Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies
Abstract
Simple Summary
Abstract
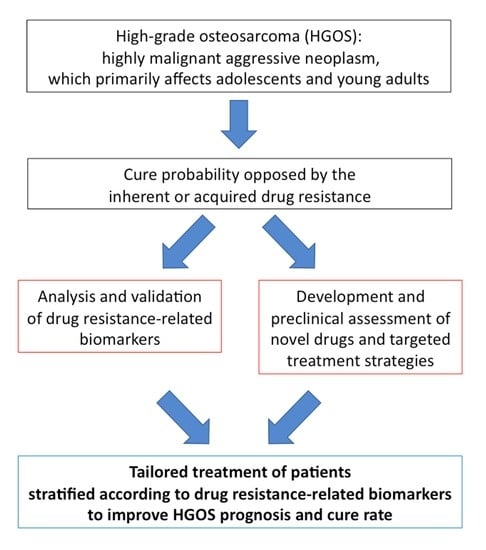
1. Introduction
2. Consolidated and Emerging Drug Resistance-Related Biomarkers
2.1. ABC Transporters
2.2. DNA Repair Factors
2.3. Methotrexate Resistance-Related Factors
2.4. Extracellular Vesicles
2.5. Non-Coding RNAs
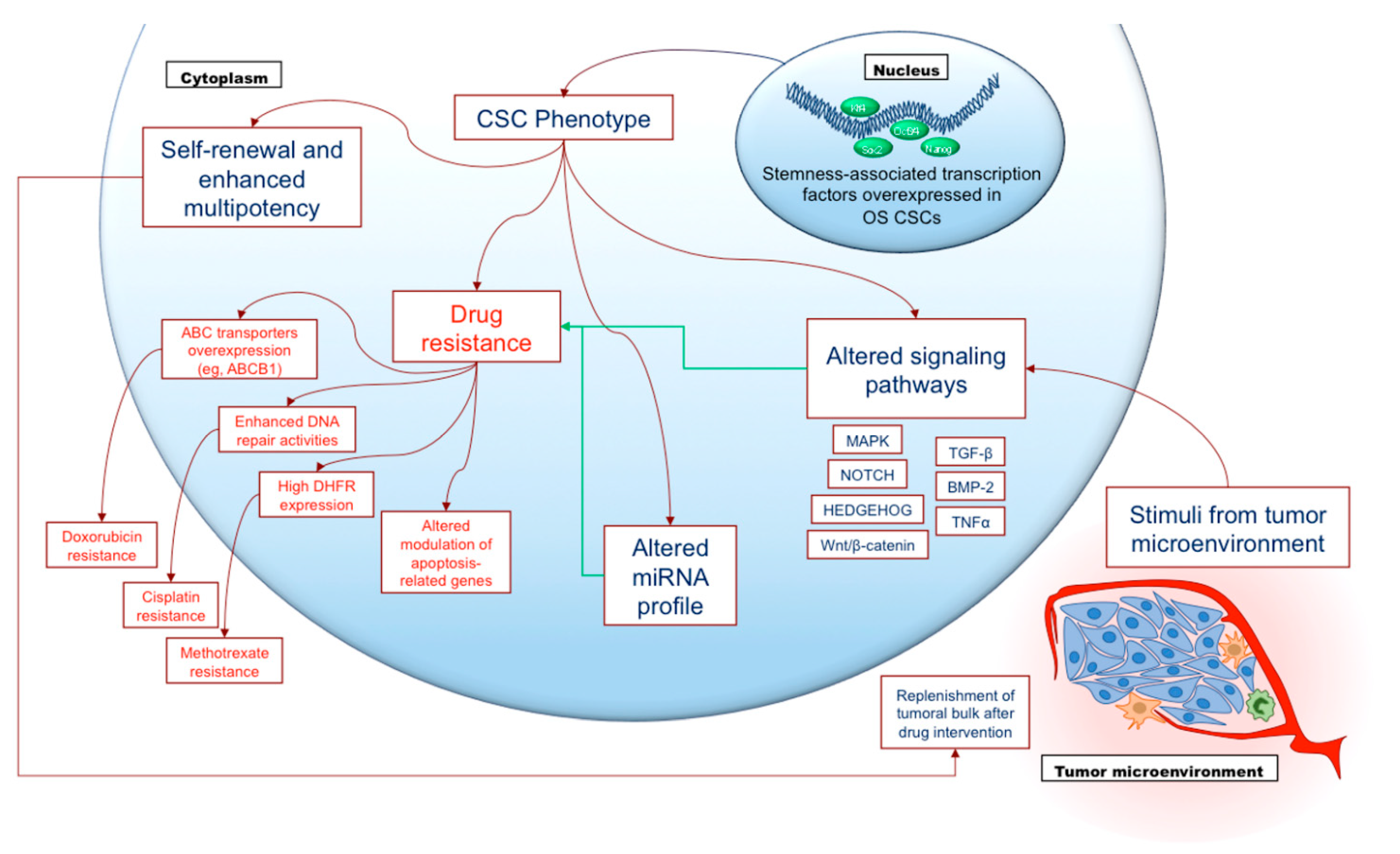
2.6. Cancer Stem Cells
3. Emerging Candidate Therapeutic Targets and Treatment Modalities
3.1. Chemorevertants and Emerging Modalities to Overcome Drug Resistance in HGOS
3.2. Modified Conventional Drugs to Overcome Resistance in HGOS
3.3. Nanocarriers and Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ABCB1 | ATP binding cassette subfamily B member 1 |
| ABCB2 | ATP binding cassette subfamily B member 2 |
| ABCB5 | ATP binding cassette subfamily B member 5 |
| ABCC1 | ATP binding cassette subfamily C member 1 |
| ABCC3 | ATP binding cassette subfamily C member 3 |
| ABCC4 | ATP binding cassette subfamily C member 4 |
| ABCC5 | ATP binding cassette subfamily C member 5 |
| ABCG1 | ATP binding cassette subfamily G member 1 |
| ABCG2 | ATP binding cassette subfamily G member 2 |
| ALCAM | anti-activated leukocyte adhesion molecule |
| ALDH1A1 | aldehyde dehydrogenase 1 family member A1 |
| APEX1 or APE1 | apurinic/apyrimidinic exonuclease 1 |
| ATR | ataxia telangiectasia and rad3 related |
| BCL2L1 | Bcl-2-like protein 1 |
| BER | base excision repair |
| BMP-2 | bone morphogenetic protein 2 |
| C/EBP-β LIP | CCAAT/enhancer binding protein- β liver inhibitory protein |
| CHOP | C/EBP-β homologous protein |
| circRNA | circular RNA |
| COL3A1 | collagen type III Alpha 1 chain |
| CPK-MB | creatine phosphokinase-MB |
| CSCs | cancer stem cells |
| DHFR | dihydrofolate reductase |
| DKK3 | dickkopf WNT signaling pathway inhibitor 3 |
| DR | direct repair |
| DSBR | double strand break repair |
| EPHA2 | ephrin alpha 2 |
| EPR | enhanced permeability and retention |
| ER | endoplasmic reticulum |
| ERAD/ERQC | ER-associated protein degradation/ER-quality control |
| ERCC | excision repair cross-complementation |
| ERCC1 | excision repair cross-complementation group 1 |
| ERCC2 | excision repair cross-complementation group 2 |
| ETC | electron transport chain |
| EVs | extracellular vesicles |
| FAO | fatty acid β-oxidation |
| FOXC2-AS1 | FOXC2 antisense RNA 1 |
| GSH | glutathione |
| GST | glutathione-S transferase |
| GSTP1 | glutathione S-transferase P1 |
| HA | hyaluronic acid |
| HGOS | high-grade osteosarcoma |
| HRR | homologous recombination repair |
| hTERT | human telomerase catalytic subunit |
| JIP1 | JNK-interacting protein 1 |
| KLF4 | Kruppel-like factor 4 |
| L-MTP-PE | liposomal muramyl tripeptide phosphatidylethanolamine |
| lncRNAs | long non-coding RNAs |
| MAPK | mitogen-activated protein kinase |
| MBTD1 | malignant brain tumor domain 1 |
| MCL1 | myeloid leukemia cell differentiation protein |
| miRNAs | micro RNAs |
| MMR | mismatch repair |
| MSCs | mesenchymal stem cells |
| ncRNAs | non-coding RNAs |
| NER | nucleotide excision repair |
| NHEJ | non homologous end joining |
| NO | nitric oxide |
| Notch1 | Notch homolog 1 |
| Oct3/4 | octamer-binding transcription factor 3/4 |
| ODRUL | osteosarcoma doxorubicin-resistance related up-regulated lncRNA |
| OXPHOS | oxidative-phosphorylation |
| PARP1 | poly(ADP-ribose) polymerase 1 |
| PLGA | poly(lactide-co-glycolide) |
| PTN | pleiotrophin |
| PUMA | p53 upregulated modulator of apoptosis |
| Rb | retinoblastoma gene |
| ROS | reactive oxygen species |
| siRNAs | small interfering RNA |
| SLC19A1 | membrane-located solute carrier family 19 (folate transporter) member 1 |
| sncRNAs | small non-coding RNAs |
| SNP | single nucleotide polymorphism |
| Sox2 | SRY (sex determining region Y)-box 2 |
| SSBR | single strand break repair |
| TCA | tricarboxylic acid |
| TGF-β1 | transforming growth factor beta 1 |
| TKIs | tyrosine kinase inhibitors |
| TNFα | tumor necrosis factor alpha |
| XIAP | X-linked inhibitor of apoptosis |
References
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Brard, C.; Piperno-Neumann, S.; Delaye, J.; Brugieres, L.; Hampson, L.V.; Le Teuff, G.; Le Deley, M.C.; Gaspar, N. Sarcome-13/OS2016 trial protocol: A multicentre, randomised, open-label, phase II trial of mifamurtide combined with postoperative chemotherapy for patients with newly diagnosed high-risk osteosarcoma. BMJ Open 2019, 9, e025877. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, R.; Stern, C.; Lisy, K.; White, S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma: A systematic review. JBI Database System. Rev. Implement Rep. 2017, 15, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Meazza, C.; Tamburini, A.; Bisogno, G.; Ferraresi, V.; Asaftei, S.; Milano, G.M.; Coccoli, L.; Manzitti, C.; Luksch, R.; et al. 1625MO: ABCB1/P-glycoprotein (Pgp) expression as stratifiation factor for treatment of patients with non metastatic extremity high grade osteosarcoma: An Italian Sarcoma Group (ISG) multicentric prospective trial (ISG/OS-2). Ann. Oncol. 2020, 31, S976. [Google Scholar] [CrossRef]
- Anninga, J.K.; Gelderblom, H.; Fiocco, M.; Kroep, J.R.; Taminiau, A.H.; Hogendoorn, P.C.; Egeler, R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur. J. Cancer 2011, 47, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Vella, S.; Tavanti, E.; Fanelli, M.; Picci, P.; Serra, M. Pharmacogenomics of second-line drugs used for treatment of unresponsive or relapsed osteosarcoma patients. Pharmacogenomics 2016, 17, 2097–2114. [Google Scholar] [CrossRef]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Roberts, R.D.; Lizardo, M.M.; Reed, D.R.; Hingorani, P.; Glover, J.; Allen-Rhoades, W.; Fan, T.; Khanna, C.; Sweet-Cordero, E.A.; Cash, T.; et al. Provocative questions in osteosarcoma basic and translational biology: A report from the Children’s Oncology Group. Cancer 2019, 125, 3514–3525. [Google Scholar] [CrossRef]
- Simpson, E.; Brown, H.L. Understanding osteosarcomas. JAAPA 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Grunewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef] [PubMed]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Baldini, N.; Scotlandi, K.; Barbanti-Brodano, G.; Manara, M.C.; Maurici, D.; Bacci, G.; Bertoni, F.; Picci, P.; Sottili, S.; Campanacci, M.; et al. Expression of P-glycoprotein in high-grade osteosarcomas in relation to clinical outcome. N. Engl. J. Med. 1995, 333, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.S.; Grogan, T.M.; Haddad, G.; DeBoer, G.; Ling, V. P-glycoprotein expression: Critical determinant in the response to osteosarcoma chemotherapy. J. Natl. Cancer Inst. 1997, 89, 1706–1715. [Google Scholar] [CrossRef][Green Version]
- Schwartz, C.L.; Gorlick, R.; Teot, L.; Krailo, M.; Chen, Z.; Goorin, A.; Grier, H.E.; Bernstein, M.L.; Meyers, P.; Children’s Oncology Group. Multiple drug resistance in osteogenic sarcoma: INT0133 from the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 2057–2062. [Google Scholar] [CrossRef]
- Serra, M.; Picci, P.; Ferrari, S.; Bacci, G. Prognostic value of P-glycoprotein in high-grade osteosarcoma. J. Clin. Oncol. 2007, 25, 4858–4860. [Google Scholar] [CrossRef]
- Serra, M.; Scotlandi, K.; Reverter-Branchat, G.; Ferrari, S.; Manara, M.C.; Benini, S.; Incaprera, M.; Bertoni, F.; Mercuri, M.; Briccoli, A.; et al. Value of P-glycoprotein and clinicopathologic factors as the basis for new treatment strategies in high-grade osteosarcoma of the extremities. J. Clin. Oncol. 2003, 21, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Z.; Zhang, Q.; De Amorim Bernstein, K.; Lozano-Calderon, S.; Choy, E.; Hornicek, F.J.; Duan, Z. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget 2016, 7, 83502–83513. [Google Scholar] [CrossRef]
- Pakos, E.E.; Ioannidis, J.P. The association of P-glycoprotein with response to chemotherapy and clinical outcome in patients with osteosarcoma. A meta-analysis. Cancer 2003, 98, 581–589. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Michelacci, F.; Sella, F.; Magagnoli, G.; Benini, S.; Gambarotti, M.; Palmerini, E.; Picci, P.; Serra, M.; Ferrari, S. Excision repair cross-complementation group 1 protein expression predicts survival in patients with high-grade, non-metastatic osteosarcoma treated with neoadjuvant chemotherapy. Histopathology 2015, 67, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Kastan, M.B. The DNA damage response: Implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med. 2015, 66, 129–143. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Luppi, S.; Magagnoli, F.; Picci, P.; Serra, M. Current understanding of pharmacogenetic implications of DNA damaging drugs used in osteosarcoma treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Nathrath, M.; Kremer, M.; Letzel, H.; Remberger, K.; Hofler, H.; Ulle, T. Expression of genes of potential importance in the response to chemotherapy in osteosarcoma patients. Klin. Padiatr. 2002, 214, 230–235. [Google Scholar] [CrossRef]
- Fanelli, M.; Tavanti, E.; Patrizio, M.P.; Vella, S.; Fernandez-Ramos, A.; Magagnoli, F.; Luppi, S.; Hattinger, C.M.; Serra, M. Cisplatin Resistance in Osteosarcoma: In vitro Validation of Candidate DNA Repair-Related Therapeutic Targets and Drugs for Tailored Treatments. Front. Oncol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Tavanti, E.; Luppi, S.; Magagnoli, F.; Picci, P.; Serra, M. Genetic testing for high-grade osteosarcoma: A guide for future tailored treatments? Expert Rev. Mol. Diagn. 2018, 18, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, D.; Cogdell, D.; Du, X.; Li, H.; Pang, Y.; Sun, Y.; Hu, L.; Sun, B.; Trent, J.; et al. APEX1 gene amplification and its protein overexpression in osteosarcoma: Correlation with recurrence, metastasis, and survival. Technol. Cancer Res. Treat. 2010, 9, 161–169. [Google Scholar] [CrossRef]
- Palmerini, E.; Setola, E.; Grignani, G.; D’Ambrosio, L.; Comandone, A.; Righi, A.; Longhi, A.; Cesari, M.; Paioli, A.; Hakim, R.; et al. High Dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma Patients: A Retrospective Series. Cells 2020, 9, 2389. [Google Scholar] [CrossRef]
- Grignani, G.; D’Ambrosio, L.; Pignochino, Y.; Palmerini, E.; Zucchetti, M.; Boccone, P.; Aliberti, S.; Stacchiotti, S.; Bertulli, R.; Piana, R.; et al. Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): An open-label, phase 1b study from the Italian Sarcoma Group. Lancet Oncol. 2018, 19, 1360–1371. [Google Scholar] [CrossRef]
- Li, X.; Dean, D.C.; Cote, G.M.; Zou, L.; Hornicek, F.J.; Yu, S.; Duan, Z. Inhibition of ATR-Chk1 signaling blocks DNA double-strand-break repair and induces cytoplasmic vacuolization in metastatic osteosarcoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920956900. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yung, B.C.; Kim, W.J.; Chen, X. Combination of nitric oxide and drug delivery systems: Tools for overcoming drug resistance in chemotherapy. J. Control Release 2017, 263, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hattinger, C.M.; Tavanti, E.; Fanelli, M.; Vella, S.; Picci, P.; Serra, M. Pharmacogenomics of genes involved in antifolate drug response and toxicity in osteosarcoma. Expert Opin. Drug Metab. Toxicol. 2017, 13, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Rucci, N. Tumour-Derived Extracellular Vesicles (EVs): A Dangerous “Message in a Bottle” for Bone. Int. J. Mol. Sci. 2019, 20, 4805. [Google Scholar] [CrossRef] [PubMed]
- Namee, N.M.; O’Driscoll, L. Extracellular vesicles and anti-cancer drug resistance. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cao, H.; Shen, B.; Feng, J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget 2015, 6, 37151–37168. [Google Scholar] [CrossRef]
- Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Baldini, N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int. J. Oncol. 2016, 49, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lin, Y.; Mi, C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol. Int. 2021, 45, 858–868. [Google Scholar] [CrossRef]
- Yoshida, A.; Fujiwara, T.; Uotani, K.; Morita, T.; Kiyono, M.; Yokoo, S.; Hasei, J.; Nakata, E.; Kunisada, T.; Ozaki, T. Clinical and Functional Significance of Intracellular and Extracellular microRNA-25-3p in Osteosarcoma. Acta Med. Okayama 2018, 72, 165–174. [Google Scholar] [CrossRef]
- Weinman, M.A.; Ramsey, S.A.; Leeper, H.J.; Brady, J.V.; Schlueter, A.; Stanisheuski, S.; Maier, C.S.; Miller, T.; Ruby, C.E.; Bracha, S. Exosomal proteomic signatures correlate with drug resistance and carboplatin treatment outcome in a spontaneous model of canine osteosarcoma. Cancer Cell Int. 2021, 21, 245. [Google Scholar] [CrossRef]
- Li, X.; Seebacher, N.A.; Hornicek, F.J.; Xiao, T.; Duan, Z. Application of liquid biopsy in bone and soft tissue sarcomas: Present and future. Cancer Lett. 2018, 439, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, Y.; Imbuluzqueta, E.; Patino-Garcia, A.; Blanco-Prieto, M.J. Antitumoral-Lipid-Based Nanoparticles: A Platform for Future Application in Osteosarcoma therapy. Curr. Pharm. Des. 2015, 21, 6104–6124. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Alvarez-Lorenzo, C.; Concheiro, A.; Santos, A.C.; Veiga, F.; Figueiras, A. Nanomedicine in osteosarcoma therapy: Micelleplexes for delivery of nucleic acids and drugs toward osteosarcoma-targeted therapies. Eur. J. Pharm. Biopharm. 2020, 148, 88–106. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zhang, R.H.; Su, X.; Qi, J.; Hu, Y.C.; Shi, J.T.; Zhang, K.; Wang, K.P.; Zhou, H.Y. Exosomes in osteosarcoma research and preclinical practice. Am. J. Transl. Res. 2021, 13, 882–897. [Google Scholar] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget 2015, 6, 8474–8490. [Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Drug resistance-related microRNAs in osteosarcoma: Translating basic evidence into therapeutic strategies. J. Cell Mol. Med. 2019, 23, 2280–2292. [Google Scholar] [CrossRef]
- Prudowsky, Z.D.; Yustein, J.T. Recent Insights into Therapy Resistance in Osteosarcoma. Cancers 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Huang, D.; Yu, C.Q.; Mou, Y.; Liu, Y.H.; Zhang, D.W.; Shi, F.J. MicroRNA-184 Modulates Doxorubicin Resistance in Osteosarcoma Cells by Targeting BCL2L1. Med. Sci. Monit. 2016, 22, 1761–1765. [Google Scholar] [CrossRef]
- Keremu, A.; Aini, A.; Maimaitirexiati, Y.; Liang, Z.; Aila, P.; Xierela, P.; Tusun, A.; Moming, H.; Yusufu, A. Overcoming cisplatin resistance in osteosarcoma through the miR-199a-modulated inhibition of HIF-1alpha. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Zhu, X.; Xu, R.; Xu, Y. miR-29 Family Inhibits Resistance to Methotrexate and Promotes Cell Apoptosis by Targeting COL3A1 and MCL1 in Osteosarcoma. Med. Sci. Monit. 2018, 24, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Long non-coding RNAs in osteosarcoma. Oncotarget 2017, 8, 20462–20475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zheng, X.; Zhong, W.; Tian, X.; Yin, B.; Tian, K.; Zhang, W. Long non-coding RNA LINC00161 sensitises osteosarcoma cells to cisplatin-induced apoptosis by regulating the miR-645-IFIT2 axis. Cancer Lett. 2016, 382, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, S.; Li, H.; Wang, J.; Wei, C.; Liu, Q. SNHG16 promotes osteosarcoma progression and enhances cisplatin resistance by sponging miR-16 to upregulate ATG4B expression. Biochem. Biophys. Res. Commun. 2019, 518, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Lu, C.; Qu, X.; Li, P.; Chen, K.; Shan, L.; Zhu, X. LncRNA TTN-AS1 regulates osteosarcoma cell apoptosis and drug resistance via the miR-134-5p/MBTD1 axis. Aging (Albany N. Y.) 2019, 11, 8374–8385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shen, X.; Zheng, M.; Zou, G.; Shen, Y. Knockdown Of lncRNA NCK-AS1 Regulates Cisplatin Resistance Through Modulating miR-137 In Osteosarcoma Cells. Onco Targets Ther. 2019, 12, 11057–11068. [Google Scholar] [CrossRef]
- Cheng, F.H.; Zhao, Z.S.; Liu, W.D. Long non-coding RNA ROR regulated ABCB1 to induce cisplatin resistance in osteosarcoma by sponging miR-153-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7256–7265. [Google Scholar] [CrossRef]
- Zhou, B.; Li, L.; Li, Y.; Sun, H.; Zeng, C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed. Pharmacother. 2018, 106, 850–857. [Google Scholar] [CrossRef]
- Han, Z.; Shi, L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem. Biophys. Res. Commun. 2018, 495, 947–953. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Q.; Wang, L.; Wang, S.; Sun, F.; Xu, D.; Jiang, J. Knockdown of the oncogene lncRNA NEAT1 restores the availability of miR-34c and improves the sensitivity to cisplatin in osteosarcoma. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.F.; Jiang, Y.Q.; Li, C.; Dai, X.K.; Wu, T.; Yin, W.Z. LncRNA-SARCC sensitizes osteosarcoma to cisplatin through the miR-143-mediated glycolysis inhibition by targeting Hexokinase 2. Cancer Biomark. 2020, 28, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Z.; Wu, S. Long non-coding RNA CTA sensitizes osteosarcoma cells to doxorubicin through inhibition of autophagy. Oncotarget 2017, 8, 31465–31477. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tian, C.; Zhang, H.; Han, K.; Zhou, M.; Gan, Z.; Zhu, H.; Min, D. Long noncoding RNA OIP5-AS1 mediates resistance to doxorubicin by regulating miR-137-3p/PTN axis in osteosarcoma. Biomed. Pharmacother. 2020, 128, 110201. [Google Scholar] [CrossRef]
- Meng, Y.; Hao, D.; Huang, Y.; Jia, S.; Zhang, J.; He, X.; Sun, L.; Liu, D. Positive feedback loop SP1/MIR17HG/miR-130a-3p promotes osteosarcoma proliferation and cisplatin resistance. Biochem. Biophys. Res. Commun. 2020, 521, 739–745. [Google Scholar] [CrossRef]
- Xie, X.; Liu, W.; Duan, Z.; Li, X.; Zhang, L.; Yang, G. LncRNA NORAD targets miR-410-3p to regulate drug resistance sensitivity of osteosarcoma. Cell Mol. Biol. 2020, 66, 143–148. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhu, K.P.; Ma, X.L. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017, 396, 66–75. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhu, K.P.; Shen, G.Q.; Zhu, Z.S. A long non-coding RNA contributes to doxorubicin resistance of osteosarcoma. Tumour. Biol. 2016, 37, 2737–2748. [Google Scholar] [CrossRef]
- Kun-Peng, Z.; Xiao-Long, M.; Chun-Lin, Z. LncRNA FENDRR sensitizes doxorubicin-resistance of osteosarcoma cells through down-regulating ABCB1 and ABCC1. Oncotarget 2017, 8, 71881–71893. [Google Scholar] [CrossRef]
- Zhu, K.P.; Zhang, C.L.; Shen, G.Q.; Zhu, Z.S. Long noncoding RNA expression profiles of the doxorubicin-resistant human osteosarcoma cell line MG63/DXR and its parental cell line MG63 as ascertained by microarray analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 8754–8773. [Google Scholar] [PubMed]
- Wang, J.Y.; Yang, Y.; Ma, Y.; Wang, F.; Xue, A.; Zhu, J.; Yang, H.; Chen, Q.; Chen, M.; Ye, L.; et al. Potential regulatory role of lncRNA-miRNA-mRNA axis in osteosarcoma. Biomed. Pharmacother. 2020, 121, 109627. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.P.; Kukekov, V.G.; Reith, J.D.; Tchigrinova, O.; Suslov, O.N.; Scott, E.W.; Ghivizzani, S.C.; Ignatova, T.N.; Steindler, D.A. Stem-like cells in bone sarcomas: Implications for tumorigenesis. Neoplasia 2005, 7, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Basu-Roy, U.; Basilico, C.; Mansukhani, A. Perspectives on cancer stem cells in osteosarcoma. Cancer Lett. 2013, 338, 158–167. [Google Scholar] [CrossRef]
- Basu-Roy, U.; Seo, E.; Ramanathapuram, L.; Rapp, T.B.; Perry, J.A.; Orkin, S.H.; Mansukhani, A.; Basilico, C. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene 2012, 31, 2270–2282. [Google Scholar] [CrossRef]
- Qi, X.T.; Li, Y.L.; Zhang, Y.Q.; Xu, T.; Lu, B.; Fang, L.; Gao, J.Q.; Yu, L.S.; Zhu, D.F.; Yang, B.; et al. KLF4 functions as an oncogene in promoting cancer stem cell-like characteristics in osteosarcoma cells. Acta Pharmacol. Sin. 2019, 40, 546–555. [Google Scholar] [CrossRef]
- Levings, P.P.; McGarry, S.V.; Currie, T.P.; Nickerson, D.M.; McClellan, S.; Ghivizzani, S.C.; Steindler, D.A.; Gibbs, C.P. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009, 69, 5648–5655. [Google Scholar] [CrossRef]
- Skoda, J.; Nunukova, A.; Loja, T.; Zambo, I.; Neradil, J.; Mudry, P.; Zitterbart, K.; Hermanova, M.; Hampl, A.; Sterba, J.; et al. Cancer stem cell markers in pediatric sarcomas: Sox2 is associated with tumorigenicity in immunodeficient mice. Tumour. Biol. 2016, 37, 9535–9548. [Google Scholar] [CrossRef]
- Yang, M.; Yan, M.; Zhang, R.; Li, J.; Luo, Z. Side population cells isolated from human osteosarcoma are enriched with tumor-initiating cells. Cancer Sci. 2011, 102, 1774–1781. [Google Scholar] [CrossRef]
- Fujii, H.; Honoki, K.; Tsujiuchi, T.; Kido, A.; Yoshitani, K.; Takakura, Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int. J. Oncol. 2009, 34, 1381–1386. [Google Scholar]
- Di Fiore, R.; Santulli, A.; Ferrante, R.D.; Giuliano, M.; De Blasio, A.; Messina, C.; Pirozzi, G.; Tirino, V.; Tesoriere, G.; Vento, R. Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J. Cell Physiol. 2009, 219, 301–313. [Google Scholar] [CrossRef]
- Roundhill, E.A.; Jabri, S.; Burchill, S.A. ABCG1 and Pgp identify drug resistant, self-renewing osteosarcoma cells. Cancer Lett. 2019, 453, 142–157. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yang, H.W.; Yang, L.C.; Lu, M.Y.; Tsai, L.L.; Yang, S.F.; Huang, Y.F.; Chou, M.Y.; Yu, C.C.; Hu, F.W. DHFR and MDR1 upregulation is associated with chemoresistance in osteosarcoma stem-like cells. Oncol. Lett. 2017, 14, 171–179. [Google Scholar] [CrossRef]
- Di Fiore, R.; Fanale, D.; Drago-Ferrante, R.; Chiaradonna, F.; Giuliano, M.; De Blasio, A.; Amodeo, V.; Corsini, L.R.; Bazan, V.; Tesoriere, G.; et al. Genetic and molecular characterization of the human osteosarcoma 3AB-OS cancer stem cell line: A possible model for studying osteosarcoma origin and stemness. J. Cell Physiol. 2013, 228, 1189–1201. [Google Scholar] [CrossRef]
- Di Fiore, R.; Drago-Ferrante, R.; Pentimalli, F.; Di Marzo, D.; Forte, I.M.; D’Anneo, A.; Carlisi, D.; De Blasio, A.; Giuliano, M.; Tesoriere, G.; et al. MicroRNA-29b-1 impairs in vitro cell proliferation, selfrenewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int. J. Oncol. 2014, 45, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Drago-Ferrante, R.; Pentimalli, F.; Di Marzo, D.; Forte, I.M.; Carlisi, D.; De Blasio, A.; Tesoriere, G.; Giordano, A.; Vento, R. Let-7d miRNA Shows Both Antioncogenic and Oncogenic Functions in Osteosarcoma-Derived 3AB-OS Cancer Stem Cells. J. Cell Physiol. 2016, 231, 1832–1841. [Google Scholar] [CrossRef]
- Gazitt, Y.; Kolaparthi, V.; Moncada, K.; Thomas, C.; Freeman, J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: Characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int. J. Oncol. 2009, 34, 551–561. [Google Scholar] [CrossRef][Green Version]
- Mu, X.; Isaac, C.; Greco, N.; Huard, J.; Weiss, K. Notch Signaling is Associated with ALDH Activity and an Aggressive Metastatic Phenotype in Murine Osteosarcoma Cells. Front. Oncol. 2013, 3, 143. [Google Scholar] [CrossRef]
- Hirotsu, M.; Setoguchi, T.; Sasaki, H.; Matsunoshita, Y.; Gao, H.; Nagao, H.; Kunigou, O.; Komiya, S. Smoothened as a new therapeutic target for human osteosarcoma. Mol. Cancer 2010, 9, 5. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Corver, W.E.; Paiva-Oliveira, D.I.; van den Akker, B.E.; Briaire-de-Bruijn, I.H.; Bovee, J.V.; Gomes, C.M.; Cleton-Jansen, A.M. Osteosarcoma Stem Cells Have Active Wnt/beta-catenin and Overexpress SOX2 and KLF4. J. Cell Physiol. 2016, 231, 876–886. [Google Scholar] [CrossRef]
- Wang, L.; Park, P.; Zhang, H.; La Marca, F.; Claeson, A.; Valdivia, J.; Lin, C.Y. BMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99-1 cell line. Cancer Biol. Ther. 2011, 11, 457–463. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Wu, H.; Zheng, J.; Yu, P.; Xu, L.; Jiang, P.; Gao, J.; Wang, H.; Zhang, Y. Transforming growth factor beta1 signal is crucial for dedifferentiation of cancer cells to cancer stem cells in osteosarcoma. Stem. Cells 2013, 31, 433–446. [Google Scholar] [CrossRef]
- Mori, T.; Sato, Y.; Miyamoto, K.; Kobayashi, T.; Shimizu, T.; Kanagawa, H.; Katsuyama, E.; Fujie, A.; Hao, W.; Tando, T.; et al. TNFalpha promotes osteosarcoma progression by maintaining tumor cells in an undifferentiated state. Oncogene 2014, 33, 4236–4241. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Fontes-Ribeiro, C.; Bovee, J.; Cleton-Jansen, A.M.; Gomes, C.M.F. IWR-1, a tankyrase inhibitor, attenuates Wnt/beta-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft. Cancer Lett. 2018, 414, 1–15. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, H.; Pan, Y.; Ren, J.; Ye, M.; Xia, F.; Huang, R.; Lin, Z.; Jiang, S.; Zhang, Y.; et al. Single-walled carbon nanotube: One specific inhibitor of cancer stem cells in osteosarcoma upon downregulation of the TGFbeta1 signaling. Biomaterials 2017, 149, 29–40. [Google Scholar] [CrossRef]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Yu, L.; Xia, K.; Gao, T.; Chen, J.; Zhang, Z.; Sun, X.; Simoes, B.M.; Eyre, R.; Fan, Z.; Guo, W.; et al. The Notch Pathway Promotes Osteosarcoma Progression through Activation of Ephrin Reverse Signaling. Mol. Cancer Res. 2019, 17, 2383–2394. [Google Scholar] [CrossRef]
- Milosevic, V.; Kopecka, J.; Salaroglio, I.C.; Libener, R.; Napoli, F.; Izzo, S.; Orecchia, S.; Ananthanarayanan, P.; Bironzo, P.; Grosso, F.; et al. Wnt/IL-1beta/IL-8 autocrine circuitries control chemoresistance in mesothelioma initiating cells by inducing ABCB5. Int. J. Cancer 2020, 146, 192–207. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, J.S. Increased multi-drug resistance and reduced apoptosis in osteosarcoma side population cells are crucial factors for tumor recurrence. Exp. Ther. Med. 2016, 12, 81–86. [Google Scholar] [CrossRef]
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Putz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014, 74, 4196–4207. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Wijers-Koster, P.M.; Abrunhosa, A.J.; Fontes-Ribeiro, C.; Bovee, J.V.; Cleton-Jansen, A.M.; Gomes, C.M. Chemotherapy induces stemness in osteosarcoma cells through activation of Wnt/beta-catenin signaling. Cancer Lett. 2016, 370, 286–295. [Google Scholar] [CrossRef]
- Gatti, L.; Beretta, G.L.; Cossa, G.; Zunino, F.; Perego, P. ABC transporters as potential targets for modulation of drug resistance. Mini Rev. Med. Chem. 2009, 9, 1102–1112. [Google Scholar] [CrossRef]
- Gillet, J.P.; Efferth, T.; Remacle, J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim. Biophys. Acta 2007, 1775, 237–262. [Google Scholar] [CrossRef]
- Fanelli, M.; Hattinger, C.M.; Vella, S.; Tavanti, E.; Michelacci, F.; Gudeman, B.; Barnett, D.; Picci, P.; Serra, M. Targeting ABCB1 and ABCC1 with their Specific Inhibitor CBT-1(R) can Overcome Drug Resistance in Osteosarcoma. Curr. Cancer Drug Targets 2016, 16, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Cao, H.; Qi, X.; Li, H.; Ye, P.; Wang, Z.; Wang, D.; Sun, M. Research Progress in Reversal of Tumor Multi-drug Resistance via Natural Products. Anticancer Agents Med. Chem. 2017, 17, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Barouch-Bentov, R.; Sauer, K. Mechanisms of drug resistance in kinases. Expert Opin. Investig. Drugs 2011, 20, 153–208. [Google Scholar] [CrossRef]
- Brozik, A.; Hegedus, C.; Erdei, Z.; Hegedus, T.; Ozvegy-Laczka, C.; Szakacs, G.; Sarkadi, B. Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: Substrates, chemosensitizers or inducers of acquired multidrug resistance? Expert Opin. Drug Metab. Toxicol. 2011, 7, 623–642. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, C.; Ozvegy-Laczka, C.; Szakacs, G.; Sarkadi, B. Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: Substrates and/or inhibitors? Curr. Cancer Drug Targets 2009, 9, 252–272. [Google Scholar] [CrossRef]
- Tavanti, E.; Sero, V.; Vella, S.; Fanelli, M.; Michelacci, F.; Landuzzi, L.; Magagnoli, G.; Versteeg, R.; Picci, P.; Hattinger, C.M.; et al. Preclinical validation of Aurora kinases-targeting drugs in osteosarcoma. Br J. Cancer 2013, 109, 2607–2618. [Google Scholar] [CrossRef]
- Melim, C.; Jarak, I.; Veiga, F.; Figueiras, A. The potential of micelleplexes as a therapeutic strategy for osteosarcoma disease. 3 Biotech 2020, 10, 147. [Google Scholar] [CrossRef]
- Scotlandi, K.; Hattinger, C.M.; Pellegrini, E.; Gambarotti, M.; Serra, M. Genomics and Therapeutic Vulnerabilities of Primary Bone Tumors. Cells 2020, 9, 968. [Google Scholar] [CrossRef]
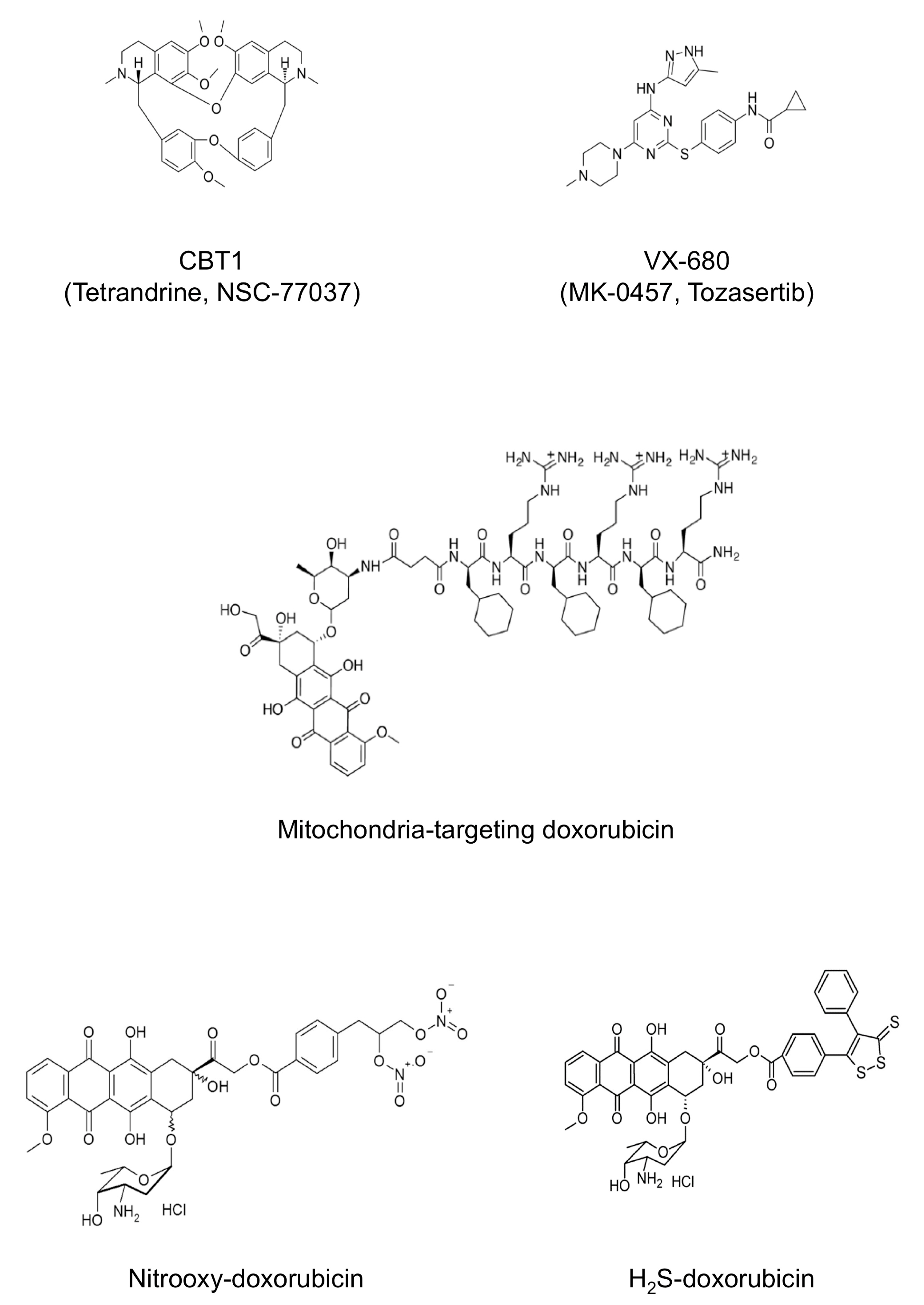
- Buondonno, I.; Gazzano, E.; Jean, S.R.; Audrito, V.; Kopecka, J.; Fanelli, M.; Salaroglio, I.C.; Costamagna, C.; Roato, I.; Mungo, E.; et al. Mitochondria-Targeted Doxorubicin: A New Therapeutic Strategy against Doxorubicin-Resistant Osteosarcoma. Mol. Cancer Ther. 2016, 15, 2640–2652. [Google Scholar] [CrossRef]
- Chamberlain, G.R.; Tulumello, D.V.; Kelley, S.O. Targeted delivery of doxorubicin to mitochondria. ACS Chem. Biol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.R.; Tulumello, D.V.; Riganti, C.; Liyanage, S.U.; Schimmer, A.D.; Kelley, S.O. Mitochondrial Targeting of Doxorubicin Eliminates Nuclear Effects Associated with Cardiotoxicity. ACS Chem. Biol. 2015, 10, 2007–2015. [Google Scholar] [CrossRef]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2013, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, E.; Rolando, B.; Chegaev, K.; Salaroglio, I.C.; Kopecka, J.; Pedrini, I.; Saponara, S.; Sorge, M.; Buondonno, I.; Stella, B.; et al. Folate-targeted liposomal nitrooxy-doxorubicin: An effective tool against P-glycoprotein-positive and folate receptor-positive tumors. J. Control Release 2018, 270, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Bratasz, A.; Weir, N.M.; Parinandi, N.L.; Zweier, J.L.; Sridhar, R.; Ignarro, L.J.; Kuppusamy, P. Reversal to cisplatin sensitivity in recurrent human ovarian cancer cells by NCX-4016, a nitro derivative of aspirin. Proc. Natl. Acad. Sci. USA 2006, 103, 3914–3919. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Kopecka, J.; Panada, E.; Barak, S.; Rubinstein, M. The role of C/EBP-beta LIP in multidrug resistance. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Salaroglio, I.C.; Panada, E.; Moiso, E.; Buondonno, I.; Provero, P.; Rubinstein, M.; Kopecka, J.; Riganti, C. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Mol. Cancer 2017, 16, 91. [Google Scholar] [CrossRef]
- Akman, M.; Belisario, D.C.; Salaroglio, I.C.; Kopecka, J.; Donadelli, M.; De Smaele, E.; Riganti, C. Hypoxia, endoplasmic reticulum stress and chemoresistance: Dangerous liaisons. J. Exp. Clin. Cancer Res. 2021, 40, 28. [Google Scholar] [CrossRef]
- Buondonno, I.; Gazzano, E.; Tavanti, E.; Chegaev, K.; Kopecka, J.; Fanelli, M.; Rolando, B.; Fruttero, R.; Gasco, A.; Hattinger, C.; et al. Endoplasmic reticulum-targeting doxorubicin: A new tool effective against doxorubicin-resistant osteosarcoma. Cell Mol. Life Sci. 2019, 76, 609–625. [Google Scholar] [CrossRef]
- Chegaev, K.; Rolando, B.; Cortese, D.; Gazzano, E.; Buondonno, I.; Lazzarato, L.; Fanelli, M.; Hattinger, C.M.; Serra, M.; Riganti, C.; et al. H2S-Donating Doxorubicins May Overcome Cardiotoxicity and Multidrug Resistance. J. Med. Chem. 2016, 59, 4881–4889. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Aller, S.G. Equilibrated atomic models of outward-facing P-glycoprotein and effect of ATP binding on structural dynamics. Sci. Rep. 2015, 5, 7880. [Google Scholar] [CrossRef]
- Gazzano, E.; Buondonno, I.; Marengo, A.; Rolando, B.; Chegaev, K.; Kopecka, J.; Saponara, S.; Sorge, M.; Hattinger, C.M.; Gasco, A.; et al. Hyaluronated liposomes containing H2S-releasing doxorubicin are effective against P-glycoprotein-positive/doxorubicin-resistant osteosarcoma cells and xenografts. Cancer Lett. 2019, 456, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Musso, L.; Dallavalle, S.; Artali, R.; Tinelli, S.; Colangelo, D.; Zunino, F.; De Cesare, M.; Beretta, G.L.; Zaffaroni, N. Design, modeling, synthesis and biological activity evaluation of camptothecin-linked platinum anticancer agents. Eur. J. Med. Chem. 2013, 63, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, J.; Ji, Z. Bifunctional Platinum(II) Complexes with Bisphosphonates Substituted Diamine Derivatives: Synthesis and In vitro Cytotoxicity. Chem. Biodivers 2017, 14. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Luo, C.; Zhu, C.; Zhang, C.; Guo, Z.; Wang, X. A Potential Bone-Targeting Hypotoxic Platinum(II) Complex with an Unusual Cytostatic Mechanism toward Osteosarcoma Cells. Inorg. Chem. 2018, 57, 3315–3322. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; Leon, I.E. In vitro and in vivo anticancer effects of two quinoline-platinum(II) complexes on human osteosarcoma models. Cancer Chemother. Pharmacol. 2019, 83, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Heymann, P.G.; Ziebart, T.; Kammerer, P.W.; Mandic, R.; Saydali, A.; Braun, A.; Neff, A.; Draenert, G.F. The enhancing effect of a laser photochemotherapy with cisplatin or zolendronic acid in primary human osteoblasts and osteosarcoma cells in vitro. J. Oral Pathol. Med. 2016, 45, 803–809. [Google Scholar] [CrossRef]
- Duchi, S.; Sotgiu, G.; Lucarelli, E.; Ballestri, M.; Dozza, B.; Santi, S.; Guerrini, A.; Dambruoso, P.; Giannini, S.; Donati, D.; et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: Effective photoinduced in vitro killing of osteosarcoma. J. Control Release 2013, 168, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Hu, H.Z.; Qing, X.C.; Zhang, Z.C.; Shao, Z.W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, W.; Yang, W.; Qing, X.; Shao, Z. Surface engineering of nanoparticles with ligands for targeted delivery to osteosarcoma. Colloids Surf. B Biointerfaces 2020, 190, 110891. [Google Scholar] [CrossRef]
- Skubitz, K.M. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in sarcoma. Cancer Investig. 2003, 21, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Haghiralsadat, F.; Amoabediny, G.; Sheikhha, M.H.; Zandieh-Doulabi, B.; Naderinezhad, S.; Helder, M.N.; Forouzanfar, T. New liposomal doxorubicin nanoformulation for osteosarcoma: Drug release kinetic study based on thermo and pH sensitivity. Chem. Biol. Drug Des. 2017, 90, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Federman, N.; Chan, J.; Nagy, J.O.; Landaw, E.M.; McCabe, K.; Wu, A.M.; Triche, T.; Kang, H.; Liu, B.; Marks, J.D.; et al. Enhanced growth inhibition of osteosarcoma by cytotoxic polymerized liposomal nanoparticles targeting the alcam cell surface receptor. Sarcoma 2012, 2012, 126906. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Nazmi, K.; De Boer, J.P.; Zandieh-Doulabi, B.; Forouzanfar, T.; Helder, M.N. EphA2 Targeted Doxorubicin-Nanoliposomes for Osteosarcoma Treatment. Pharm. Res. 2017, 34, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.L.; Spinelli, F.M.; Del Dago, D.; Icardi, A.; Demarchi, G.; Caon, I.; Garcia, M.; Bolontrade, M.F.; Passi, A.; Cristina, C.; et al. Co-treatment of tumor cells with hyaluronan plus doxorubicin affects endothelial cell behavior independently of VEGF expression. Oncotarget 2018, 9, 36585–36602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haghiralsadat, F.; Amoabediny, G.; Naderinezhad, S.; Forouzanfar, T.; Helder, M.N.; Zandieh-Doulabi, B. Preparation of PEGylated cationic nanoliposome-siRNA complexes for cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 684–692. [Google Scholar] [CrossRef]
- Posthumadeboer, J.; van Egmond, P.W.; Helder, M.N.; de Menezes, R.X.; Cleton-Jansen, A.M.; Belien, J.A.; Verheul, H.M.; van Royen, B.J.; Kaspers, G.J.; van Beusechem, V.W. Targeting JNK-interacting-protein-1 (JIP1) sensitises osteosarcoma to doxorubicin. Oncotarget 2012, 3, 1169–1181. [Google Scholar] [CrossRef]
- Niu, G.; Yousefi, B.; Qujeq, D.; Marjani, A.; Asadi, J.; Wang, Z.; Mir, S.M. Melatonin and doxorubicin co-delivered via a functionalized graphene-dendrimeric system enhances apoptosis of osteosarcoma cells. Mater. Sci. Eng. C Mater Biol. Appl. 2021, 119, 111554. [Google Scholar] [CrossRef]
- Low, S.A.; Yang, J.; Kopecek, J. Bone-targeted acid-sensitive doxorubicin conjugate micelles as potential osteosarcoma therapeutics. Bioconjug Chem. 2014, 25, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Jana, S.K.; Pramanik, N. Biomedical Application of Doxorubicin Coated Hydroxyapatite-Poly(lactide-co-glycolide) Nanocomposite for Controlling Osteosarcoma Therapeutics. J. Nanosci. Nanotechnol. 2020, 20, 3994–4004. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Zhao, L.; Chang, Y.; Zhang, Y.; Cui, Y.; Zhang, Z. Calcium-mineralized polypeptide nanoparticle for intracellular drug delivery in osteosarcoma chemotherapy. Bioact. Mater. 2020, 5, 721–731. [Google Scholar] [CrossRef]
- Morton, S.W.; Shah, N.J.; Quadir, M.A.; Deng, Z.J.; Poon, Z.; Hammond, P.T. Osteotropic therapy via targeted layer-by-layer nanoparticles. Adv. Healthc. Mater. 2014, 3, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef]
- Feng, S.; Wu, Z.X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z. Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar] [CrossRef]
- El-Shafie, S.; Fahmy, S.A.; Ziko, L.; Elzahed, N.; Shoeib, T.; Kakarougkas, A. Encapsulation of Nedaplatin in Novel PEGylated Liposomes Increases Its Cytotoxicity and Genotoxicity against A549 and U2OS Human Cancer Cells. Pharmaceutics 2020, 12, 863. [Google Scholar] [CrossRef]
- Son, K.D.; Kim, Y.J. Anticancer activity of drug-loaded calcium phosphate nanocomposites against human osteosarcoma. Biomater. Res. 2017, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Sumathra, M.; Sadasivuni, K.K.; Kumar, S.S.; Rajan, M. Cisplatin-Loaded Graphene Oxide/Chitosan/Hydroxyapatite Composite as a Promising Tool for Osteosarcoma-Affected Bone Regeneration. ACS Omega 2018, 3, 14620–14633. [Google Scholar] [CrossRef] [PubMed]
- Meshkini, A.; Oveisi, H. Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf. B Biointerfaces 2017, 158, 319–330. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.H.; Kim, J.H. Tangeretin-Assisted Platinum Nanoparticles Enhance the Apoptotic Properties of Doxorubicin: Combination Therapy for Osteosarcoma Treatment. Nanomaterials 2019, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, C.; Cheng, Y.; Yang, Z.; Zang, J.; Liu, J.; Chen, X. Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. ACS Appl. Mater. Interfaces 2015, 7, 27040–27048. [Google Scholar] [CrossRef] [PubMed]

| Name | Expression | Mechanism | References |
|---|---|---|---|
| LncRNA Targeting miRNAs | |||
| LINC00161 | Down-regulated | Promotes apoptosis by sponging miR-645 and upregulating IFIT2 | [54] |
| SNHG16 | Up-regulated | Increases cisplatin-resistance upregulating ATG4B by sponging miR-16 | [55] |
| TTN-AS1 | Up-regulated | Increases cisplatin-resistance promoting MBTD1 expression by targeting miR-134-5p | [56] |
| NCK-AS1 | Up-regulated | Increases cisplatin-resistance upregulating MRP1 by targeting miR-137 | [57] |
| ROR | Up-regulated | Increases cisplatin-resistance upregulating ABCB1 by targeting miR-153-3p | [58] |
| SNHG12 | Up-regulated | Increases doxorubicin resistance promoting the expression of MCL1 by targeting miR-320a | [59] |
| LUCAT1 | Up-regulated | Increases methotrexate resistance upregulating ABCB1 by targeting miR-200c | [60] |
| NEAT1 | Up-regulated | Increases cisplatin resistance sponging miR-34c | [61] |
| SARCC | Down-regulated | Increases cisplatin sensitivity promoting miR-43 expression, promoting down regulation of Hexokinase 2 | [62] |
| CTA | Down-regulated | Increases Doxorubicin sensitivity promoting apoptosis by binding miR-210 and inhibiting autophagy | [63] |
| OIP5-AS1 | Up-regulated | Increases doxorubicin resistance upregulating PTN by targeting miR-137-3p | [64] |
| MIR17HG | Up-regulated | Increases cisplatin resistance suppressing miR-130-3p and upregulating SP1 | [65] |
| NORAD | Up-regulated | Increases cisplatin resistance targeting miR-410-3p | [66] |
| LncRNA Targeting ABC Transporters | |||
| FOXC2-AS1 | Up-regulated | Increases doxorubicin resistance upregulating ABCB1 by increasing FOXC2 | [67] |
| ODRUL | Up-regulated | Increases doxorubicin resistance increasing ABCB1 expression | [68] |
| FENDRR | Down-regulated | Increases doxorubicin sensitivity promoting apoptosis and down regulating ABCB1 and ABCC1 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattinger, C.M.; Patrizio, M.P.; Fantoni, L.; Casotti, C.; Riganti, C.; Serra, M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers 2021, 13, 2878. https://doi.org/10.3390/cancers13122878
Hattinger CM, Patrizio MP, Fantoni L, Casotti C, Riganti C, Serra M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers. 2021; 13(12):2878. https://doi.org/10.3390/cancers13122878
Chicago/Turabian StyleHattinger, Claudia Maria, Maria Pia Patrizio, Leonardo Fantoni, Chiara Casotti, Chiara Riganti, and Massimo Serra. 2021. "Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies" Cancers 13, no. 12: 2878. https://doi.org/10.3390/cancers13122878
APA StyleHattinger, C. M., Patrizio, M. P., Fantoni, L., Casotti, C., Riganti, C., & Serra, M. (2021). Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers, 13(12), 2878. https://doi.org/10.3390/cancers13122878