The Immunotherapy Landscape in Adrenocortical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Adrenocortical Carcinoma: Epidemiology, Pathophysiology, and Treatment
2.1. Staging and Prognosis
2.2. Current Therapeutic Approaches
2.3. Genomics
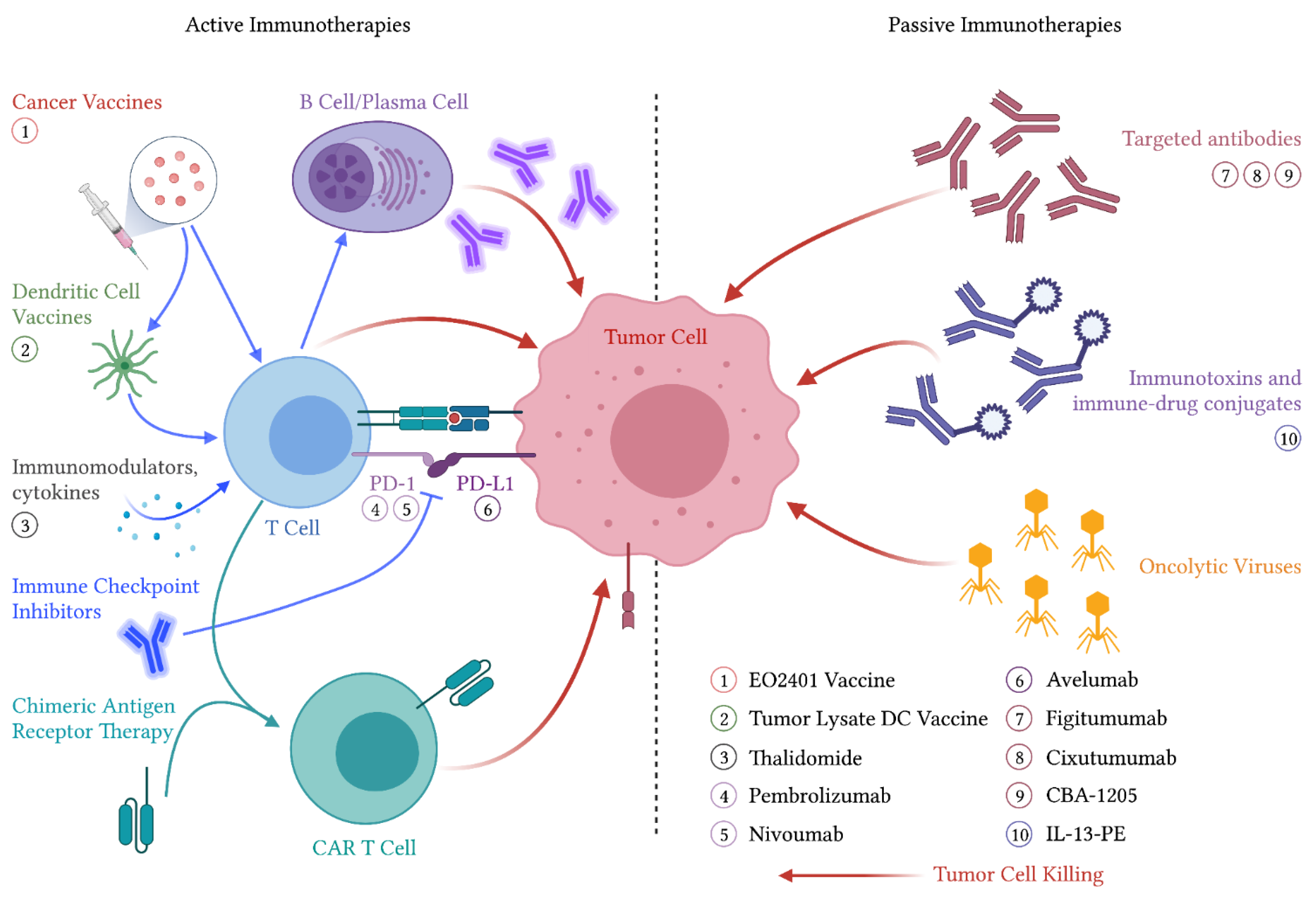
3. Immunotherapeutic Approaches to ACC
3.1. Active Immunotherapies
3.1.1. Cancer Vaccines
3.1.2. Dendritic Cell Therapies
3.1.3. Immune Checkpoint Inhibitors
3.1.4. Immune Checkpoint Inhibitors in ACC
3.1.5. Immune Checkpoint Inhibitors Combined with Targeted Therapies
3.1.6. Immune Modulators
3.2. Passive Immunotherapies
3.2.1. Targeted Antibodies
3.2.2. Immunotoxins and Antibody–Drug Conjugates
| Identifier | Experimental Arm | Phase | Treatment Line | Primary Endpoint | ORR | PFS | Ref |
|---|---|---|---|---|---|---|---|
| Active immunotherapies | |||||||
| Unregistered | DCV: tumor lysate | n/a | 2nd | POC | 0% (0/2) | [59] | |
| NCT02673333 | Pembrolizumab | 2 | All | ORR | 23% (9/39) | 2.1 mos | [66] |
| NCT02720484 | Nivolumab | 2 | 2nd | ORR | 10% (1/10) * | 1.8 mos | [69] |
| NCT01772004 | Avelumab | 1b | 2nd | Safety, ORR | 6% (3/50) | 2.6 mos | [73] |
| Passive immunotherapies | |||||||
| Unregistered | Figitumumab | 1 | 2nd+ | Safety, tolerability | 0% (0/14) | [89] | |
| NCT00778817 | Cixutumumab + mitotane | 2 | 1st | PFS | 5% (1/20) | 6 wks | [90] |
| NCT01832974 | Interleukin-13-Psm exotoxin | 1 | 2nd+ | Safety, tolerability | 0% (0/5) | [98] | |
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753. [Google Scholar] [CrossRef]
- Cutler, S.; Young, J. Third National Cancer Survey: Incidence Data. Natl. Cancer Inst. Monogr. 1975, i-x, 1–454. [Google Scholar]
- Kebebew, E.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J. Surg. 2006, 30, 872–878. [Google Scholar] [CrossRef]
- Ng, L.; Libertino, J.M. Adrenocortical carcinoma: Diagnosis, evaluation and treatment. J. Urol. 2003, 169, 5–11. [Google Scholar] [CrossRef]
- Roman, S. Adrenocortical carcinoma. Curr. Opin. Oncol. 2006, 18, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Roses, R.E. Surgical Management of Adrenocortical Carcinoma: An Evidence-Based Approach. Surg. Oncol. Clin. N. Am. 2016, 25, 153–170. [Google Scholar] [CrossRef]
- Berruti, A.; Fassnacht, M.; Haak, H.; Else, T.; Baudin, E.; Sperone, P.; Kroiss, M.; Kerkhofs, T.; Williams, A.R.; Ardito, A.; et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur. Urol. 2014, 65, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Abiven, G.; Coste, J.; Groussin, L.; Anract, P.; Tissier, F.; Legmann, P.; Dousset, B.; Bertagna, X.; Bertherat, J. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J. Clin. Endocrinol. Metab. 2006, 91, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best Pr. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Paton, B.L.; Novitsky, Y.W.; Zerey, M.; Harrell, A.G.; Norton, H.J.; Asbun, H.; Kercher, K.W.; Heniford, B.T. Outcomes of adrenal cortical carcinoma in the United States. Surgery 2006, 140, 914–920; discussion 919–920. [Google Scholar] [CrossRef]
- Sturgeon, C.; Shen, W.T.; Clark, O.H.; Duh, Q.Y.; Kebebew, E. Risk assessment in 457 adrenal cortical carcinomas: How much does tumor size predict the likelihood of malignancy? J. Am. Coll. Surg. 2006, 202, 423–430. [Google Scholar] [CrossRef]
- Luton, J.P.; Cerdas, S.; Billaud, L.; Thomas, G.; Guilhaume, B.; Bertagna, X.; Laudat, M.H.; Louvel, A.; Chapuis, Y.; Blondeau, P.; et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N. Engl. J. Med. 1990, 322, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a Revised TNM Classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Dackiw, A.P.; Lee, J.E.; Gagel, R.F.; Evans, D.B. Adrenal cortical carcinoma. World J. Surg. 2001, 25, 914–926. [Google Scholar] [CrossRef]
- Terzolo, M.; Berruti, A. Adjunctive treatment of adrenocortical carcinoma. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 221–226. [Google Scholar] [CrossRef]
- Lubitz, J.A.; Freeman, L.; Okun, R. Mitotane Use in Inoperable Adrenal Cortical Carcinoma. JAMA 1973, 223, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.V.; Kim, L.; Yen, T.W.F.; Yang, A.; Grubbs, E.G.; Patel, D.; Solórzano, C.C. Adjuvant and Neoadjuvant Therapy, Treatment for Advanced Disease, and Genetic Considerations for Adrenocortical Carcinoma: An Update from the SSO Endocrine and Head and Neck Disease Site Working Group. Ann. Surg. Oncol. 2018, 25, 3453–3459. [Google Scholar] [CrossRef] [PubMed]
- Wängberg, B.; Khorram-Manesh, A.; Jansson, S.; Nilsson, B.; Nilsson, O.; Jakobsson, C.E.; Lindstedt, S.; Odén, A.; Ahlman, H. The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocr. Relat. Cancer 2010, 17, 265–272. [Google Scholar] [CrossRef]
- Polat, B.; Fassnacht, M.; Pfreundner, L.; Guckenberger, M.; Bratengeier, K.; Johanssen, S.; Kenn, W.; Hahner, S.; Allolio, B.; Flentje, M. Radiotherapy in adrenocortical carcinoma. Cancer 2009, 115, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.S.; Imam, H.; Juhlin, C.; Skogseid, B.; Gröndal, S.; Tibblin, S.; Wilander, E.; Oberg, K.; Eriksson, B. Streptozocin and o,p’DDD in the treatment of adrenocortical cancer patients: Long-term survival in its adjuvant use. Ann. Oncol. 2000, 11, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef]
- Fassnacht, M.; Berruti, A.; Baudin, E.; Demeure, M.J.; Gilbert, J.; Haak, H.; Kroiss, M.; Quinn, D.I.; Hesseltine, E.; Ronchi, C.L.; et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: A double-blind, randomised, phase 3 study. Lancet Oncol. 2015, 16, 426–435. [Google Scholar] [CrossRef]
- Kroiss, M.; Quinkler, M.; Johanssen, S.; van Erp, N.P.; Lankheet, N.; Pöllinger, A.; Laubner, K.; Strasburger, C.J.; Hahner, S.; Müller, H.H.; et al. Sunitinib in refractory adrenocortical carcinoma: A phase II, single-arm, open-label trial. J. Clin. Endocrinol. Metab. 2012, 97, 3495–3503. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Edgerly, M.; Velarde, M.; Wilkerson, J.; Venkatesan, A.M.; Pittaluga, S.; Yang, S.X.; Nguyen, D.; Balasubramaniam, S.; Fojo, T. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J. Clin. Endocrinol. Metab. 2014, 99, 1291–1297. [Google Scholar] [CrossRef]
- Berends, M.J.; Cats, A.; Hollema, H.; Karrenbeld, A.; Beentjes, J.A.; Sijmons, R.H.; Mensink, R.G.; Hofstra, R.M.; Verschueren, R.C.; Kleibeuker, J.H. Adrenocortical adenocarcinoma in an MSH2 carrier: Coincidence or causal relation? Hum. Pathol. 2000, 31, 1522–1527. [Google Scholar] [CrossRef]
- Broaddus, R.R.; Lynch, P.M.; Lu, K.H.; Luthra, R.; Michelson, S.J. Unusual tumors associated with the hereditary nonpolyposis colorectal cancer syndrome. Mod. Pathol. 2004, 17, 981–989. [Google Scholar] [CrossRef][Green Version]
- Else, T.; Lerario, A.M.; Everett, J.; Haymon, L.; Wham, D.; Mullane, M.; Wilson, T.L.; Rainville, I.; Rana, H.; Worth, A.J.; et al. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: An observational case series. Eur. J. Endocrinol. 2017, 177, 439–444. [Google Scholar] [CrossRef]
- Fienman, N.L.; Yakovac, W.C. Neurofibromatosis in childhood. J. Pediatr. 1970, 76, 339–346. [Google Scholar] [CrossRef]
- Pinto, E.M.; Chen, X.; Easton, J.; Finkelstein, D.; Liu, Z.; Pounds, S.; Rodriguez-Galindo, C.; Lund, T.C.; Mardis, E.R.; Wilson, R.K.; et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015, 6, 6302. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.A.; Mulvihill, J.J.; Nielsen, A. Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N. Engl. J. Med. 1986, 314, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.A.; Mulvihill, J.J.; Nielsen, A. On the natural history of von Recklinghausen neurofibromatosis. Ann. N. Y. Acad. Sci. 1986, 486, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.S.; Fleitz, J.M.; Kleinschmidt-Demasters, B.K. Pediatric adrenal cortical carcinoma: Brain metastases and relationship to NF-1, case reports and review of the literature. J. Neurooncol. 2005, 75, 127–133. [Google Scholar] [CrossRef]
- Assié, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; René-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014, 46, 607–612. [Google Scholar] [CrossRef]
- Barzon, L.; Chilosi, M.; Fallo, F.; Martignoni, G.; Montagna, L.; Palù, G.; Boscaro, M. Molecular analysis of CDKN1C and TP53 in sporadic adrenal tumors. Eur. J. Endocrinol. 2001, 145, 207–212. [Google Scholar] [CrossRef][Green Version]
- Gaujoux, S.; Grabar, S.; Fassnacht, M.; Ragazzon, B.; Launay, P.; Libé, R.; Chokri, I.; Audebourg, A.; Royer, B.; Sbiera, S.; et al. β-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin. Cancer Res. 2011, 17, 328–336. [Google Scholar] [CrossRef]
- Gicquel, C.; Bertagna, X.; Schneid, H.; Francillard-Leblond, M.; Luton, J.P.; Girard, F.; Le Bouc, Y. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 1994, 78, 1444–1453. [Google Scholar] [CrossRef]
- Gicquel, C.; Raffin-Sanson, M.L.; Gaston, V.; Bertagna, X.; Plouin, P.F.; Schlumberger, M.; Louvel, A.; Luton, J.P.; Le Bouc, Y. Structural and functional abnormalities at 11p15 are associated with the malignant phenotype in sporadic adrenocortical tumors: Study on a series of 82 tumors. J. Clin. Endocrinol. Metab. 1997, 82, 2559–2565. [Google Scholar] [CrossRef]
- Libè, R.; Groussin, L.; Tissier, F.; Elie, C.; René-Corail, F.; Fratticci, A.; Jullian, E.; Beck-Peccoz, P.; Bertagna, X.; Gicquel, C.; et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin. Cancer Res. 2007, 13, 844–850. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P.; Heitz, P.U. p53 mutations in sporadic adrenocortical tumors. Int. J. Cancer 1993, 54, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Reincke, M.; Karl, M.; Travis, W.H.; Mastorakos, G.; Allolio, B.; Linehan, H.M.; Chrousos, G.P. p53 mutations in human adrenocortical neoplasms: Immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 1994, 78, 790–794. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sullivan, M.; Boileau, M.; Hodges, C.V. Adrenal cortical carcinoma. J. Urol. 1978, 120, 660–665. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016, 29, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Raymond, V.M.; Everett, J.N.; Furtado, L.V.; Gustafson, S.L.; Jungbluth, C.R.; Gruber, S.B.; Hammer, G.D.; Stoffel, E.M.; Greenson, J.K.; Giordano, T.J.; et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3012–3018. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.-M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.-P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Basu, P.; Banerjee, D.; Singh, P.; Bhattacharya, C.; Biswas, J. Efficacy and safety of human papillomavirus vaccine for primary prevention of cervical cancer: A review of evidence from phase III trials and national programs. S. Asian J. Cancer 2013, 2, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Reincke, M.; Beuschlein, F.; Latronico, A.C.; Arlt, W.; Chrousos, G.P.; Allolio, B. Expression of adrenocorticotrophic hormone receptor mRNA in human adrenocortical neoplasms: Correlation with P450scc expression. Clin. Endocrinol. 1997, 46, 619–626. [Google Scholar] [CrossRef]
- Schubert, B.; Fassnacht, M.; Beuschlein, F.; Zenkert, S.; Allolio, B.; Reincke, M. Angiotensin II type 1 receptor and ACTH receptor expression in human adrenocortical neoplasms. Clin. Endocrinol. 2001, 54, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Fassnacht, M.; Klink, A.; Allolio, B.; Reincke, M. ACTH-receptor expression, regulation and role in adrenocortial tumor formation. Eur. J. Endocrinol. 2001, 144, 199–206. [Google Scholar] [CrossRef]
- Zenkert, S.; Schubert, B.; Fassnacht, M.; Beuschlein, F.; Allolio, B.; Reincke, M. Steroidogenic acute regulatory protein mRNA expression in adrenal tumours. Eur. J. Endocrinol. 2000, 142, 294–299. [Google Scholar] [CrossRef][Green Version]
- Ortmann, D.; Hausmann, J.; Beuschlein, F.; Schmenger, K.; Stahl, M.; Geissler, M.; Reincke, M. Steroidogenic acute regulatory (StAR)-directed immunotherapy protects against tumor growth of StAR-expressing Sp2-0 cells in a rodent adrenocortical carcinoma model. Endocrinology 2004, 145, 1760–1766. [Google Scholar] [CrossRef][Green Version]
- National Library of Medicine (U.S.). A Novel Therapeutic Vaccine (EO2401) in Metastatic Adreno-cortical Carcinoma, or Malignant Pheochromocytoma/Paraganglioma. Identifier NCT04187404. December 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04187404?id=NCT04187404 (accessed on 14 April 2021).
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Reincke, M.; Ortmann, D.; Hausmann, J.; Beuschlein, F. Cytotoxic T-cell response against steroidogenic acute regulatory protein using DNA vaccination followed by vaccinia virus infection in a mouse adrenal carcinoma model. Horm. Metab. Res. 2004, 36, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Shimizu, K.; Mulé, J.J. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Papewalis, C.; Fassnacht, M.; Willenberg, H.S.; Domberg, J.; Fenk, R.; Rohr, U.P.; Schinner, S.; Bornstein, S.R.; Scherbaum, W.A.; Schott, M. Dendritic cells as potential adjuvant for immunotherapy in adrenocortical carcinoma. Clin. Endocrinol. 2006, 65, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Gupta, M.; Sahasranaman, S. Immune Checkpoint inhibitors: An introduction to the next-generation cancer immunotherapy. J. Clin. Pharm. 2016, 56, 157–169. [Google Scholar] [CrossRef]
- Melero, I.; Hervas-Stubbs, S.; Glennie, M.; Pardoll, D.M.; Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 2007, 7, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I.; et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J. Clin. Oncol. 2020, 38, 71–80. [Google Scholar] [CrossRef]
- Habra, M.A.; Stephen, B.; Campbell, M.; Hess, K.; Tapia, C.; Xu, M.; Rodon Ahnert, J.; Jimenez, C.; Lee, J.E.; Perrier, N.D.; et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. Immunother. Cancer 2019, 7, 253. [Google Scholar] [CrossRef]
- Head, L.; Kiseljak-Vassiliades, K.; Clark, T.J.; Somerset, H.; King, J.; Raeburn, C.; Albuja-Cruz, M.; Weyant, M.; Cleveland, J.; Wierman, M.E.; et al. Response to Immunotherapy in Combination With Mitotane in Patients With Metastatic Adrenocortical Cancer. J. Endocr. Soc. 2019, 3, 2295–2304. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Konda, B.; Costa, R.B.; Costa, R.L.B.; Sagar, V.; Gursel, D.B.; Kirschner, L.S.; Chae, Y.K.; Abdulkadir, S.A.; Rademaker, A.; et al. Nivolumab in Metastatic Adrenocortical Carcinoma: Results of a Phase 2 Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- Angelo, S.P.; Bhatia, S.; Brohl, A.S.; Hamid, O.; Mehnert, J.M.; Terheyden, P.; Shih, K.C.; Brownell, I.; Lebbé, C.; Lewis, K.D.; et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: Long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J. Immunother. Cancer 2020, 8, e000674. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Ellerton, J.A.; Infante, J.R.; Agrawal, M.; Gordon, M.S.; Aljumaily, R.; Gourdin, T.; Dirix, L.; Lee, K.-W.; Taylor, M.H.; et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J. Immunother. Cancer 2020, 8, e001246. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Hoimes, C.; Zarwan, C.; Wong, D.J.; Bauer, S.; Claus, R.; Wermke, M.; Hariharan, S.; von Heydebreck, A.; Kasturi, V.; et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J. Immunother. Cancer 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Bedrose, S.; Miller, K.C.; Altameemi, L.; Ali, M.S.; Nassar, S.; Garg, N.; Daher, M.; Eaton, K.D.; Yorio, J.T.; Daniel, D.B.; et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (U.S.). Study of Relacorilant in Combination with Pembrolizumab for Patients with Adrenocortical Carcinoma with Excess Glucocorticoid Production. Identifier NCT04373265. May 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04373265 (accessed on 14 April 2021).
- Bartlett, J.B.; Dredge, K.; Dalgleish, A.G. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat. Rev. Cancer 2004, 4, 314–322. [Google Scholar] [CrossRef]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Anagnostopoulos, A.; Weber, D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J. Clin. Oncol. 2003, 21, 4444–4454. [Google Scholar] [CrossRef] [PubMed]
- Kritharis, A.; Coyle, M.; Sharma, J.; Evens, A.M. Lenalidomide in non-Hodgkin lymphoma: Biological perspectives and therapeutic opportunities. Blood 2015, 125, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Deutschbein, T.; Schlötelburg, W.; Ronchi, C.L.; Hescot, S.; Körbl, D.; Megerle, F.; Beuschlein, F.; Neu, B.; Quinkler, M.; et al. Treatment of Refractory Adrenocortical Carcinoma with Thalidomide: Analysis of 27 Patients from the European Network for the Study of Adrenal Tumours Registry. Exp. Clin. Endocrinol. Diabetes 2019, 127, 578–584. [Google Scholar] [CrossRef]
- Breedveld, F.C. Therapeutic monoclonal antibodies. Lancet 2000, 355, 735–740. [Google Scholar] [CrossRef]
- Temming, A.R.; de Taeye, S.W.; de Graaf, E.L.; de Neef, L.A.; Dekkers, G.; Bruggeman, C.W.; Koers, J.; Ligthart, P.; Nagelkerke, S.Q.; Zimring, J.C.; et al. Functional Attributes of Antibodies, Effector Cells, and Target Cells Affecting NK Cell–Mediated Antibody-Dependent Cellular Cytotoxicity. J. Immunol. 2019, 203, 3126–3135. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.L.; Simcock, R.; Rea, D.; et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef]
- Guren, T.K.; Thomsen, M.; Kure, E.H.; Sorbye, H.; Glimelius, B.; Pfeiffer, P.; Österlund, P.; Sigurdsson, F.; Lothe, I.M.B.; Dalsgaard, A.M.; et al. Cetuximab in treatment of metastatic colorectal cancer: Final survival analyses and extended RAS data from the NORDIC-VII study. Br. J. Cancer 2017, 116, 1271–1278. [Google Scholar] [CrossRef]
- van Oers, M.H.J.; Klasa, R.; Marcus, R.E.; Wolf, M.; Kimby, E.; Gascoyne, R.D.; Jack, A.; van’t Veer, M.; Vranovsky, A.; Holte, H.; et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood 2006, 108, 3295–3301. [Google Scholar] [CrossRef]
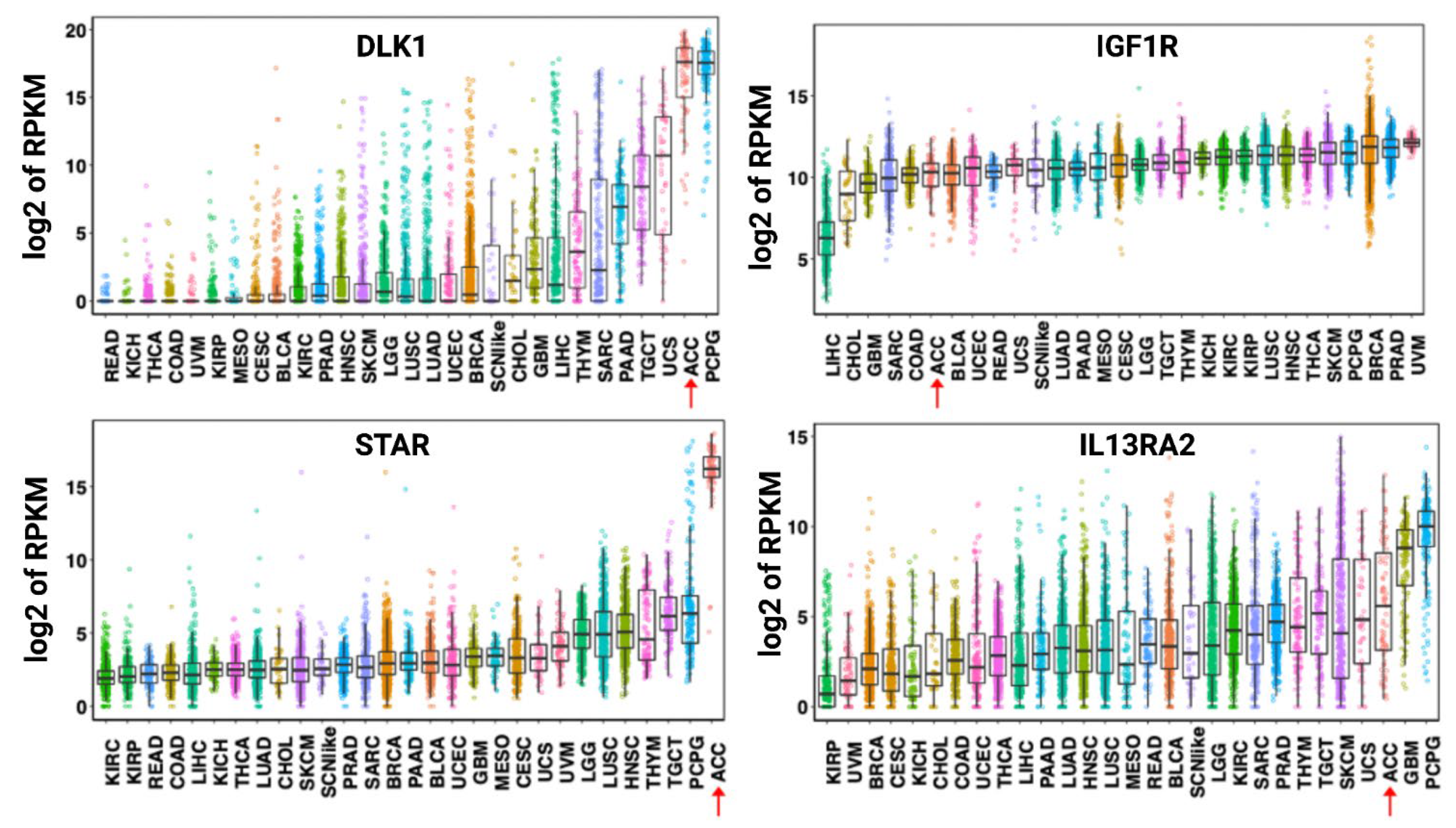
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Cohen, B.D.; Baker, D.A.; Soderstrom, C.; Tkalcevic, G.; Rossi, A.M.; Miller, P.E.; Tengowski, M.W.; Wang, F.; Gualberto, A.; Beebe, J.S.; et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin. Cancer Res. 2005, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Haluska, P.; Worden, F.; Olmos, D.; Yin, D.; Schteingart, D.; Batzel, G.N.; Paccagnella, M.L.; de Bono, J.S.; Gualberto, A.; Hammer, G.D. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother. Pharm. 2010, 65, 765–773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lerario, A.M.; Worden, F.P.; Ramm, C.A.; Hesseltine, E.A.; Stadler, W.M.; Else, T.; Shah, M.H.; Agamah, E.; Rao, K.; Hammer, G.D. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: A multi-institutional NCI-sponsored trial. Horm. Cancer 2014, 5, 232–239. [Google Scholar] [CrossRef]
- Hadjidemetriou, I.; Mariniello, K.; Ruiz-Babot, G.; Pittaway, J.; Mancini, A.; Mariannis, D.; Gomez-Sanchez, C.E.; Parvanta, L.; Drake, W.M.; Chung, T.-T.; et al. DLK1/PREF1 marks a novel cell population in the human adrenal cortex. J. Steroid Biochem. Mol. Biol. 2019, 193, 105422. [Google Scholar] [CrossRef]
- Chiome Bioscience. Chiome to Launch First-in-Human Trial for Anti-DLK-1 Antibody. Available online: https://pj.jiho.jp/article/242559 (accessed on 10 April 2021).
- Kreitman, R.J.; Dearden, C.E.; Zinzani, P.L.L.; Delgado, J.; Robak, T.; le Coutre, P.D.; Gjertsen, B.T.; Troussard, X.; Roboz, G.J.; Karlin, L.; et al. Moxetumomab Pasudotox-Tdfk in Heavily Pretreated Patients with Relapsed/Refractory Hairy Cell Leukemia (HCL): Long-Term Follow-up from the Pivotal Phase 3 Trial. Blood 2019, 134, 2808. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.R.; Obiri, N.I.; Gill, P.; Zheng, T.; Pastan, I.; Debinski, W.; Puri, R.K. Receptor for interleukin 13 on AIDS-associated Kaposi’s sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin. Cancer Res. 1997, 3, 151–156. [Google Scholar] [PubMed]
- Jain, M.; Zhang, L.; He, M.; Patterson, E.E.; Nilubol, N.; Fojo, A.T.; Joshi, B.; Puri, R.; Kebebew, E. Interleukin-13 receptor alpha2 is a novel therapeutic target for human adrenocortical carcinoma. Cancer 2012, 118, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Liu-Chittenden, Y.; Jain, M.; Kumar, P.; Patel, D.; Aufforth, R.; Neychev, V.; Sadowski, S.; Gara, S.K.; Joshi, B.H.; Cottle-Delisle, C.; et al. Phase I trial of systemic intravenous infusion of interleukin-13-Pseudomonas exotoxin in patients with metastatic adrenocortical carcinoma. Cancer Med. 2015, 4, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020, 105, 26–42. [Google Scholar] [CrossRef]
| Stage | Description | Survival (%) |
|---|---|---|
| I | Disease < 5 cm, without local invasion, nodal or metastatic spread | 82 |
| II | Disease > 5 cm, without local invasion, nodal or metastatic spread | 61 |
| III | Tumor with local, lymphatic, vena cava, or renal vein invasion | 50 |
| IV | Distantly metastatic disease | 13 |
| Tumor staging by the 8th edition of the American Joint Committee on Cancer TNM Staging System and European Network for the Study of Adrenal Tumors | ||
| NCT Identifier | Phase | Intervention | Key Inclusion Criteria |
|---|---|---|---|
| NCT04373265 | I | Relacorilant with pembrolizumab | Advanced unresectable or metastatic ACC, hormonally active |
| NCT04187404 | I/II | Therapeutic vaccine (EO2401) with or without nivolumab | Advanced unresectable or metastatic ACC |
| NCT02721732 | II | Pembrolizumab | Advanced unresectable or metastatic rare solid tumor malignancies, including ACC |
| NCT04318730 | II | Camrelizumab with Apatinib | Advanced unresectable or metastatic ACC, progressive after first-line therapy |
| NCT03333616 | II | Nivolumab with Ipilimumab | Advanced unresectable or metastatic ACC |
| NCT02834013 | II | Nivolumab with Ipilimumab | Advanced unresectable or metastatic ACC, progressive after first-line therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegna, G.J.; Roper, N.; Kaplan, R.N.; Bergsland, E.; Kiseljak-Vassiliades, K.; Habra, M.A.; Pommier, Y.; Del Rivero, J. The Immunotherapy Landscape in Adrenocortical Cancer. Cancers 2021, 13, 2660. https://doi.org/10.3390/cancers13112660
Pegna GJ, Roper N, Kaplan RN, Bergsland E, Kiseljak-Vassiliades K, Habra MA, Pommier Y, Del Rivero J. The Immunotherapy Landscape in Adrenocortical Cancer. Cancers. 2021; 13(11):2660. https://doi.org/10.3390/cancers13112660
Chicago/Turabian StylePegna, Guillaume J., Nitin Roper, Rosandra N. Kaplan, Emily Bergsland, Katja Kiseljak-Vassiliades, Mouhammed Amir Habra, Yves Pommier, and Jaydira Del Rivero. 2021. "The Immunotherapy Landscape in Adrenocortical Cancer" Cancers 13, no. 11: 2660. https://doi.org/10.3390/cancers13112660
APA StylePegna, G. J., Roper, N., Kaplan, R. N., Bergsland, E., Kiseljak-Vassiliades, K., Habra, M. A., Pommier, Y., & Del Rivero, J. (2021). The Immunotherapy Landscape in Adrenocortical Cancer. Cancers, 13(11), 2660. https://doi.org/10.3390/cancers13112660







