Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men
Abstract
Simple Summary
Abstract
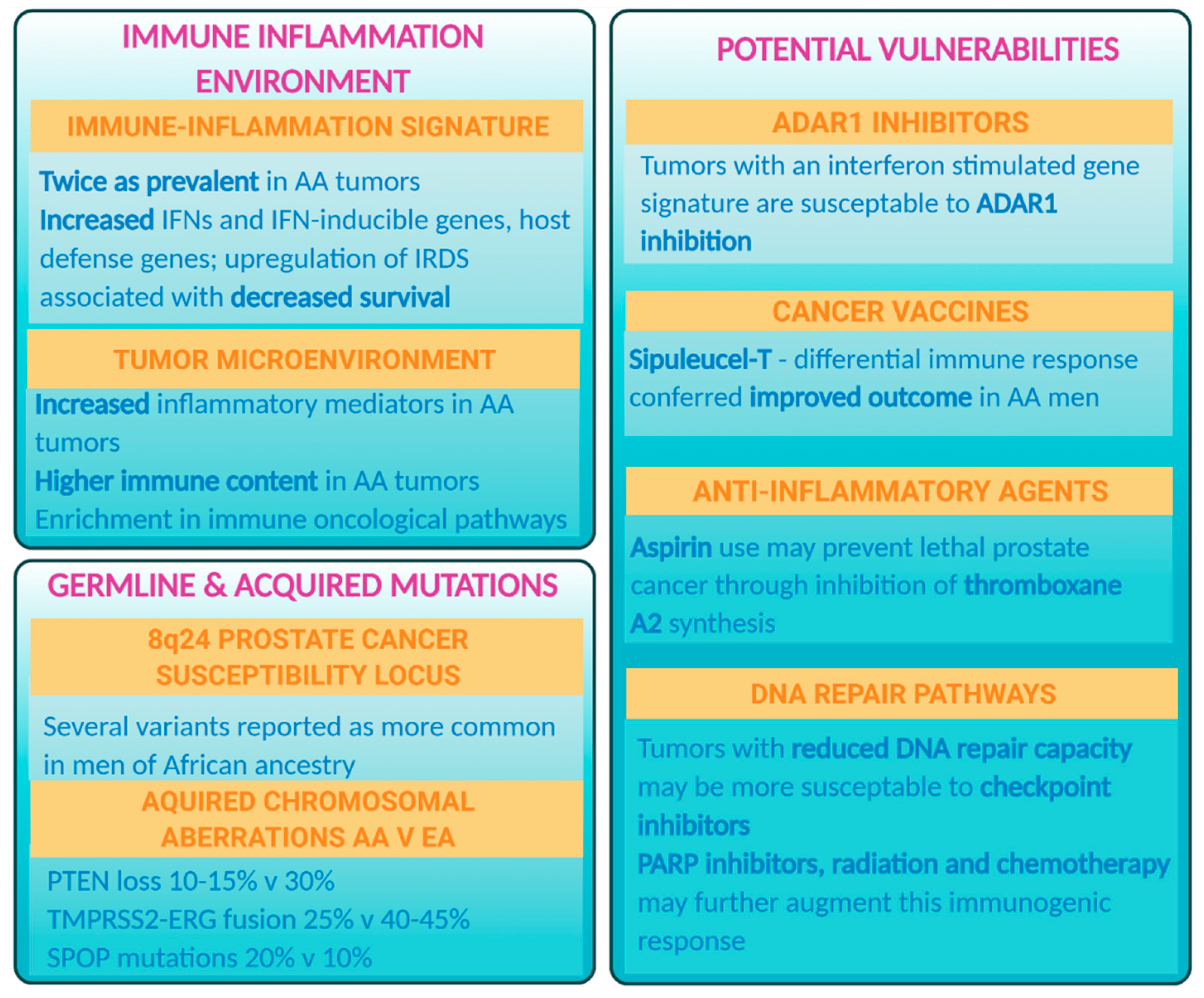
1. The Mutational and Immune–Oncologic Landscape of Prostate Tumors Differs between Populations
2. Inflammation as a Possible Driver of Aggressive Prostate Cancer in African American Men
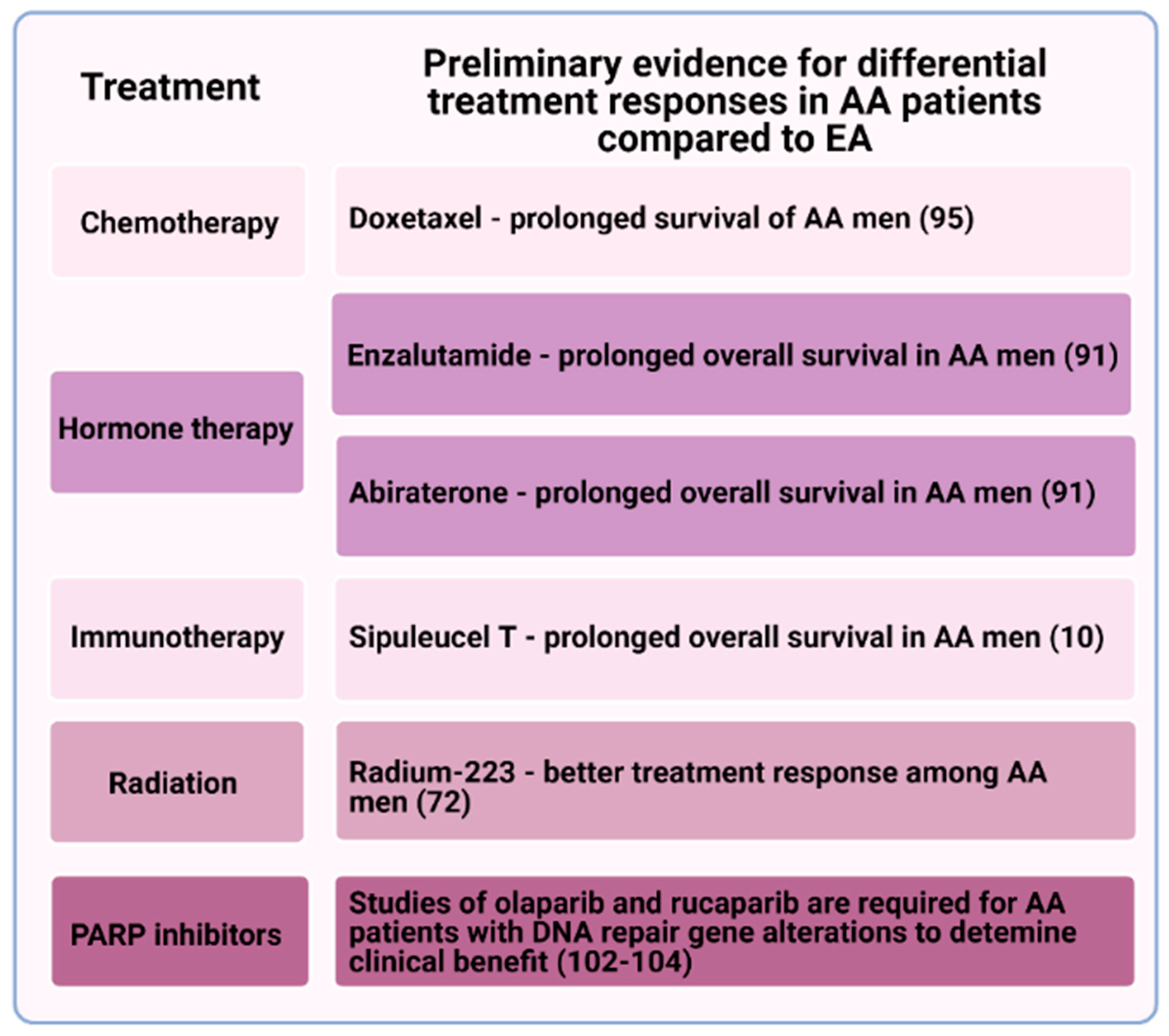
3. African American Men May Have a Differential Response to Certain Therapies for Metastatic Prostate Cancer
4. Radiation
5. Immunotherapy
6. Other Treatment Opportunities
7. Germline and Somatic Mutations in DNA Repair Pathways
8. Anti-Inflammatory Drug Aspirin for Prevention of Adverse Outcomes in African American Men with Prostate Cancer
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Butler, E.N.; Kelly, S.P.; Coupland, V.H.; Rosenberg, P.S.; Cook, M.B. Fatal prostate cancer incidence trends in the United States and England by race, stage, and treatment. Br. J. Cancer 2020, 123, 1–8. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Devesa, S.S.; Chang, B.L.; Bunker, C.H.; Cheng, I.; Cooney, K.; Eeles, R.; Fernandez, P.; Giri, V.N.; Gueye, S.M.; et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer 2013, 2013, 560857. [Google Scholar] [CrossRef]
- Tewari, A.; Horninger, W.; Pelzer, A.E.; Demers, R.; Crawford, E.D.; Gamito, E.J.; Divine, G.; Johnson, C.C.; Bartsch, G.; Menon, M. Factors contributing to the racial differences in prostate cancer mortality. BJU Int. 2005, 96, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Dess, R.T.; Hartman, H.E.; Mahal, B.A.; Soni, P.D.; Jackson, W.C.; Cooperberg, M.R.; Amling, C.L.; Aronson, W.J.; Kane, C.J.; Terris, M.K. Association of black race with prostate cancer–specific and other-cause mortality. JAMA Oncol. 2019, 5, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Faisal, F.A.; Sundi, D.; Tosoian, J.J.; Choeurng, V.; Alshalalfa, M.; Ross, A.E.; Klein, E.; Den, R.; Dicker, A.; Erho, N.; et al. Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur. Urol. 2016, 70, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Magi-Galluzzi, C.; Tsusuki, T.; Elson, P.; Simmerman, K.; Lafargue, C.; Esgueva, R.; Klein, E.; Rubin, M.A.; Zhou, M. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate 2011, 71, 489–497. [Google Scholar] [CrossRef]
- Rosen, P.; Sesterhenn, I.A.; Brassell, S.A.; McLeod, D.G.; Srivastava, S.; Dobi, A. Clinical potential of the ERG oncoprotein in prostate cancer. Nat. Rev. Urol. 2012, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.; Lee, H.J.; Ren, S.; Zi, X.; Zhang, Z.; Wang, H.; Yu, Y.; Yang, C.; Gao, X.; et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature 2020, 580, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Armstrong, A.J.; Ahaghotu, C.; McLeod, D.G.; Cooperberg, M.R.; Penson, D.F.; Kantoff, P.W.; Vogelzang, N.J.; Hussain, A.; Pieczonka, C.M.; et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: Outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 2020, 23, 517–526. [Google Scholar] [CrossRef]
- Hsing, A.W.; Chokkalingam, A.P. Prostate cancer epidemiology. Front. Biosci. 2006, 11, 1388–1413. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Platz, E.A.; Stampfer, M.J.; Willett, W.C. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J. Cancer 2007, 121, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Amundadottir, L.T.; Sulem, P.; Gudmundsson, J.; Helgason, A.; Baker, A.; Agnarsson, B.A.; Sigurdsson, A.; Benediktsdottir, K.R.; Cazier, J.B.; Sainz, J.; et al. A common variant associated with prostate cancer in European and African populations. Nat.Genet. 2006, 38, 652–658. [Google Scholar] [CrossRef]
- Zheng, S.L.; Sun, J.; Wiklund, F.; Smith, S.; Stattin, P.; Li, G.; Adami, H.O.; Hsu, F.C.; Zhu, Y.; Balter, K.; et al. Cumulative association of five genetic variants with prostate cancer. N. Engl. J. Med. 2008, 358, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Tsao, L.; Devesa, S.S. International trends and patterns of prostate cancer incidence and mortality. Int. J. Cancer 2000, 85, 60–67. [Google Scholar] [CrossRef]
- Shimizu, H.; Ross, R.K.; Bernstein, L.; Yatani, R.; Henderson, B.E.; Mack, T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer 1991, 63, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Mangtani, P.; Rachet, B.; Leon, D.A.; Coleman, M.P.; dos Santos Silva, I. Cancer incidence in South Asian migrants to England, 1986–2004: Unraveling ethnic from socioeconomic differentials. Int. J. Cancer 2013, 132, 1886–1894. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Alexander, D.D. A review and meta-analysis of prospective studies of red and processed meat, meat cooking methods, heme iron, heterocyclic amines and prostate cancer. Nutr. J. 2015, 14, 1–18. [Google Scholar] [CrossRef]
- Warner, W.A.; Lee, T.Y.; Fang, F.; Llanos, A.A.M.; Bajracharya, S.; Sundaram, V.; Badal, K.; Sookdeo, V.D.; Roach, V.; Lamont-Greene, M.; et al. The burden of prostate cancer in Trinidad and Tobago: One of the highest mortality rates in the world. Cancer Causes Control. 2018, 29, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.L.; Haiman, C.A.; Patterson, N.; McDonald, G.J.; Tandon, A.; Waliszewska, A.; Penney, K.; Steen, R.G.; Ardlie, K.; John, E.M.; et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl. Acad. Sci. USA 2006, 103, 14068–14073. [Google Scholar] [CrossRef]
- Lachance, J.; Berens, A.J.; Hansen, M.E.B.; Teng, A.K.; Tishkoff, S.A.; Rebbeck, T.R. Genetic Hitchhiking and Population Bottlenecks Contribute to Prostate Cancer Disparities in Men of African Descent. Cancer Res. 2018, 78, 2432–2443. [Google Scholar] [CrossRef]
- Maruthappu, M.; Barnes, I.; Sayeed, S.; Ali, R. Incidence of prostate and urological cancers in England by ethnic group, 2001-2007: A descriptive study. BMC Cancer 2015, 15, 753. [Google Scholar] [CrossRef] [PubMed]
- Petersen, D.C.; Jaratlerdsiri, W.; van Wyk, A.; Chan, E.K.F.; Fernandez, P.; Lyons, R.J.; Mutambirw, S.B.A.; van der Merwe, A.; Venter, P.A.; Bates, W.; et al. African KhoeSan ancestry linked to high-risk prostate cancer. BMC Med. Genom. 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Conti, D.V.; Darst, B.F.; Moss, L.C.; Saunders, E.J.; Sheng, X.; Chou, A.; Schumacher, F.R.; Olama, A.A.A.; Benlloch, S.; Dadaev, T.; et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 2021, 53, 65–75. [Google Scholar] [CrossRef]
- Heyns, C.F.; Fisher, M.; Lecuona, A.; van der Merwe, A. Prostate cancer among different racial groups in the Western Cape: Presenting features and management. S. Afr. Med. J. 2011, 101, 267–270. [Google Scholar] [CrossRef]
- Wallace, T.A.; Martin, D.N.; Ambs, S. Interactions among genes, tumor biology and the environment in cancer health disparities: Examining the evidence on a national and global scale. Carcinogenesis 2011, 32, 1107–1121. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prostate Cancer Disparities by Race and Ethnicity: From Nucleotide to Neighborhood. Cold Spring Harb. Perspect Med. 2018, 8. [Google Scholar] [CrossRef]
- Giri, V.N.; Beebe-Dimmer, J.L. Familial prostate cancer. Semin. Oncol. 2016, 43, 560–565. [Google Scholar] [CrossRef]
- Carpten, J.; Nupponen, N.; Isaacs, S.; Sood, R.; Robbins, C.; Xu, J.; Faruque, M.; Moses, T.; Ewing, C.; Gillanders, E.; et al. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat. Genet. 2002, 30, 181–184. [Google Scholar] [CrossRef]
- Rennert, H.; Zeigler-Johnson, C.M.; Addya, K.; Finley, M.J.; Walker, A.H.; Spangler, E.; Leonard, D.G.; Wein, A.; Malkowicz, S.B.; Rebbeck, T.R. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African American men. Cancer Epidemiol. Biomark. Prev. 2005, 14, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Ewing, C.M.; Ray, A.M.; Lange, E.M.; Zuhlke, K.A.; Robbins, C.M.; Tembe, W.D.; Wiley, K.E.; Isaacs, S.D.; Johng, D.; Wang, Y.; et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 2012, 366, 141–149. [Google Scholar] [CrossRef]
- Raymond, V.M.; Mukherjee, B.; Wang, F.; Huang, S.C.; Stoffel, E.M.; Kastrinos, F.; Syngal, S.; Cooney, K.A.; Gruber, S.B. Elevated risk of prostate cancer among men with Lynch syndrome. J. Clin. Oncol. 2013, 31, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Implications for RNase L in prostate cancer biology. Biochemistry 2003, 42, 1805–1812. [Google Scholar] [CrossRef]
- Haiman, C.A.; Chen, G.K.; Blot, W.J.; Strom, S.S.; Berndt, S.I.; Kittles, R.A.; Rybicki, B.A.; Isaacs, W.B.; Ingles, S.A.; Stanford, J.L.; et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011, 7, e1001387. [Google Scholar] [CrossRef]
- Haiman, C.A.; Patterson, N.; Freedman, M.L.; Myers, S.R.; Pike, M.C.; Waliszewska, A.; Neubauer, J.; Tandon, A.; Schirmer, C.; McDonald, G.J.; et al. Multiple regions within 8q24 independently affect risk for prostate cancer. Nat. Genet. 2007, 39, 638–644. [Google Scholar] [CrossRef]
- Robbins, C.; Torres, J.B.; Hooker, S.; Bonilla, C.; Hernandez, W.; Candreva, A.; Ahaghotu, C.; Kittles, R.; Carpten, J. Confirmation study of prostate cancer risk variants at 8q24 in African Americans identifies a novel risk locus. Genome Res. 2007, 17, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kibel, A.S.; Hu, J.J.; Turner, A.R.; Pruett, K.; Zheng, S.L.; Sun, J.; Isaacs, S.D.; Wiley, K.E.; Kim, S.T.; et al. Prostate cancer risk associated loci in African Americans. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2145–2149. [Google Scholar] [CrossRef]
- Cropp, C.D.; Robbins, C.M.; Sheng, X.; Hennis, A.J.; Carpten, J.D.; Waterman, L.; Worrell, R.; Schwantes-An, T.H.; Trent, J.M.; Haiman, C.A.; et al. 8q24 risk alleles and prostate cancer in African-Barbadian men. Prostate 2014, 74, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.; Sulem, P.; Manolescu, A.; Amundadottir, L.T.; Gudbjartsson, D.; Helgason, A.; Rafnar, T.; Bergthorsson, J.T.; Agnarsson, B.A.; Baker, A.; et al. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat. Genet. 2007, 39, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Rand, K.A.; Hazelett, D.J.; Ingles, S.A.; Kittles, R.A.; Strom, S.S.; Rybicki, B.A.; Nemesure, B.; Isaacs, W.B.; Stanford, J.L.; et al. Prostate Cancer Susceptibility in Men of African Ancestry at 8q24. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Sabelnykova, V.Y.; Yamaguchi, T.N.; Heisler, L.E.; Livingstone, J.; Huang, V.; Shiah, Y.J.; Yousif, F.; Lin, X.; Masella, A.P.; et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017, 541, 359–364. [Google Scholar] [CrossRef]
- Abate-Shen, C.; Shen, M.M. Molecular genetics of prostate cancer. Genes Dev. 2000, 14, 2410–2434. [Google Scholar] [CrossRef]
- Khani, F.; Mosquera, J.M.; Park, K.; Blattner, M.; O’Reilly, C.; MacDonald, T.Y.; Chen, Z.; Srivastava, A.; Tewari, A.K.; Barbieri, C.E.; et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin. Cancer Res. 2014, 20, 4925–4934. [Google Scholar] [CrossRef]
- Blackburn, J.; Vecchiarelli, S.; Heyer, E.E.; Patrick, S.M.; Lyons, R.J.; Jaratlerdsiri, W.; van Zyl, S.; Bornman, M.S.R.; Mercer, T.R.; Hayes, V.M. TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: A focus on Africa. Prostate 2019, 79, 1191–1196. [Google Scholar] [CrossRef]
- Koga, Y.; Song, H.; Chalmers, Z.R.; Newberg, J.; Kim, E.; Carrot-Zhang, J.; Piou, D.; Polak, P.; Abdulkadir, S.A.; Ziv, E.; et al. Genomic Profiling of Prostate Cancers from Men with African and European Ancestry. Clin. Cancer Res. 2020, 26, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Lucia, M.S.; Thompson, I.M., Jr.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar] [CrossRef]
- Klink, J.C.; Banez, L.L.; Gerber, L.; Lark, A.; Vollmer, R.T.; Freedland, S.J. Intratumoral inflammation is associated with more aggressive prostate cancer. World J. Urol. 2013, 31, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Dorsey, T.H.; Tang, W.; Jordan, S.V.; Loffredo, C.A.; Ambs, S. Aspirin Use Reduces the Risk of Aggressive Prostate Cancer and Disease Recurrence in African-American Men. Cancer Epidemiol. Biomark. Prev. 2017, 26, 845–853. [Google Scholar] [CrossRef]
- Van Dyke, A.L.; Cote, M.L.; Wenzlaff, A.S.; Land, S.; Schwartz, A.G. Cytokine SNPs: Comparison of allele frequencies by race and implications for future studies. Cytokine 2009, 46, 236–244. [Google Scholar] [CrossRef]
- Nedelec, Y.; Sanz, J.; Baharian, G.; Szpiech, Z.A.; Pacis, A.; Dumaine, A.; Grenier, J.C.; Freiman, A.; Sams, A.J.; Hebert, S.; et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 2016, 167, 657–669 e621. [Google Scholar] [CrossRef]
- Wallace, T.A.; Prueitt, R.L.; Yi, M.; Howe, T.M.; Gillespie, J.W.; Yfantis, H.G.; Stephens, R.M.; Caporaso, N.E.; Loffredo, C.A.; Ambs, S. Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res. 2008, 68, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Reams, R.R.; Agrawal, D.; Davis, M.B.; Yoder, S.; Odedina, F.T.; Kumar, N.; Higginbotham, J.M.; Akinremi, T.; Suther, S.; Soliman, K.F. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: A pilot project study. Infect. Agent. Cancer 2009, 4 (Suppl. 1), S3. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Kensler, K.H.; Hu, Z.; Zhang, Y.; Zhang, T.; Jiang, J.; Xu, M.; Pan, Y.; Long, M.; Montone, K.T.; et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 2020, 16, e1008641. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wallace, T.A.; Yi, M.; Magi-Galluzzi, C.; Dorsey, T.H.; Onabajo, O.O.; Obajemu, A.; Jordan, S.V.; Loffredo, C.A.; Stephens, R.M.; et al. IFNL4-DeltaG Allele Is Associated with an Interferon Signature in Tumors and Survival of African-American Men with Prostate Cancer. Clin. Cancer Res. 2018, 24, 5471–5481. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Ishwaran, H.; Yoon, T.; Nuyten, D.S.; Baker, S.W.; Khodarev, N.; Su, A.W.; Shaikh, A.Y.; Roach, P.; Kreike, B. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 18490–18495. [Google Scholar] [CrossRef]
- Gannon, H.S.; Zou, T.; Kiessling, M.K.; Gao, G.F.; Cai, D.; Choi, P.S.; Ivan, A.P.; Buchumenski, I.; Berger, A.C.; Goldstein, J.T. Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Golji, J.; Brodeur, L.K.; Chung, F.S.; Chen, J.T.; deBeaumont, R.S.; Bullock, C.P.; Jones, M.D.; Kerr, G.; Li, L. Tumor-derived IFN triggers chronic pathway agonism and sensitivity to ADAR loss. Nat. Med. 2019, 25, 95–102. [Google Scholar] [CrossRef]
- Prokunina-Olsson, L.; Muchmore, B.; Tang, W.; Pfeiffer, R.M.; Park, H.; Dickensheets, H.; Hergott, D.; Porter-Gill, P.; Mumy, A.; Kohaar, I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet. 2013, 45, 164. [Google Scholar] [CrossRef]
- Minas, T.Z.; Tang, W.; Smith, C.J.; Onabajo, O.O.; Obajemu, A.; Dorsey, T.H.; Jordan, S.V.; Obadi, O.M.; Ryan, B.M.; Prokunina-Olsson, L.; et al. IFNL4-ΔG is associated with prostate cancer among men at increased risk of sexually transmitted infections. Commun. Biol. 2018, 1, 191. [Google Scholar] [CrossRef]
- Vidal, A.C.; Oyekunle, T.; Howard, L.E.; Shivappa, N.; De Hoedt, A.; Figueiredo, J.C.; Taioli, E.; Fowke, J.H.; Lin, P.H.; Hebert, J.R.; et al. Dietary inflammatory index (DII) and risk of prostate cancer in a case-control study among Black and White US Veteran men. Prostate Cancer Prostatic Dis. 2019, 22, 580–587. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.A.; Downey, R.F.; Seufert, C.J.; Schetter, A.; Dorsey, T.H.; Johnson, C.A.; Goldman, R.; Loffredo, C.A.; Yan, P.; Sullivan, F.J.; et al. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis 2014, 35, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Javier, R.; Ji, Y.; Zheng, S.L.; Xu, J.; Brendler, C.B.; Crawford, S.E.; Pierce, B.L.; Vander Griend, D.J.; Franco, O.E. Elevation of stromal-derived mediators of inflammation promote prostate cancer progression in African-American men. Cancer Res. 2018, 78, 6134–6145. [Google Scholar] [CrossRef]
- Awasthi, S.; Berglund, A.; Abraham-Miranda, J.; Rounbehler, R.J.; Kensler, K.; Serna, A.; Vidal, A.; You, S.; Freeman, M.R.; Davicioni, E. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin. Cancer Res. 2021, 27, 320–329. [Google Scholar] [CrossRef]
- Nonomura, N.; Takayama, H.; Nakayama, M.; Nakai, Y.; Kawashima, A.; Mukai, M.; Nagahara, A.; Aozasa, K.; Tsujimura, A. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011, 107, 1918–1922. [Google Scholar] [CrossRef]
- Weiner, A.B.; Vidotto, T.; Liu, Y.; Mendes, A.A.; Salles, D.C.; Faisal, F.A.; Murali, S.; McFarlane, M.; Imada, E.L.; Zhao, X. Plasma cells are enriched in localized prostate cancer in Black men and are associated with improved outcomes. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kinseth, M.A.; Jia, Z.; Rahmatpanah, F.; Sawyers, A.; Sutton, M.; Wang-Rodriguez, J.; Mercola, D.; McGuire, K.L. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int. J. Cancer 2014, 134, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Prueitt, R.L.; Wallace, T.A.; Glynn, S.A.; Yi, M.; Tang, W.; Luo, J.; Dorsey, T.H.; Stagliano, K.E.; Gillespie, J.W.; Hudson, R.S.; et al. An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers. Cancer Res. 2016, 76, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, L.B.; Quintana-Murci, L. Evolutionary and population (epi)genetics of immunity to infection. Hum. Genet. 2020, 139, 723–732. [Google Scholar] [CrossRef]
- Coe, C.L.; Love, G.D.; Karasawa, M.; Kawakami, N.; Kitayama, S.; Markus, H.R.; Tracy, R.P.; Ryff, C.D. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav. Immun. 2011, 25, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Hong, C.C.; Ruiz-Narvaez, E.A.; Evans, S.S.; Zhu, Q.; Schaefer, B.A.; Yan, L.; Coignet, M.V.; Lunetta, K.L.; Sucheston-Campbell, L.E.; et al. Genetic ancestry and population differences in levels of inflammatory cytokines in women: Role for evolutionary selection and environmental factors. PLoS Genet. 2018, 14, e1007368. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Daw, J. Contribution of Four Comorbid Conditions to Racial/Ethnic Disparities in Mortality Risk. Am. J. Prev. Med. 2017, 52, S95–S102. [Google Scholar] [CrossRef]
- Geiss, L.S.; Wang, J.; Cheng, Y.J.; Thompson, T.J.; Barker, L.; Li, Y.; Albright, A.L.; Gregg, E.W. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014, 312, 1218–1226. [Google Scholar] [CrossRef]
- Panigrahi, G.; Ambs, S. How Comorbidities Shape Cancer Biology and Survival. Trends Cancer 2021, 7, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Minas, T.Z.; Kiely, M.; Ajao, A.; Ambs, S. An overview of cancer health disparities: New approaches and insights and why they matter. Carcinogenesis 2021, 42, 2–13. [Google Scholar] [CrossRef]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Howard, L.E.; De Hoedt, A.; Terris, M.K.; Amling, C.L.; Kane, C.J.; Cooperberg, M.R.; Aronson, W.J.; Klaassen, Z.; Polascik, T.J. Racial Discrepancies in Overall Survival among Men Treated with 223Radium. J. Urol. 2020, 203, 331–337. [Google Scholar] [CrossRef]
- Van der Doelen, M.J.; Velho, P.I.; Slootbeek, P.H.; Naga, S.P.; Bormann, M.; van Helvert, S.; Kroeze, L.I.; van Oort, I.M.; Gerritsen, W.R.; Antonarakis, E.S. Impact of DNA damage repair defects on response to radium-223 and overall survival in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2020, 136, 16–24. [Google Scholar] [CrossRef]
- Ramos, J.D.; Mostaghel, E.A.; Pritchard, C.C.; Evan, Y.Y. DNA repair pathway alterations in metastatic castration-resistant prostate cancer responders to radium-223. Clin. Genitourin. Cancer 2018, 16, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, A.E.; Cotogno, P.; Ledet, E.M.; Lewis, B.; Sartor, O. Exceptional duration of radium-223 in prostate cancer with a BRCA2 mutation. Clin. Genitourin. Cancer 2017, 15, e69–e71. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized Phase II Trial of Sipuleucel-T with or without Radium-223 in Men with Bone-metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 2020, 38, 395. [Google Scholar] [CrossRef]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; Van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Salk, H.M.; Lambert, N.D.; Ovsyannikova, I.G.; Kennedy, R.B.; Warner, N.D.; Pankratz, V.S.; Poland, G.A. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 2014, 32, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Kurupati, R.; Kossenkov, A.; Haut, L.; Kannan, S.; Xiang, Z.; Li, Y.; Doyle, S.; Liu, Q.; Schmader, K.; Showe, L. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct pre-vaccination gene expression profiles in blood. Oncotarget 2016, 7, 62898. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A. The immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. JNCI J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; Brahmer, J.R.; McDermott, D.F.; Smith, D.C.; Gettinger, S.N.; Taube, J.M.; Drake, C.G.; Pardoll, D.M.; Powderly, J.D. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in Patients with Advanced Solid Tumors: Survival and Long-Term Safety in a Phase I Trial; American Society of Clinical Oncology: Alexandria, VA, USA, 2013; p. 3002. [Google Scholar]
- Haffner, M.C.; Guner, G.; Taheri, D.; Netto, G.J.; Palsgrove, D.N.; Zheng, Q.; Guedes, L.B.; Kim, K.; Tsai, H.; Esopi, D.M. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am. J. Pathol. 2018, 188, 1478–1485. [Google Scholar] [CrossRef]
- Fankhauser, C.D.; Schüffler, P.J.; Gillessen, S.; Omlin, A.; Rupp, N.J.; Rueschoff, J.H.; Hermanns, T.; Poyet, C.; Sulser, T.; Moch, H. Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer. Oncotarget 2018, 9, 10284. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Zhang, Y.; Sun, G.; Ding, B.; Yan, L.; Liu, H.; Guan, W.; Hu, Z.; Wang, S. The immune checkpoint regulator PDL1 is an independent prognostic biomarker for biochemical recurrence in prostate cancer patients following adjuvant hormonal therapy. J. Cancer 2019, 10, 3102. [Google Scholar] [CrossRef]
- Petitprez, F.; Fossati, N.; Vano, Y.; Freschi, M.; Becht, E.; Lucianò, R.; Calderaro, J.; Guédet, T.; Lacroix, L.; Rancoita, P.M. PD-L1 expression and CD8+ T-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur. Urol. Focus 2019, 5, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ness, N.; Andersen, S.; Khanehkenari, M.R.; Nordbakken, C.V.; Valkov, A.; Paulsen, E.E.; Nordby, Y.; Bremnes, R.M.; Donnem, T.; Busund, L.T.; et al. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) in a large, multicenter prostate cancer cohort. Oncotarget 2017, 8, 26789–26801. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234. [Google Scholar] [CrossRef]
- Calagua, C.; Russo, J.; Sun, Y.; Schaefer, R.; Lis, R.; Zhang, Z.; Mahoney, K.; Bubley, G.J.; Loda, M.; Taplin, M.E.; et al. Expression of PD-L1 in Hormone-naïve and Treated Prostate Cancer Patients Receiving Neoadjuvant Abiraterone Acetate plus Prednisone and Leuprolide. Clin. Cancer Res. 2017, 23, 6812–6822. [Google Scholar] [CrossRef]
- McNamara, M.A.; George, D.J.; Ramaswamy, K.; Lechpammer, S.; Mardekian, J.; Schultz, N.M.; Wang, L.; Baser, O.; Huang, A.; Freedland, S.J. Overall survival by race in chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate or enzalutamide. J. Clin. Oncol. 2019, 37, 212. [Google Scholar] [CrossRef]
- Brandon, D.T.; Isaac, L.A.; LaVeist, T.A. The legacy of Tuskegee and trust in medical care: Is Tuskegee responsible for race differences in mistrust of medical care? J. Natl. Med. Assoc. 2005, 97, 951. [Google Scholar]
- Duma, N.; Vera Aguilera, J.; Paludo, J.; Haddox, C.L.; Gonzalez Velez, M.; Wang, Y.; Leventakos, K.; Hubbard, J.M.; Mansfield, A.S.; Go, R.S. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J. Oncol. Pract. 2018, 14, e1–e10. [Google Scholar] [CrossRef]
- Loree, J.M.; Anand, S.; Dasari, A.; Unger, J.M.; Gothwal, A.; Ellis, L.M.; Varadhachary, G.; Kopetz, S.; Overman, M.J.; Raghav, K. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. 2019, 5, e191870. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Dutta, S.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Thompson, I.M., Jr.; Chi, K.N.; Araujo, J.C.; Logothetis, C.; Quinn, D.I.; et al. Overall Survival of Black and White Men With Metastatic Castration-Resistant Prostate Cancer Treated With Docetaxel. J. Clin. Oncol. 2019, 37, 403–410. [Google Scholar] [CrossRef]
- Powell, I.J.; Bock, C.H.; Ruterbusch, J.J.; Sakr, W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol. 2010, 183, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Fantus, R.J.; Helfand, B.T. Germline Genetics of Prostate Cancer: Time to Incorporate Genetics into Early Detection Tools. Clin. Chem. 2019, 65, 74–79. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Yadav, S.; Anbalagan, M.; Baddoo, M.; Chellamuthu, V.K.; Mukhopadhyay, S.; Woods, C.; Jiang, W.; Moroz, K.; Flemington, E.K.; Makridakis, N. Somatic mutations in the DNA repairome in prostate cancers in African Americans and Caucasians. Oncogene 2020, 39, 4299–4311. [Google Scholar] [CrossRef]
- Taylor, R.A.; Fraser, M.; Livingstone, J.; Espiritu, S.M.G.; Thorne, H.; Huang, V.; Lo, W.; Shiah, Y.-J.; Yamaguchi, T.N.; Sliwinski, A. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Narod, S.; Neuhausen, S.; Vichodez, G.; Armel, S.; Lynch, H.; Ghadirian, P.; Cummings, S.; Olopade, O.; Stoppa-Lyonnet, D.; Couch, F. Rapid progression of prostate cancer in men with a BRCA2 mutation. Br. J. Cancer 2008, 99, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Petrovics, G.; Price, D.K.; Lou, H.; Chen, Y.; Garland, L.; Bass, S.; Jones, K.; Kohaar, I.; Ali, A.; Ravindranath, L. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis. 2019, 22, 406–410. [Google Scholar] [CrossRef]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 162–174. [Google Scholar] [CrossRef]
- Mateo, J.; McKay, R.; Abida, W.; Aggarwal, R.; Alumkal, J.; Alva, A.; Feng, F.; Gao, X.; Graff, J.; Hussain, M. Accelerating precision medicine in metastatic prostate cancer. Nat. Cancer 2020, 1, 1041–1053. [Google Scholar] [CrossRef]
- Ratta, R.; Guida, A.; Scotté, F.; Neuzillet, Y.; Teillet, A.B.; Lebret, T.; Beuzeboc, P. PARP inhibitors as a new therapeutic option in metastatic prostate cancer: A systematic review. Prostate Cancer Prostatic Dis. 2020, 23, 549–560. [Google Scholar] [CrossRef]
- Bever, K.M.; Le, D.T. DNA repair defects and implications for immunotherapy. J. Clin. Investig. 2018, 128, 4236–4242. [Google Scholar] [CrossRef]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Härtlova, A.; Erttmann, S.F.; Raffi, F.A.; Schmalz, A.M.; Resch, U.; Anugula, S.; Lienenklaus, S.; Nilsson, L.M.; Kröger, A.; Nilsson, J.A. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 2015, 42, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Cañadas, I.; Roehle, K.; Heckler, M. EZH2 inhibition activates a dsRNA–STING–interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat. Cancer 2021, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.M.; Papaevangelou, E.; Dasgupta, P.; Galustian, C. Combination of Interleukin-15 with a STING agonist, ADU-S100 analog: A potential immunotherapy for prostate cancer. Front. Oncol. 2021, 11, 621550. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Gupta, P.D.; Chaudagar, K.; Sharma-Saha, S.; Bynoe, K.; Maillat, L.; Heiss, B.; Stadler, W.M.; Patnaik, A. PARP and PI3K inhibitor combination therapy eradicates c-MYC-driven murine prostate cancers via cGAS/STING pathway activation within tumor-associated macrophages. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Muirhead, G.; Krastev, D.B.; Adam, J.; Morel, D.; Garrido, M.; Lamb, A.; Hénon, C.; Dorvault, N.; Rouanne, M. PARP inhibition enhances tumor cell–intrinsic immunity in ERCC1-deficient non–small cell lung cancer. J. Clin. Investig. 2019, 129, 1211–1228. [Google Scholar] [CrossRef]
- Chubak, J.; Whitlock, E.P.; Williams, S.B.; Kamineni, A.; Burda, B.U.; Buist, D.S.; Anderson, M.L. Aspirin for the prevention of cancer incidence and mortality: Systematic evidence reviews for the US Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2016, 164, 836–845. [Google Scholar] [CrossRef]
- Dehmer, S.P.; Maciosek, M.V.; Flottemesch, T.J.; LaFrance, A.B.; Whitlock, E.P. Aspirin for the primary prevention of cardiovascular disease and colorectal cancer: A decision analysis for the US Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Osborn, V.W.; Chen, S.C.; Weiner, J.; Schwartz, D.; Schreiber, D. Impact of aspirin on clinical outcomes for African American men with prostate cancer undergoing radiation. Tumori 2016, 102, 65–70. [Google Scholar] [CrossRef]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Gil-Bernabé, A.M.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A 2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fowke, J.H.; Hurwitz, L.M.; Steinwandel, M.; Blot, W.J.; Ambs, S. Aspirin Use and Prostate Cancer among African-American Men in the Southern Community Cohort Study. Cancer Epidemiol. Prev. Biomark. 2021, 30, 539–544. [Google Scholar] [CrossRef]
- Hurwitz, L.M.; Joshu, C.E.; Barber, J.R.; Prizment, A.E.; Vitolins, M.Z.; Jones, M.R.; Folsom, A.R.; Han, M.; Platz, E.A. Aspirin and non-aspirin NSAID use and prostate cancer incidence, mortality, and case fatality in the atherosclerosis risk in communities study. Cancer Epidemiol. Prev. Biomark. 2019, 28, 563–569. [Google Scholar] [CrossRef]
- Bitting, R.L.; Goodman, M.; George, D.J. Racial Disparity in Response to Prostate Cancer Systemic Therapies. Curr. Oncol. Rep. 2020, 22, 1–5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiely, M.; Ambs, S. Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men. Cancers 2021, 13, 2874. https://doi.org/10.3390/cancers13122874
Kiely M, Ambs S. Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men. Cancers. 2021; 13(12):2874. https://doi.org/10.3390/cancers13122874
Chicago/Turabian StyleKiely, Maeve, and Stefan Ambs. 2021. "Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men" Cancers 13, no. 12: 2874. https://doi.org/10.3390/cancers13122874
APA StyleKiely, M., & Ambs, S. (2021). Immune Inflammation Pathways as Therapeutic Targets to Reduce Lethal Prostate Cancer in African American Men. Cancers, 13(12), 2874. https://doi.org/10.3390/cancers13122874





