Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay
Abstract
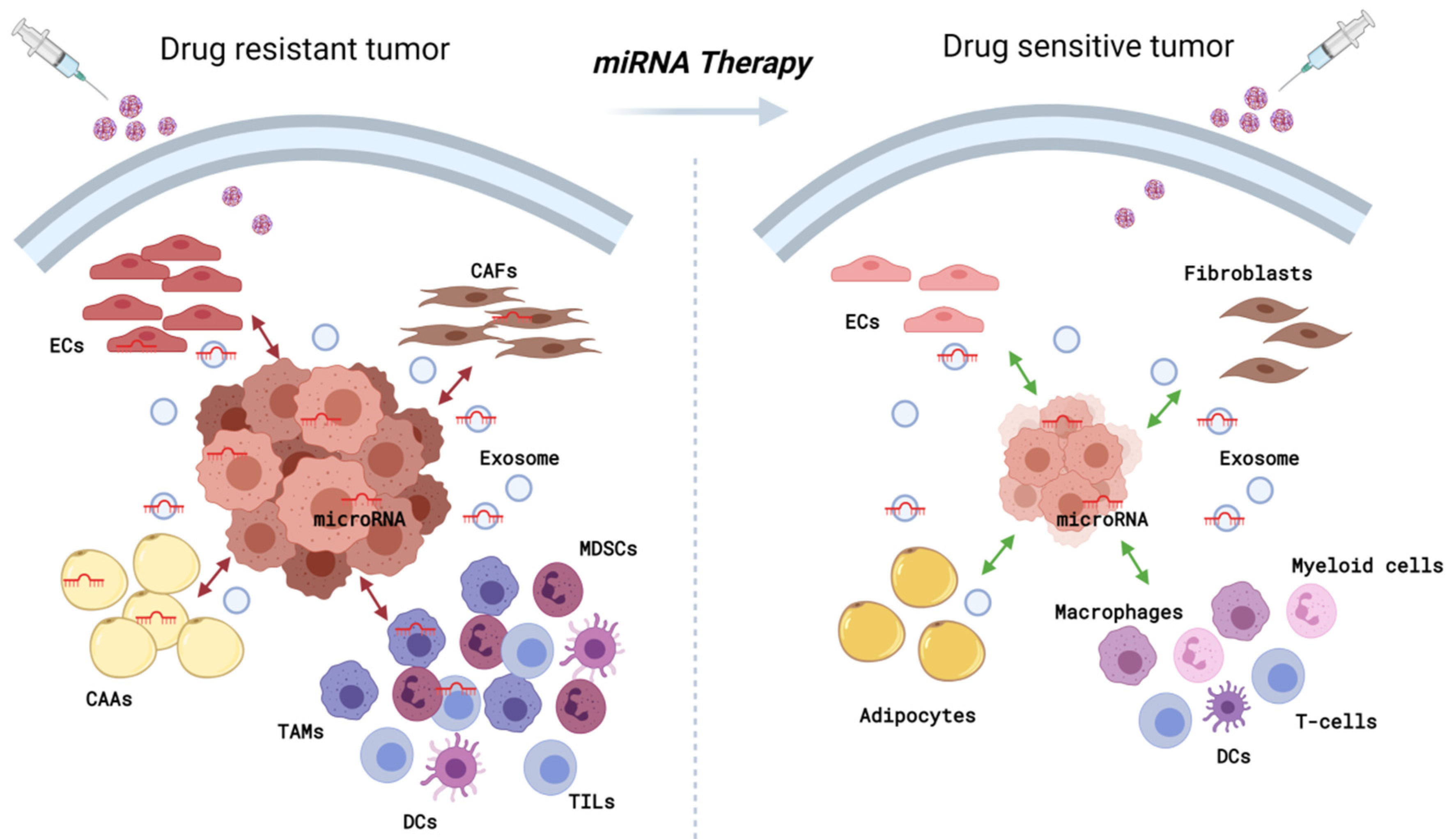
Simple Summary
Abstract
1. Introduction
1.1. MicroRNAs
1.2. The Tumor Microenvironment
2. Role of miRNAs in Modulating Breast Cancer Microenvironment
2.1. Fibroblasts
2.2. Immune Cells
2.2.1. Macrophages
2.2.2. Myeloid-Derived Suppressor Cells
2.2.3. T-Cells
2.2.4. Dendritic Cells
2.3. Endothelial Cells
2.4. Adipocytes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between Different Polychemotherapy Regimens for Early Breast Cancer: Meta-Analyses of Long-Term Outcome among 100,000 Women in 123 Randomised Trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Fortunato, O.; Iorio, M.V. The Therapeutic Potential of MicroRNAs in Cancer: Illusion or Opportunity? Pharmaceuticals 2020, 13, 438. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, J.; Chen, X.; Liu, L. Role of MicroRNA in Anticancer Drug Resistance. Int. J. Cancer 2010, 126, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.C.; Rueff, J.; Rodrigues, A.S. MicroRNAs and Cancer Drug Resistance. Methods Mol. Biol. 2016, 1395, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of MiRNA Expression across Human Tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Plantamura, I.; Cosentino, G.; Cataldo, A. MicroRNAs and DNA-Damaging Drugs in Breast Cancer: Strength in Numbers. Front. Oncol. 2018, 8, 352. [Google Scholar] [CrossRef]
- O’Neill, C.P.; Dwyer, R.M. Nanoparticle-Based Delivery of Tumor Suppressor MicroRNA for Cancer Therapy. Cells 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Kavishwar, A.; Ross, A.; Wang, P.; Tabassum, D.P.; Polyak, K.; Barteneva, N.; Petkova, V.; Pantazopoulos, P.; Tena, A.; et al. Combining MiR-10b-Targeted Nanotherapy with Low-Dose Doxorubicin Elicits Durable Regressions of Metastatic Breast Cancer. Cancer Res. 2015, 75, 4407–4415. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Silvestri, M.; Baselga, J.; Piccart, M.; Huober, J.; Izquierdo, M.; de la Pena, L.; Hilbers, F.S.; et al. Early Modulation of Circulating MicroRNAs Levels in HER2-Positive Breast Cancer Patients Treated with Trastuzumab-Based Neoadjuvant Therapy. Int. J. Mol. Sci. 2020, 21, 1386. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, F.; Jiao, Y.; Feng, J.; Tang, W.; Yao, H.; Gong, C.; Chen, J.; Su, F.; Zhang, Y.; et al. Reduced MiR-128 in Breast Tumor-Initiating Cells Induces Chemotherapeutic Resistance via Bmi-1 and ABCC5. Clin. Cancer Res. 2011, 17, 7105–7115. [Google Scholar] [CrossRef]
- Gao, M.; Miao, L.; Liu, M.; Li, C.; Yu, C.; Yan, H.; Yin, Y.; Wang, Y.; Qi, X.; Ren, J. MiR-145 Sensitizes Breast Cancer to Doxorubicin by Targeting Multidrug Resistance-Associated Protein-1. Oncotarget 2016, 7, 59714–59726. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Li, S.; Ma, R.; Cao, H.; Ji, M.; Jing, C.; Tang, J. The Function Role of MiR-181a in Chemosensitivity to Adriamycin by Targeting Bcl-2 in Low-Invasive Breast Cancer Cells. Cell Physiol. Biochem. 2013, 32, 1225–1237. [Google Scholar] [CrossRef]
- The Overexpression of Hypomethylated MiR-663 Induces Chemotherapy Resistance in Human Breast Cancer Cells by Targeting Heparin Sulfate Proteoglycan 2 (HSPG2)—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23436656/ (accessed on 1 June 2021).
- Pogribny, I.P.; Filkowski, J.N.; Tryndyak, V.P.; Golubov, A.; Shpyleva, S.I.; Kovalchuk, O. Alterations of MicroRNAs and Their Targets Are Associated with Acquired Resistance of MCF-7 Breast Cancer Cells to Cisplatin. Int. J. Cancer 2010, 127, 1785–1794. [Google Scholar] [CrossRef]
- Cataldo, A.; Cheung, D.G.; Balsari, A.; Tagliabue, E.; Coppola, V.; Iorio, M.V.; Palmieri, D.; Croce, C.M. MiR-302b Enhances Breast Cancer Cell Sensitivity to Cisplatin by Regulating E2F1 and the Cellular DNA Damage Response. Oncotarget 2016, 7, 786–797. [Google Scholar] [CrossRef]
- Cataldo, A.; Romero-Cordoba, S.; Plantamura, I.; Cosentino, G.; Hidalgo-Miranda, A.; Tagliabue, E.; Iorio, M.V. MiR-302b as a Combinatorial Therapeutic Approach to Improve Cisplatin Chemotherapy Efficacy in Human Triple-Negative Breast Cancer. Cancers 2020, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Bai, W.; Zhu, H.; Zhang, X.; Chen, Y.; Wang, L.; Yang, A.; Zhao, J.; Jia, L. MiR-221 Promotes Trastuzumab-Resistance and Metastasis in HER2-Positive Breast Cancers by Targeting PTEN. BMB Rep. 2014, 47, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.-D.; Ye, X.-M.; Zhang, M.-Y.; Zhu, H.-Y.; Xi, W.-J.; Huang, X.; Zhao, J.; Gu, B.; Zheng, G.-X.; Yang, A.-G.; et al. MiR-200c Suppresses TGF-β Signaling and Counteracts Trastuzumab Resistance and Metastasis by Targeting ZNF217 and ZEB1 in Breast Cancer. Int. J. Cancer 2014, 135, 1356–1368. [Google Scholar] [CrossRef]
- Cataldo, A.; Piovan, C.; Plantamura, I.; D’Ippolito, E.; Camelliti, S.; Casalini, P.; Giussani, M.; Déas, O.; Cairo, S.; Judde, J.-G.; et al. MiR-205 as Predictive Biomarker and Adjuvant Therapeutic Tool in Combination with Trastuzumab. Oncotarget 2018, 9, 27920–27928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast Cancer-Secreted MiR-939 Downregulates VE-Cadherin and Destroys the Barrier Function of Endothelial Monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Sahai, E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Morsing, M.; Kim, J.; Villadsen, R.; Goldhammer, N.; Jafari, A.; Kassem, M.; Petersen, O.W.; Rønnov-Jessen, L. Fibroblasts Direct Differentiation of Human Breast Epithelial Progenitors. Breast Cancer Res. 2020, 22, 102. [Google Scholar] [CrossRef]
- Rybinska, I.; Agresti, R.; Trapani, A.; Tagliabue, E.; Triulzi, T. Adipocytes in Breast Cancer, the Thick and the Thin. Cells 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Danforth, D.N. The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin. Breast Cancer 2021, 21, e63–e73. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Gonzalez, L.O.; Fraile, M.; Cid, S.; Schneider, J.; Vizoso, F.J. Breast Cancer Tumor Stroma: Cellular Components, Phenotypic Heterogeneity, Intercellular Communication, Prognostic Implications and Therapeutic Opportunities. Cancers 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Esposito, A.; Curigliano, G. Tumor-Stroma Crosstalk: Targeting Stroma in Breast Cancer. Curr. Opin. Oncol. 2014, 26, 551–555. [Google Scholar] [CrossRef]
- De Sanctis, F.; Ugel, S.; Facciponte, J.; Facciabene, A. The Dark Side of Tumor-Associated Endothelial Cells. Semin. Immunol. 2018, 35, 35–47. [Google Scholar] [CrossRef]
- Rybinska, I.; Mangano, N.; Tagliabue, E.; Triulzi, T. Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences. Int. J. Mol. Sci. 2021, 22, 3775. [Google Scholar] [CrossRef]
- Kim, I.S.; Zhang, X.H.-F. One Microenvironment Does Not Fit All: Heterogeneity beyond Cancer Cells. Cancer Metastasis Rev. 2016, 35, 601–629. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and Functionally Distinct Subclasses of Breast Cancer-Associated Fibroblasts Revealed by Single Cell RNA Sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Koo, J.S. Clinicopathologic Characteristics of Breast Cancer According to the Infiltrating Immune Cell Subtypes. Int. J. Mol. Sci. 2020, 21, 4438. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J.; Chen, H.; Li, J.; Zhang, C.; Jiang, X.; Ni, C. The Immunomodulatory Effects of Endocrine Therapy in Breast Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 19. [Google Scholar] [CrossRef]
- Triulzi, T.; Regondi, V.; De Cecco, L.; Cappelletti, M.R.; Di Modica, M.; Paolini, B.; Lollini, P.L.; Di Cosimo, S.; Sfondrini, L.; Generali, D.; et al. Early Immune Modulation by Single-Agent Trastuzumab as a Marker of Trastuzumab Benefit. Br. J. Cancer 2018, 119, 1487–1494. [Google Scholar] [CrossRef]
- Park, Y.H.; Lal, S.; Lee, J.E.; Choi, Y.-L.; Wen, J.; Ram, S.; Ding, Y.; Lee, S.-H.; Powell, E.; Lee, S.K.; et al. Chemotherapy Induces Dynamic Immune Responses in Breast Cancers That Impact Treatment Outcome. Nat. Commun. 2020, 11, 6175. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating MicroRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of Extracellular Circulating MicroRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Remaley, A.T. Lipid-Based Carriers of MicroRNAs and Intercellular Communication. Curr. Opin. Lipidol. 2012, 23, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Su, G.; Casper, C.; He, R.; Rehrauer, W.; Friedl, A. Heterogeneity of Gene Expression in Stromal Fibroblasts of Human Breast Carcinomas and Normal Breast. Oncogene 2010, 29, 1732–1740. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, L.; Hou, Y.; Sun, Y.; Wang, L.; Luo, H.; Peng, H.; Liu, M. Biological Characteristics and Genetic Heterogeneity between Carcinoma-Associated Fibroblasts and Their Paired Normal Fibroblasts in Human Breast Cancer. PLoS ONE 2013, 8, e60321. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Ma, L.; Liu, W.; Shao, C.; Shu, Y.; Shen, H. Exosomal MiRNAs and MiRNA Dysregulation in Cancer-Associated Fibroblasts. Mol. Cancer 2017, 16, 148. [Google Scholar] [CrossRef]
- Donnarumma, E.; Fiore, D.; Nappa, M.; Roscigno, G.; Adamo, A.; Iaboni, M.; Russo, V.; Affinito, A.; Puoti, I.; Quintavalle, C.; et al. Cancer-Associated Fibroblasts Release Exosomal MicroRNAs That Dictate an Aggressive Phenotype in Breast Cancer. Oncotarget 2017, 8, 19592–19608. [Google Scholar] [CrossRef]
- Baroni, S.; Romero-Cordoba, S.; Plantamura, I.; Dugo, M.; D’Ippolito, E.; Cataldo, A.; Cosentino, G.; Angeloni, V.; Rossini, A.; Daidone, M.G.; et al. Exosome-Mediated Delivery of MiR-9 Induces Cancer-Associated Fibroblast-like Properties in Human Breast Fibroblasts. Cell Death Dis. 2016, 7, e2312. [Google Scholar] [CrossRef]
- Cosentino, G.; Romero-Cordoba, S.; Plantamura, I.; Cataldo, A.; Iorio, M.V. MiR-9-Mediated Inhibition of EFEMP1 Contributes to the Acquisition of Pro-Tumoral Properties in Normal Fibroblasts. Cells 2020, 9, 2143. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Zeng, C.; Liu, C.; Hao, Q.; Li, W.; Zhang, K.; Zhang, W.; Wang, S.; Zhao, H.; et al. CD63+ Cancer-Associated Fibroblasts Confer Tamoxifen Resistance to Breast Cancer Cells through Exosomal MiR-22. Adv. Sci. (Weinh) 2020, 7, 2002518. [Google Scholar] [CrossRef]
- Sansone, P.; Berishaj, M.; Rajasekhar, V.K.; Ceccarelli, C.; Chang, Q.; Strillacci, A.; Savini, C.; Shapiro, L.; Bowman, R.L.; Mastroleo, C.; et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles. Cancer Res. 2017, 77, 1927–1941. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sun, X.; Su, Q.; You, C. Down-Regulation of MiR-29b in Carcinoma Associated Fibroblasts Promotes Cell Growth and Metastasis of Breast Cancer. Oncotarget 2017, 8, 39559–39570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Du, J.; Lin, D.; Li, F. MiR-3613-3p from Carcinoma-Associated Fibroblasts Exosomes Promoted Breast Cancer Cell Proliferation and Metastasis by Regulating SOCS2 Expression. IUBMB Life 2020, 72, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.-H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-Cell-Secreted Exosomal MiR-105 Promotes Tumour Growth through the MYC-Dependent Metabolic Reprogramming of Stromal Cells. Nat. Cell Biol. 2018, 20, 597–609. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Ireland, L.V.; Mielgo, A. Macrophages and Fibroblasts, Key Players in Cancer Chemoresistance. Front. Cell Dev. Biol. 2018, 6, 131. [Google Scholar] [CrossRef]
- Squadrito, M.L.; Etzrodt, M.; De Palma, M.; Pittet, M.J. MicroRNA-Mediated Control of Macrophages and Its Implications for Cancer. Trends Immunol. 2013, 34, 350–359. [Google Scholar] [CrossRef]
- Syed, S.N.; Frank, A.-C.; Raue, R.; Brüne, B. MicroRNA-A Tumor Trojan Horse for Tumor-Associated Macrophages. Cells 2019, 8, 1482. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.-Y.; Zen, K. Re-Polarization of Tumor-Associated Macrophages to pro-Inflammatory M1 Macrophages by MicroRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; So, A.Y.-L.; Sinha, N.; Gibson, W.S.J.; Taganov, K.D.; O’Connell, R.M.; Baltimore, D. MicroRNA-125b Potentiates Macrophage Activation. J. Immunol. 2011, 187, 5062–5068. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.-H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p Inhibits Breast Cancer Progression and Metastasis by Inducing Macrophage Polarization through Downregulated Expression of Fra-1 Proto-Oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Lee, J.-K.; Jeon, Y.-K.; Kim, C.-W. Exosome Derived from Epigallocatechin Gallate Treated Breast Cancer Cells Suppresses Tumor Growth by Inhibiting Tumor-Associated Macrophage Infiltration and M2 Polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal MiRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.-C.; Ebersberger, S.; Fink, A.F.; Lampe, S.; Weigert, A.; Schmid, T.; Ebersberger, I.; Syed, S.N.; Brüne, B. Apoptotic Tumor Cell-Derived MicroRNA-375 Uses CD36 to Alter the Tumor-Associated Macrophage Phenotype. Nat. Commun. 2019, 10, 1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Guo, J.; He, H.; Mi, X.; Chen, C.; Xie, J.; Wang, S.; Wu, P.; Cao, F.; et al. MiR-100 Maintains Phenotype of Tumor-Associated Macrophages by Targeting MTOR to Promote Tumor Metastasis via Stat5a/IL-1ra Pathway in Mouse Breast Cancer. Oncogenesis 2018, 7, 97. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Bobien, A.; Molnar, C.; Schmid, S.; Strotbek, M.; Boerries, M.; Busch, H.; Olayioye, M.A. MiR-149 Suppresses Breast Cancer Metastasis by Blocking Paracrine Interactions with Macrophages. Cancer Res. 2020, 80, 1330–1341. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Shi, B.; Ma, S.; Xu, Z.; Ge, Y.; Liu, Y.; Zheng, D.; Shi, J. Functions of MiR-146a and MiR-222 in Tumor-Associated Macrophages in Breast Cancer. Sci. Rep. 2015, 5, 18648. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for Myeloid-Derived Suppressor Cell Nomenclature and Characterization Standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Role of Tumor-Associated Myeloid Cells in Breast Cancer. Cells 2020, 9, 1785. [Google Scholar] [CrossRef]
- Kim, S.; Song, J.H.; Kim, S.; Qu, P.; Martin, B.K.; Sehareen, W.S.; Haines, D.C.; Lin, P.C.; Sharan, S.K.; Chang, S. Loss of Oncogenic MiR-155 in Tumor Cells Promotes Tumor Growth by Enhancing C/EBP-β-Mediated MDSC Infiltration. Oncotarget 2016, 7, 11094–11112. [Google Scholar] [CrossRef]
- Rong, Y.; Yuan, C.-H.; Qu, Z.; Zhou, H.; Guan, Q.; Yang, N.; Leng, X.-H.; Bu, L.; Wu, K.; Wang, F.-B. Doxorubicin Resistant Cancer Cells Activate Myeloid-Derived Suppressor Cells by Releasing PGE2. Sci. Rep. 2016, 6, 23824. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Zhuang, X.; Samykutty, A.; Mu, J.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Exosomes MiR-126a Released from MDSC Induced by DOX Treatment Promotes Lung Metastasis. Oncogene 2017, 36, 639–651. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, L.; Chen, Q.; Song, Y.; Xu, S.; Ma, F.; Wang, X.; Wang, J.; Yu, H.; Cao, X.; et al. MicroRNA-494 Is Required for the Accumulation and Functions of Tumor-Expanded Myeloid-Derived Suppressor Cells via Targeting of PTEN. J. Immunol. 2012, 188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, W.; Zhang, R.; Liu, P.; Ye, Y.; Yu, W.; Guo, X.; Yu, J. Cancer Exosome-Derived MiR-9 and MiR-181a Promote the Development of Early-Stage MDSCs via Interfering with SOCS3 and PIAS3 Respectively in Breast Cancer. Oncogene 2020, 39, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
- Burugu, S.; Asleh-Aburaya, K.; Nielsen, T.O. Immune Infiltrates in the Breast Cancer Microenvironment: Detection, Characterization and Clinical Implication. Breast Cancer 2017, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Mognol, G.P.; Spreafico, R.; Wong, V.; Scott-Browne, J.P.; Togher, S.; Hoffmann, A.; Hogan, P.G.; Rao, A.; Trifari, S. Exhaustion-Associated Regulatory Regions in CD8+ Tumor-Infiltrating T Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2776–E2785. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, D.; Shi, Y.; Wang, Y.; Joshi, R.; Yu, Q.; Liu, D.; Alotaibi, F.; Zhang, Y.; Wang, H.; et al. MiR-149-3p Reverses CD8+ T-Cell Exhaustion by Reducing Inhibitory Receptors and Promoting Cytokine Secretion in Breast Cancer Cells. Open Biol. 2019, 9, 190061. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Liu, F.; Yin, L.; Yu, B.; Wu, J. MicroRNA-155 May Affect Allograft Survival by Regulating the Expression of Suppressor of Cytokine Signaling 1. Med. Hypotheses 2011, 77, 682–684. [Google Scholar] [CrossRef]
- Ji, Y.; Fioravanti, J.; Zhu, W.; Wang, H.; Wu, T.; Hu, J.; Lacey, N.E.; Gautam, S.; Le Gall, J.B.; Yang, X.; et al. MiR-155 Harnesses Phf19 to Potentiate Cancer Immunotherapy through Epigenetic Reprogramming of CD8+ T Cell Fate. Nat. Commun. 2019, 10, 2157. [Google Scholar] [CrossRef]
- Hong, B.S.; Ryu, H.S.; Kim, N.; Kim, J.; Lee, E.; Moon, H.; Kim, K.H.; Jin, M.-S.; Kwon, N.H.; Kim, S.; et al. Tumor Suppressor MiRNA-204-5p Regulates Growth, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Cancer Res. 2019, 79, 1520–1534. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, C.; Li, Y.; Zhao, J.; Chen, C.; Zhou, Y.; Tao, Y.; Guo, M.; Qin, N.; Ren, T.; et al. MiR-21 Controls in Situ Expansion of CCR6+ Regulatory T Cells through PTEN/AKT Pathway in Breast Cancer. Immunol. Cell Biol. 2015, 93, 753–764. [Google Scholar] [CrossRef]
- Qin, A.; Wen, Z.; Zhou, Y.; Li, Y.; Li, Y.; Luo, J.; Ren, T.; Xu, L. MicroRNA-126 Regulates the Induction and Function of CD4(+) Foxp3(+) Regulatory T Cells through PI3K/AKT Pathway. J. Cell Mol. Med. 2013, 17, 252–264. [Google Scholar] [CrossRef]
- Palucka, K.; Coussens, L.M.; O’Shaughnessy, J. Dendritic Cells, Inflammation, and Breast Cancer. Cancer J. 2013, 19, 511–516. [Google Scholar] [CrossRef]
- Garg, A.D.; Vara Perez, M.; Schaaf, M.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Dendritic Cell-Based Anticancer Immunotherapy. Oncoimmunology 2017, 6, e1328341. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Iwanowycz, S.; Yu, F.; Jia, X.; Leng, S.; Wang, Y.; Li, W.; Huang, S.; Ai, W.; Fan, D. MicroRNA-155 Deficiency Impairs Dendritic Cell Function in Breast Cancer. Oncoimmunology 2016, 5, e1232223. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.; Wang, F.; Wang, J.; Liu, Q.; Saaoud, F.; Wang, Y.; Singh, U.P.; Chen, H.; Luo, M.; Ai, W.; et al. Overexpression of MicroRNA-155 Enhances the Efficacy of Dendritic Cell Vaccine against Breast Cancer. Oncoimmunology 2020, 9, 1724761. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gan, J.; Long, Z.; Guo, G.; Shi, X.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; et al. Targeted Delivery of Let-7b to Reprogramme Tumor-Associated Macrophages and Tumor Infiltrating Dendritic Cells for Tumor Rejection. Biomaterials 2016, 90, 72–84. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.; Zhang, Y.; Wang, Y.; Alotaibi, F.; Qiu, L.; Wang, H.; Peng, S.; Liu, Y.; Li, Q.; et al. MiRNA-5119 Regulates Immune Checkpoints in Dendritic Cells to Enhance Breast Cancer Immunotherapy. Cancer Immunol. Immunother. 2020, 69, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The Vascular Endothelium and Human Diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Liang, Z.; Bian, X.; Shim, H. Downregulation of MicroRNA-206 Promotes Invasion and Angiogenesis of Triple Negative Breast Cancer. Biochem. Biophys. Res. Commun. 2016, 477, 461–466. [Google Scholar] [CrossRef]
- D’Ippolito, E.; Plantamura, I.; Bongiovanni, L.; Casalini, P.; Baroni, S.; Piovan, C.; Orlandi, R.; Gualeni, A.V.; Gloghini, A.; Rossini, A.; et al. MiR-9 and MiR-200 Regulate PDGFRβ-Mediated Endothelial Differentiation of Tumor Cells in Triple-Negative Breast Cancer. Cancer Res. 2016, 76, 5562–5572. [Google Scholar] [CrossRef]
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J. MiRNA-29b Suppresses Tumor Growth through Simultaneously Inhibiting Angiogenesis and Tumorigenesis by Targeting Akt3. Cancer Lett. 2017, 397, 111–119. [Google Scholar] [CrossRef]
- Liby, T.A.; Spyropoulos, P.; Buff Lindner, H.; Eldridge, J.; Beeson, C.; Hsu, T.; Muise-Helmericks, R.C. Akt3 Controls Vascular Endothelial Growth Factor Secretion and Angiogenesis in Ovarian Cancer Cells. Int. J. Cancer 2012, 130, 532–543. [Google Scholar] [CrossRef]
- Luengo-Gil, G.; Gonzalez-Billalabeitia, E.; Perez-Henarejos, S.A.; Navarro Manzano, E.; Chaves-Benito, A.; Garcia-Martinez, E.; Garcia-Garre, E.; Vicente, V.; Ayala de la Peña, F. Angiogenic Role of MiR-20a in Breast Cancer. PLoS ONE 2018, 13, e0194638. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine Functions of VEGF in Breast Tumor Cells: Adhesion, Survival, Migration and Invasion. Cell Adhes. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernández-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; van Solingen, C.; Yu, J.; Fernández-Hernando, C.; et al. VEGF-Induced Expression of MiR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p Inhibits Invasion and Angiogenesis through Targeting VEGF-A in Breast Cancer. Cancer Gene. 2017, 24, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qiu, Y.; Yagüe, E.; Ji, W.; Liu, J.; Zhang, J. MiRNA-205 Targets VEGFA and FGF2 and Regulates Resistance to Chemotherapeutics in Breast Cancer. Cell Death Dis. 2016, 7, e2291. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Lu, M.M.; Qu, L.Y.; Xu, S.G.; Li, Y.Z.; Wang, M.Y.; Zhu, H.F.; Zhang, Z.Y.; He, G.Y.; et al. EPAS1 Targeting by MiR-152-3p in Paclitaxel-Resistant Breast Cancer. J. Cancer 2020, 11, 5822–5830. [Google Scholar] [CrossRef]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A MicroRNA Regulon That Mediates Endothelial Recruitment and Metastasis by Cancer Cells. Nature 2011, 481, 190–194. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Liang, H.; Ge, F.; Xu, Y.; Xiao, J.; Zhou, Z.; Liu, R.; Chen, C. MiR-153 Inhibits the Migration and the Tube Formation of Endothelial Cells by Blocking the Paracrine of Angiopoietin 1 in Breast Cancer Cells. Angiogenesis 2018, 21, 849–860. [Google Scholar] [CrossRef]
- McCann, J.V.; Xiao, L.; Kim, D.J.; Khan, O.F.; Kowalski, P.S.; Anderson, D.G.; Pecot, C.V.; Azam, S.H.; Parker, J.S.; Tsai, Y.S.; et al. Endothelial MiR-30c Suppresses Tumor Growth via Inhibition of TGF-β-Induced Serpine1. J. Clin. Investig. 2019, 129, 1654–1670. [Google Scholar] [CrossRef]
- Cui, Y.-X.; Bradbury, R.; Flamini, V.; Wu, B.; Jordan, N.; Jiang, W.G. MicroRNA-7 Suppresses the Homing and Migration Potential of Human Endothelial Cells to Highly Metastatic Human Breast Cancer Cells. Br. J. Cancer 2017, 117, 89–101. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J. Regulation of IL-6 Signaling by MiR-125a and Let-7e in Endothelial Cells Controls Vasculogenic Mimicry Formation of Breast Cancer Cells. BMB Rep. 2019, 52, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Qiu, W.; Xiao, Y.; Ma, J.; Xu, F.; Zhang, K.; Gao, Y.; Chen, Q.; Li, Y.; Li, H.; et al. MiR-199b-5p Suppresses Tumor Angiogenesis Mediated by Vascular Endothelial Cells in Breast Cancer by Targeting ALK1. Front. Genet. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Bovy, N.; Blomme, B.; Frères, P.; Dederen, S.; Nivelles, O.; Lion, M.; Carnet, O.; Martial, J.A.; Noël, A.; Thiry, M.; et al. Endothelial Exosomes Contribute to the Antitumor Response during Breast Cancer Neoadjuvant Chemotherapy via MicroRNA Transfer. Oncotarget 2015, 6, 10253–10266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-Associated Adipocytes: Key Players in Breast Cancer Progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Bandini, E.; Rossi, T.; Gallerani, G.; Fabbri, F. Adipocytes and MicroRNAs Crosstalk: A Key Tile in the Mosaic of Breast Cancer Microenvironment. Cancers 2019, 11, 1451. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Li, Z.; Sun, S.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Zhang, Y.; Sun, S.; et al. Exosomes from the Tumour-Adipocyte Interplay Stimulate Beige/Brown Differentiation and Reprogram Metabolism in Stromal Adipocytes to Promote Tumour Progression. J. Exp. Clin. Cancer Res. 2019, 38, 223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, S.; Li, Z.; Yang, Q.; Li, B.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Yang, C.; et al. Tumour-Originated Exosomal MiR-155 Triggers Cancer-Associated Cachexia to Promote Tumour Progression. Mol. Cancer 2018, 17, 155. [Google Scholar] [CrossRef]
- Lee, J.; Hong, B.S.; Ryu, H.S.; Lee, H.-B.; Lee, M.; Park, I.A.; Kim, J.; Han, W.; Noh, D.-Y.; Moon, H.-G. Transition into Inflammatory Cancer-Associated Adipocytes in Breast Cancer Microenvironment Requires MicroRNA Regulatory Mechanism. PLoS ONE 2017, 12, e0174126. [Google Scholar] [CrossRef]
- Rajarajan, D.; Selvarajan, S.; Charan Raja, M.R.; Kar Mahapatra, S.; Kasiappan, R. Genome-Wide Analysis Reveals MiR-3184-5p and MiR-181c-3p as a Critical Regulator for Adipocytes-Associated Breast Cancer. J. Cell Physiol. 2019, 234, 17959–17974. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Pan, C.; Drews-Elger, K.; Jang, K.; Besser, A.H.; Zhao, D.; Morata-Tarifa, C.; Kim, M.; Ince, T.A.; Azzam, D.J.; et al. Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/MiR-302b-Mediated Malignant Progression. Cancer Res. 2016, 76, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting Exosomes from Preadipocytes Inhibits Preadipocyte to Cancer Stem Cell Signaling in Early-Stage Breast Cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef] [PubMed]
| MicroRNA Expression | Cell Type | Roles |
|---|---|---|
| hsa-miR-9 | Fibroblasts (CAFs), MDSCs, tumor cells | Tumor growth, drug resistance [46,47]. MDSC activation and immunosuppression [69]. Promotion of vascular lacunae formation [82] |
| hsa-miR-22 | Fibroblast (CAFs) | Drug resistance [39] |
| hsa-miR-221 | Fibroblast (CAFs) | Drug resistance [40] |
| hsa-miR-29a | Fibroblast (CAFs) | Cell growth, drug resistance and metastasis [41] |
| hsa-miR-3613-3p | Fibroblast (CAFs) | Drug resistance, ROS production and metastasis [42] |
| hsa-miRNA-105 | Fibroblast (CAFs) | Metabolic reprogramming [43] |
| hsa-miR-155 | Macrophages, MDSCs, T-cells, Dendritic cells. | Inflammatory role [50,61,69,70,76,77,103] |
| hsa-miR-125b | Macrophages | Immune response [51] |
| hsa-miR-19a-3p | Macrophages | M2 phenotype inhibition [52] |
| hsa-miR-16 | Macrophages | M2 phenotype inhibition [53] |
| hsa-miR-503 | Macrophages | M2 phenotype induction [54] |
| hsa-miR-375 | Macrophages | Migration and infiltration into the tumor [55] |
| hsa-miR-100 | Macrophages | TAM phenotype Maintenance and immunosuppression [56] |
| hsa-miR-149-5p | Macrophages | M2 phenotype inhibition and lung metastasis reduction [57] |
| hsa-miR-146 | Macrophages | M2 phenotype induction [58] |
| hsa-miR-222 | Macrophages | Inhibition of TAM recruitment and of tumor growth [58] |
| hsa-miR-10 | Tumor cells | Activation of MDSC and drug resistance [62] |
| hsa-miR-126a | MDSCs, T-cells | Immunosuppression and induction of Th2 cells [63] |
| hsa-miR-494 | MDSCs | MDSC activation [64] |
| hsa-miR-181 | MDSCs | MDSC activation and immunosuppression [69] |
| hsa-miR-149-3p | T cells | Reversion of CD8+ T-cell exhaustion [68] |
| hsa-miR-204-5p | Tumor cells | Reduction of MDSCs, macrophages, and natural killer (NK) cells and increase of CD8+ and CD4+ cells [71] |
| hsa-miR-21 | T cells | Treg proliferation [72] |
| hsa-miR-126 | T cells | Treg proliferation [73] |
| hsa-let-7b | Dendritic cells and macrophages | Reprogramming of the functions of both tumor-infiltrating DCs and tumor-associated macrophages [78] |
| hsa-miR-5119 | Dendritic cells | Reduction of T-cell exhaustion and suppressed breast tumor growth [79] |
| hsa-miR-206 | Tumor cells | Inhibition of tumor invasion and angiogenesis [81] |
| hsa-miR-200c | Tumor cells | Inhibition of vascular lacunae formation [82] |
| hsa-miR-29b | Endothelial cells | Anti-angiogenesis [83,84] |
| hsa-miR-20a | Tumor cells | Angiogenesis [85,86] |
| hsa-miR-17-92 | Tumor cells | Angiogenesis [87] |
| hsa-miR-205 | Tumor cells | Anti-angiogenesis and drug sensitivity [89] |
| hsa-miR-152-3p | Tumor cells | Anti-angiogenesis and drug sensitivity [90] |
| hsa-miR-126 | Tumor cells, adipocytes | Inhibition of endothelial cell recruitment [91], metabolic reprogramming of adipocytes [102] |
| hsa-miR-140 | Tumor cells | Anti-angiogenesis and tumor inhibition [88] |
| hsa-miR-105 | Endothelial cells | Angiogenesis and metastasis formation [92] |
| hsa-miR-939 | Tumor cells, endothelial cells | Endothelial monolayer permeability and trans-endothelial migration of tumor cells [93] |
| hsa-miR-153 | Tumor cells | Inhibition of migration and of tube formation of endothelial cells [94] |
| hsa-miR-30c | Tumor cells, endothelial cells | Suppression of vascularization and inhibition of tumor growth [95] |
| hsa-miR-7 | Endothelial cells | Inhibition of proliferative, chemotactic and angiogenic-like homing characteristics of endothelial cells [96] |
| hsa-miR-125a | Endothelial cells | Downregulated by cisplatin treatment, induction of vasculogenic mimicry [97] |
| hsa-let-7e | Endothelial cells | Downregulated by cisplatin treatment, induction of vasculogenic mimicry [97] |
| hsa-miR-199b-5p | Tumor cells, endothelial cells | Inhibition of tumor growth, angiogenesis, formation of capillary-like tubular structures and migration [98] |
| hsa-miR-503 | Endothelial cells | Inhibition of tumor growth [99] |
| hsa-miR-155 | Tumor cells, adipocytes | Differentiation and alteration of the metabolism of surrounding adipocytes, tumor progression [103] |
| hsa-miR-144 | Tumor cells, adipocytes | Metabolic reprogramming of adipocytes [102] |
| mmu-miR-5112 | Adipocytes | Acquisition of cancer-associated-adipocytes inflammatory phenotypes [104] |
| hsa-miR-3184-5p | Adipocytes | Regulation of neoplastic transformation of BC cells [105] |
| hsa-miR-181c-3p | Adipocytes | Regulation of neoplastic transformation of BC cells [105] |
| hsa-miR-302b | Adipocytes | Production of pro-inflammatory cytokines [106] |
| hsa-miR-140 | Adipocytes | Regulation of neoplastic transformation of BC cells [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosentino, G.; Plantamura, I.; Tagliabue, E.; Iorio, M.V.; Cataldo, A. Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay. Cancers 2021, 13, 3691. https://doi.org/10.3390/cancers13153691
Cosentino G, Plantamura I, Tagliabue E, Iorio MV, Cataldo A. Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay. Cancers. 2021; 13(15):3691. https://doi.org/10.3390/cancers13153691
Chicago/Turabian StyleCosentino, Giulia, Ilaria Plantamura, Elda Tagliabue, Marilena V. Iorio, and Alessandra Cataldo. 2021. "Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay" Cancers 13, no. 15: 3691. https://doi.org/10.3390/cancers13153691
APA StyleCosentino, G., Plantamura, I., Tagliabue, E., Iorio, M. V., & Cataldo, A. (2021). Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay. Cancers, 13(15), 3691. https://doi.org/10.3390/cancers13153691









