Evolving Treatment of Advanced Hepatocellular Carcinoma in the Asia–Pacific Region: A Review and Multidisciplinary Expert Opinion
Abstract
Simple Summary
Abstract
1. Introduction
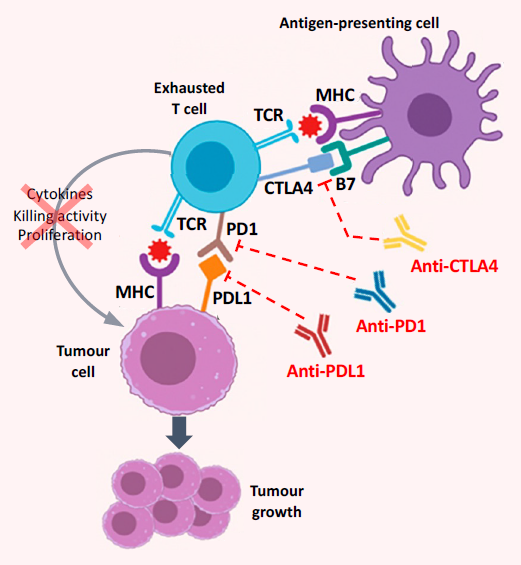
2. Current and Emerging Systemic Therapies in Advanced HCC
3. Healthcare and Reimbursement Systems in the Asia–Pacific Region
4. Expert Opinion: Treating Advanced HCC in the Asia–Pacific Region
4.1. Sorafenib and Lenvatinib Remain First-Line Treatment Choices
4.2. The IMbrave150 Trial Results Are Practice-Changing
4.3. A Snapshot of Advanced HCC: The Different Perspectives of Patients, Physicians, and Payer Systems
4.3.1. The Patient’s Perspective
4.3.2. The Physician’s Perspective
4.3.3. The Payer System’s Perspective
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2020, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Korean Liver Cancer Association; National Cancer Center Korea. Practice guidelines for the management of hepatocellular carcinoma. Korean J. Radiol. 2019, 20, 1042–1113. [Google Scholar] [CrossRef]
- Richani, M.; Kolly, P.; Knoepfli, M.; Herrmann, E.; Zweifel, M.; von Tengg-Kobligk, H.; Candinas, D.; Dufour, J.-F. Treatment allocation in hepatocellular carcinoma: Assessment of the BCLC algorithm. Ann. Hepatol. 2016, 15, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, Y.; Duan, W.; Li, C.; Meng, X.; Dong, J. A concise review of current guidelines for the clinical management of hepatocellular carcinoma in Asia. Transl. Cancer Res. 2017, 6, 1214–1225. [Google Scholar] [CrossRef]
- Bruix, J.; da Fonseca, L.G.; Reig, M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 617–630. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Poon, D.; Anderson, B.O.; Chen, L.-T.; Tanaka, K.; Lau, W.Y.; Van Cutsem, E.; Singh, H.; Chow, W.C.; Ooi, L.L.; Chow, P.; et al. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009, 10, 1111–1118. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Chen, L.-T.; Martinelli, E.; Cheng, A.-L.; Pentheroudakis, G.; Qin, S.; Bhattacharyya, G.S.; Ikeda, M.; Lim, H.-Y.; Ho, G.F.; Choo, S.P.; et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: A TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann. Oncol. 2020, 31, 334–351. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, H.; Wang, Z.; Cong, W.; Wang, J.; Zeng, M.; Zhou, W.; Bie, P.; Liu, L.; Wen, T.; et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer 2020, 9, 682–720. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Choo, S.P.; Ng, D.C.E.; Lo, R.H.G.; Wang, M.L.C.; Toh, H.C.; Tai, D.W.M.; Goh, B.K.P.; Wong, J.S.; Tay, K.H.; et al. National Cancer Centre Singapore consensus guidelines for hepatocellular carcinoma. Liver Cancer 2016, 5, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-Y.; Wang, S.-Y.; Lin, S.-M.; Diagnosis Group; Systemic Therapy Group. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2020, 120, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Tang, V.Y.F.; Yao, T.-J.; Fan, S.-T.; Lo, C.-M.; Poon, R.T.P. Development of Hong Kong liver cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014, 146, 1691–1700. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific Region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar] [CrossRef]
- McNamara, M.G.; Slagter, A.E.; Nuttall, C.; Frizziero, M.; Pihlak, R.; Lamarca, A.; Tariq, N.; Valle, J.W.; Hubner, R.A.; Knox, J.J.; et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma—A meta-analysis. Eur. J. Cancer 2018, 105, 1–9. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA Grants Accelerated Approval to Nivolumab and Ipilimumab Combination for Hepatocellular Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma (accessed on 18 March 2020).
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Yau, T.; Kang, Y.-K.; Kim, T.-Y.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (AHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 2021, 39, 269. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Doycheva, I.; Thuluvath, P.J. Systemic therapy for advanced hepatocellular carcinoma: An update of a rapidly evolving field. J. Clin. Exp. Hepatol. 2019, 9, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Federico, P.; Petrillo, A.; Giordano, P.; Bosso, D.; Fabbrocini, A.; Ottaviano, M.; Rosanova, M.; Silvestri, A.; Tufo, A.; Cozzolino, A.; et al. Immune checkpoint inhibitors in hepatocellular carcinoma: Current status and novel perspectives. Cancers 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K. The novel regimen of tremelimumab in combination with durvalumab provides a favorable safety profile and clinical activity for patients with advanced hepatocellular carcinoma (AHCC). In Proceedings of the International Liver Cancer Association, Virtual Annual Conference, 11–13 September 2020; abstract O-22. pp. 13–14. [Google Scholar]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Cheng, A.-L.; Finn, R.S.; Galle, P.R.; Kaneko, S.; Meyer, T.; Qin, S.; Dutcus, C.E.; Chen, E.; et al. Lenvatinib (Len) plus pembrolizumab (Pembro) for the first-line treatment of patients (Pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J. Clin. Oncol. 2019, 37, 15. [Google Scholar] [CrossRef]
- Abd El Aziz, M.A.; Facciorusso, A.; Nayfeh, T.; Saadi, S.; Elnaggar, M.; Cotsoglou, C.; Sacco, R. Immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Vaccines 2020, 8, 616. [Google Scholar] [CrossRef]
- Giannini, E.G.; Aglitti, A.; Borzio, M.; Gambato, M.; Guarino, M.; Iavarone, M.; Lai, Q.; Levi Sandri, G.B.; Melandro, F.; Morisco, F.; et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI.CA cohort derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers 2019, 11, 1689. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (Atezo) + bevacizumab (Bev) vs sorafenib (Sor) in patients (Pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, Z.; Dang, H.; Li, P.; Chuong, L.; Yue, D.; Wen, J.; Zhao, R.; Li, W.; Kominski, G. Towards universal health coverage: Achievements and challenges of 10 years of healthcare reform in China. BMJ Glob. Health 2020, 5, e002087. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, G.; Garabrant, A.; Adyas, A.; Muga, R.; Otoo, N. Moving towards universal health coverage: Health insurance reforms in nine developing countries in Africa and Asia. Lancet 2012, 380, 933–943. [Google Scholar] [CrossRef]
- Liu, G.; Wu, E.Q.; Ahn, J.; Kamae, I.; Xie, J.; Yang, H. The development of health technology assessment in Asia: Current status and future trends. Value Health Reg. Issues 2020, 21, 39–44. [Google Scholar] [CrossRef]
- Wang, B.C.M. The rise of health economics and outcomes research in healthcare decision-making. Leuk. Res. 2013, 37, 238–239. [Google Scholar] [CrossRef]
- Ngorsuraches, S.; Meng, W.; Kim, B.-Y.; Kulsomboon, V. Drug reimbursement decision-making in Thailand, China, and South Korea. Value Health 2012, 15, S120–S125. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Hatanaka, T.; Tada, T.; Kariyama, K.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; et al. Therapeutic efficacy of lenvatinib as third line treatment following regorafenib for unresectable hepatocellular carcinoma progression. Hepatol. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Casadei-Gardini, A.; Scartozzi, M.; Tada, T.; Yoo, C.; Shimose, S.; Masi, G.; Lonardi, S.; Frassineti, L.G.; Nicola, S.; Piscaglia, F.; et al. Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: An inverse probability of treatment weighting analysis. Liver Int. 2021, 41, 1389–1397. [Google Scholar] [CrossRef]
- Wang, E.; Xia, D.; Bai, W.; Wang, Z.; Wang, Q.; Liu, L.; Wang, W.; Yuan, J.; Li, X.; Chen, H.; et al. Hand-foot-skin reaction of grade ≥ 2 within sixty days as the optimal clinical marker best help predict survival in sorafenib therapy for HCC. Invest. New Drugs 2019, 37, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Lu, S.-N.; Rau, K.-M.; Huang, C.-S.; Lee, K.-T. Increased cumulative doses and appearance of hand-foot skin reaction prolonged progression free survival in sorafenib-treated advanced hepatocellular carcinoma patients. Kaohsiung J. Med. Sci. 2018, 34, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Kim, Y.J.; Huang, Y.-H.; Shao, G.; Kim, D.Y.; Cho, S.B.; Hsu, C.-H.; Lin, S.-M.; Jeng, L.-B.; Kuo, K.-K.; et al. 1009P Regorafenib in patients (Pts) with unresectable hepatocellular carcinoma (UHCC) in real-world practice in Asia: Interim results from the observational REFINE study. Ann. Oncol. 2020, 31, S699. [Google Scholar] [CrossRef]
- Merle, P.; Lim, H.Y.; Finn, R.S.; Ikeda, M.; Kudo, M.; Frenette, C.; Masi, G.; Kim, Y.J.; Gerolami, R.; Kurosaki, M.; et al. 1010P Real-world dosing of regorafenib (REG) in patients (Pts) with unresectable hepatocellular carcinoma (UHCC): Interim analysis (IA) of the observational REFINE study. Ann. Oncol. 2020, 31, S699–S700. [Google Scholar] [CrossRef]
- Merle, P.; Lim, H.Y.; Finn, R.S.; Ikeda, M.; Kudo, M.; Frenette, C.T.; Masi, G.; Kim, Y.J.; Gerolami, R.; Kurosaki, M.; et al. Sequential treatment with sorafenib (SOR) followed by regorafenib (REG) in patients (Pts) with unresectable hepatocellular carcinoma (HCC): Interim analysis of the observational REFINE study. J. Clin. Oncol. 2020, 38, e16680. [Google Scholar] [CrossRef]
- Prevention of Infection Related Cancer (PIRCA) Group; Specialized Committee of Cancer Prevention and Control; Chinese Preventive Medicine Association; Non-communicable & Chronic Disease Control and Prevention Society; Chinese Preventive Medicine Association; Health Communication Society, Chinese Preventive Medicine Association. Strategies of primary prevention of liver cancer in China: Expert consensus (2018). Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Kudo, M.; Izumi, N.; Kubo, S.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Tateishi, R.; Nakashima, O.; Murakami, T.; Matsuyama, Y.; et al. Report of the 20th nationwide follow-up survey of primary liver cancer in Japan. Hepatol. Res. 2020, 50, 15–46. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef]
- Goh, G.B.-B.; Li, J.W.; Chang, P.-E.; Chow, K.-Y.; Tan, C.-K. Deciphering the epidemiology of hepatocellular carcinoma through the passage of time: A study of 1401 patients across 3 decades. Hepatol. Commun. 2017, 1, 564–571. [Google Scholar] [CrossRef]
- Liew, Z.-H.; Goh, G.B.-B.; Hao, Y.; Chang, P.-E.; Tan, C.-K. Comparison of hepatocellular carcinoma in patients with cryptogenic versus hepatitis B etiology: A study of 1079 cases over 3 decades. Dig. Dis. Sci. 2019, 64, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Susanibaradaniya, S.P.; Siegel, E.R. Effect of hepatitis C virus (HCV) on the outcome of hepatocellular carcinoma (HCC). J. Clin. Oncol. 2016, 34, e15634. [Google Scholar] [CrossRef]
- Jackson, R.; Psarelli, E.-E.; Berhane, S.; Khan, H.; Johnson, P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: A meta-analysis of randomized phase III trials. J. Clin. Oncol. 2017, 35, 622–628. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.-L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Verghese, N.R.; Barrenetxea, J.; Bhargava, Y.; Agrawal, S.; Finkelstein, E.A. Government pharmaceutical pricing strategies in the Asia-Pacific region: An overview. J. Mark. Access Health Policy 2019, 7, 1601060. [Google Scholar] [CrossRef]
- Ren, Z.; Fan, J.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Liu, B.; Li, Q.; Lu, Y.; et al. LBA2: Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32). Ann. Oncol. 2020, 31, S1287. [Google Scholar] [CrossRef]
- Tai, D.; Choo, S.P.; Chew, V. Rationale of immunotherapy in hepatocellular carcinoma and its potential biomarkers. Cancers 2019, 11, 1926. [Google Scholar] [CrossRef] [PubMed]
- Labgaa, I.; Villanueva, A. Liquid biopsy in liver cancer. Discov. Med. 2015, 19, 263–273. [Google Scholar]
- Yang, J.D.; Liu, M.C.; Kisiel, J.B. Circulating tumor DNA and hepatocellular carcinoma. Semin. Liver Dis. 2019, 39, 452–462. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, J.H.; Ryu, M.-H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.-S.; Lee, H.C.; Lee, J.; et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: A multinational, multicenter retrospective study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef] [PubMed]
| Country or Region of Origin of the Guideline | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Asia Pacific (APASL) [11] | China [14] |
Hong Kong (HKLCS) [17] |
Japan (JSH) [13] |
Korea (KLCSG) [5] |
Singapore (NCCS) [15] |
Taiwan (TLCA) [16] | ||||||||
| Disease features | MVI+ | EHM+ | MVI+ (IIIa) | EHM+ (IIIb) | Int. I or IIa + Vp 1–3 LA IIb or IIIa + Vp 1–3 | EVM (IVa, IVb) Vp 4 or EHM (or both) | MVI+ | EHM+ | MVI+ (IIc, IIIb, IVa) Single Multiple | EHM+ IVa (LN), IVb (others) | MVI+ | EHM+ | MVI+ | EHM+ |
| First-line treatment | Systemic therapy, TACE | CP A or B: systemic therapy CP C: best supportive care | TACE ± (MKI or FOLFOX4, LR, RT) | MKI or FOLFOX4 ± (TACE, RT) | IIb or IIIa, CP A: LR | Systemic therapy | TACE, LR, HAIC, MKI | MKI | TACE (SIRT) ± EBRT Sorafenib Lenvatinib | Sorafenib Lenvatinib | SIRT, TACE | Systemic therapy | LR, MKI | Systemic therapy ± (TACE, SIRT, LR) |
| Second-line treatment | MKI | MKI | IIb or IIIa, CP B, or IIIb: TACE | (Vp 1–3: LR x 1–3) | (TACE, EBRT) | Systemic therapy | TACE + RT | Chemo-therapy | ||||||
| Later line of treatment | (CT, HAIC if MKI failed or not available) | SIRT, HAIC | ||||||||||||
| Country or Territory | First Line | Second Line (After Sorafenib) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sor | Len | Atezo + Bev | Cabo | Rego | Ramu | Nivo | Pembro | Nivo + Ipi | Camre | |
| China | A, R | A, R | A, NR a | NA, NR | A, R | NA, NR | NA, NR | NA, NR | NA, NR | A, R |
| Japan | A, R | A, R | A, R | A, R | A, R | A, R | NA, NR | NA, NR | NA, NR | NA, NR |
| Korea | A, R | A, R | A, NR | A, NR | A, R | A, NR | NA, b NR | NA, NR | NA, NR | NA, NR |
| Singapore | A, NR | A, NR | A, NR | A, NR | A, NR | A, NR | NA, c NR | NA, c NR | NA, NR | NA, NR |
| Taiwan | A, R | A, R | A, NR | A, NR | A, R | A, NR | A, d NR | A, d NR | NA, NR | NA, NR |
| Study Drug (s) | Control Arm | Key Eligibility Criteria | Clinical Trials Identifier (Study Name) | Mechanism of Study Drug | Status |
|---|---|---|---|---|---|
| Atezolizumab + bevacizumab | Sorafenib | ECOG PS ≤1, CP A, ≥1 measurable lesion | NCT03434379 (IMbrave150) | Anti-PDL1 Anti-VEGF | Active, not recruiting |
| Lenvatinib + pembrolizumab | Lenvatinib + placebo | ECOG PS ≤1, BCLC stage B or C, CP A, ≥1 measurable lesion | NCT03713593 (LEAP-002) | MKI Anti-PD1 | Active, not recruiting |
| Sintilimab + bevacizumab biosimilar IBI305 | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP score ≤7, ≥1 measurable lesion | NCT03794440 (ORIENT-32) | Anti-PD1 Anti-VEGF | Active, not recruiting |
| Cabozantinib + atezolizumab | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP A, measurable disease | NCT03755791 (COSMIC-312) | Anti-VEGFR Anti-PDL1 | Recruiting |
| Camrelizumab (SHR-1210) + apatinib | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP A, ≥1 measurable lesion | NCT03764293 | Anti-PD1 Anti-VEGFR2 | Recruiting |
| Camrelizumab (SHR-1210) + FOLFOX4 | Placebo + FOLFOX4 | ECOG PS ≤1, CP score ≤7, measurable disease | NCT03605706 | Anti-PD1 Chemotherapy | Recruiting |
| Durvalumab ± tremelimumab | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP A | NCT03298451 (HIMALAYA) | Anti-PDL1 Anti-CTLA4 | Recruiting |
| IBI310 + sintilimab | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP score ≤6, ≥1 measurable lesion | NCT04720716 | Anti-CTLA4 Anti-PD1 | Recruiting |
| Lenvatinib ± CS1003 | Placebo | ECOG PS ≤1, BCLC stage B or C, CP A, ≥1 measurable lesion | NCT04194775 | MKI Anti-PD1 | Recruiting |
| Nivolumab + ipilimumab | Sorafenib or lenvatinib | ECOG PS ≤1, CP A, ≥1 measurable lesion | NCT04039607 (CheckMate 9DW) | Anti-PD1 Anti-CTLA4 | Recruiting |
| SCT-I10A + bevacizumab biosimilar SCT-510 | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP score ≤7, ≥1 measurable lesion | NCT04560894 | Anti-PD1 Anti-VEGF | Recruiting |
| HLX10 + HLX04 | Sorafenib | BCLC stage B or C, ≥1 measurable lesion | NCT04465734 | Anti-PD1 Anti-VEGF | Not yet recruiting |
| Penpulimab injection + anlotinib | Sorafenib | ECOG PS ≤1, BCLC stage B or C, CP score ≤7, ≥1 measurable lesion | NCT04344158 | Anti-PD1 MKI | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogasawara, S.; Choo, S.-P.; Li, J.-T.; Yoo, C.; Wang, B.; Lee, D.; Chow, P.K.H. Evolving Treatment of Advanced Hepatocellular Carcinoma in the Asia–Pacific Region: A Review and Multidisciplinary Expert Opinion. Cancers 2021, 13, 2626. https://doi.org/10.3390/cancers13112626
Ogasawara S, Choo S-P, Li J-T, Yoo C, Wang B, Lee D, Chow PKH. Evolving Treatment of Advanced Hepatocellular Carcinoma in the Asia–Pacific Region: A Review and Multidisciplinary Expert Opinion. Cancers. 2021; 13(11):2626. https://doi.org/10.3390/cancers13112626
Chicago/Turabian StyleOgasawara, Sadahisa, Su-Pin Choo, Jiang-Tao Li, Changhoon Yoo, Bruce Wang, Dee Lee, and Pierce K. H. Chow. 2021. "Evolving Treatment of Advanced Hepatocellular Carcinoma in the Asia–Pacific Region: A Review and Multidisciplinary Expert Opinion" Cancers 13, no. 11: 2626. https://doi.org/10.3390/cancers13112626
APA StyleOgasawara, S., Choo, S.-P., Li, J.-T., Yoo, C., Wang, B., Lee, D., & Chow, P. K. H. (2021). Evolving Treatment of Advanced Hepatocellular Carcinoma in the Asia–Pacific Region: A Review and Multidisciplinary Expert Opinion. Cancers, 13(11), 2626. https://doi.org/10.3390/cancers13112626







