaldo, S.; Liberati, F.R.; Lauro, C.; Limatola, C.; et al. Cytosolic serine hydroxymethyltransferase controls lung adenocarcinoma cells migratory ability by modulating AMP kinase activity. Cell Death Dis. 2020. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Elimination of substances from the brain parenchyma: Efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 2018, 15, 30. [Google Scholar] [CrossRef]
- Hosoya, K.I.; Ohtsuki, S.; Terasaki, T. Recent advances in the brain-to-blood efflux transport across the blood-brain barrier. Int. J. Pharm. 2002, 248, 15–29. [Google Scholar] [CrossRef]
- Cohen-Kashi-Malina, K.; Cooper, I.; Teichberg, V.I. Mechanisms of glutamate efflux at the blood-brain barrier: Involvement of glial cells. J. Cereb. Blood Flow Metab. 2012. [Google Scholar] [CrossRef]
- Fares, J.; Kanojia, D.; Rashidi, A.; Ulasov, I.; Lesniak, M.S. Genes that Mediate Metastasis across the Blood–Brain Barrier. Trends Cancer 2020, 6, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Fazakas, C.; Wilhelm, I.; Nagyoszi, P.; Farkas, A.E.; Haskó, J.; Molnár, J.; Bauer, H.; Bauer, H.C.; Ayaydin, F.; Dung, N.T.K.; et al. Transmigration of melanoma cells through the blood-brain barrier: Role of endothelial tight junctions and melanoma-released serine proteases. PLoS ONE 2011, 6, e20758. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci. Rep. 2018. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutruzzolà, F.; Bouzidi, A.; Liberati, F.R.; Spizzichino, S.; Boumis, G.; Macone, A.; Rinaldo, S.; Giardina, G.; Paone, A. The Emerging Role of Amino Acids of the Brain Microenvironment in the Process of Metastasis Formation. Cancers 2021, 13, 2891. https://doi.org/10.3390/cancers13122891
Cutruzzolà F, Bouzidi A, Liberati FR, Spizzichino S, Boumis G, Macone A, Rinaldo S, Giardina G, Paone A. The Emerging Role of Amino Acids of the Brain Microenvironment in the Process of Metastasis Formation. Cancers. 2021; 13(12):2891. https://doi.org/10.3390/cancers13122891
Chicago/Turabian StyleCutruzzolà, Francesca, Amani Bouzidi, Francesca Romana Liberati, Sharon Spizzichino, Giovanna Boumis, Alberto Macone, Serena Rinaldo, Giorgio Giardina, and Alessio Paone. 2021. "The Emerging Role of Amino Acids of the Brain Microenvironment in the Process of Metastasis Formation" Cancers 13, no. 12: 2891. https://doi.org/10.3390/cancers13122891
APA StyleCutruzzolà, F., Bouzidi, A., Liberati, F. R., Spizzichino, S., Boumis, G., Macone, A., Rinaldo, S., Giardina, G., & Paone, A. (2021). The Emerging Role of Amino Acids of the Brain Microenvironment in the Process of Metastasis Formation. Cancers, 13(12), 2891. https://doi.org/10.3390/cancers13122891









