Nutritional Care in Patients with Head and Neck Cancer during Chemoradiotherapy (CRT) and Bioradiotherapy (BRT) Provides Better Compliance with the Treatment Plan
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Treatment Regimen
2.2. Nutritional Care Programme
2.3. Statistics
3. Results
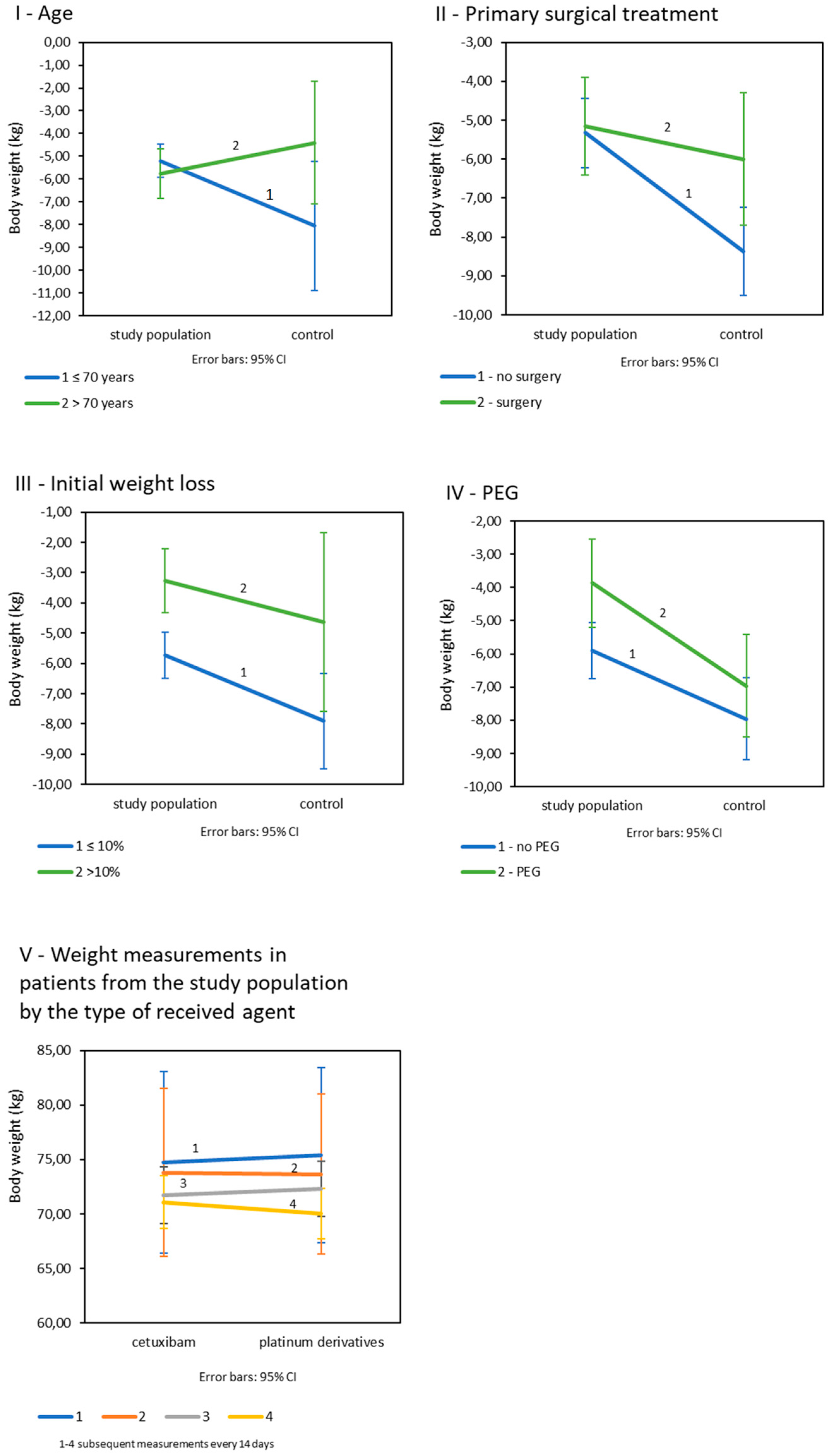
3.1. Influence of Nutritional Care on the Weight Loss and Deterioration of Nutritional Status Markers during Therapy
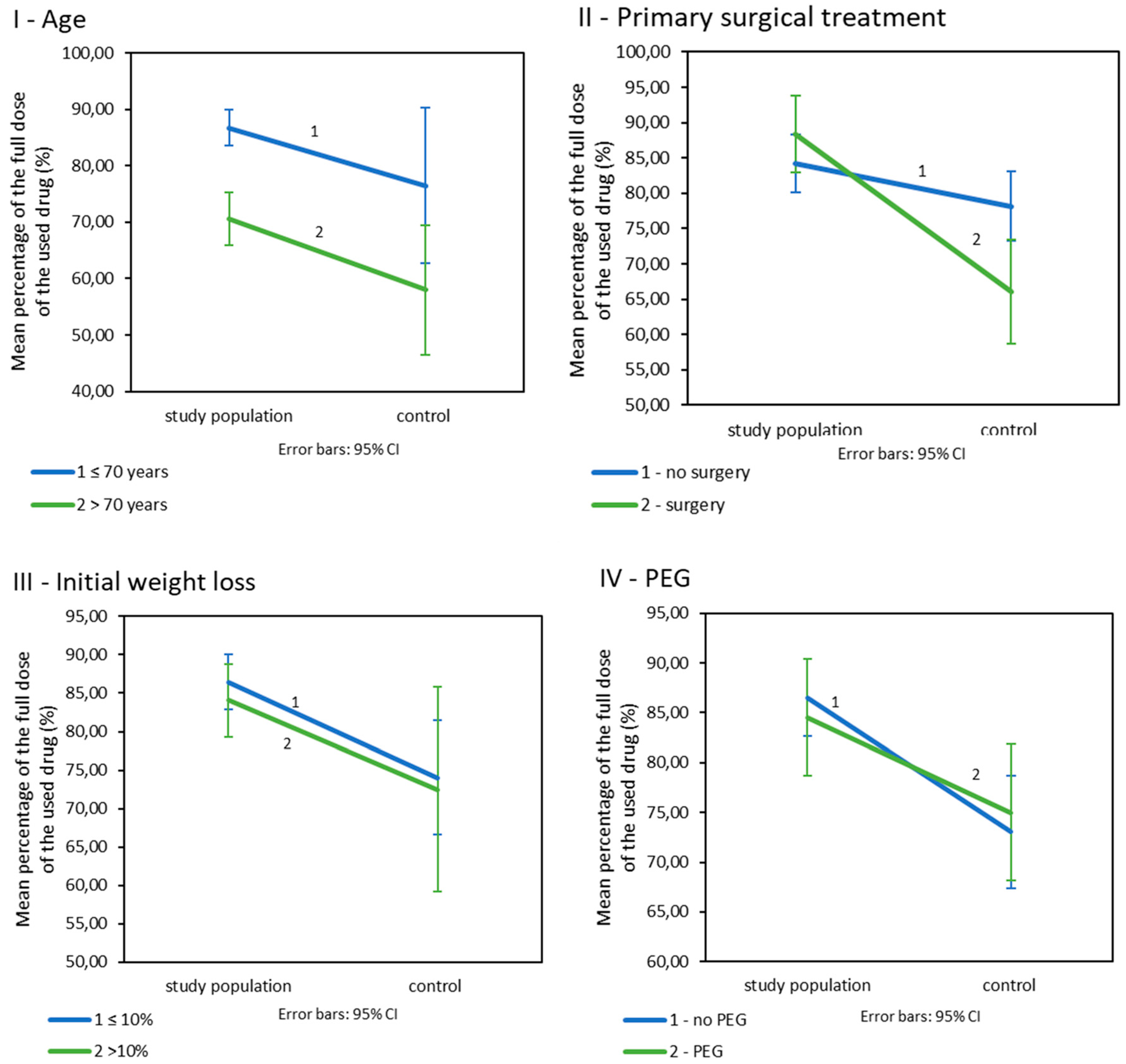
3.2. Influence of Nutritional Care on the Doses of Drugs Used during CRT/BRT
3.3. Influence of Nutrition Care on the Rate of Adverse Events during CRT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, L.; Millán, I.; Torre, A.; Aragón, G.; Otero, J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer: A multivariate study of 492 cases. Cancer 2010, 69, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; Forastiere, A.; Ang, K.K. Factors Associated with Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Lee, J.; Kim, E.; Lippman, S.M.; Khuri, F.R.; Spitz, M.R.; Lotan, R.; Hong, W.K.; Wu, X. MicroRNA-related genetic variations as predictors for risk of second primary tumor and/or recurrence in patients with early-stage head and neck cancer. Carcinogenesis 2010, 31, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Baselga, J.; Trigo, J.M.; Bourhis, J.; Tortochaux, J.; Cortés-Funes, H.; Hitt, R.; Gascón, P.; Amellal, N.; Harstrick, A.; Eckardt, A. Phase II Multicenter Study of the Antiepidermal Growth Factor Receptor Monoclonal Antibody Cetuximab in Combination with Platinum-Based Chemotherapy in Patients with Platinum-Refractory Metastatic and/or Recurrent Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2005, 23, 5568–5577. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Keum, K.C.; Shin, S.J.; Choi, H.J.; Lee, Y.J.; Kim, S.H.; Choi, E.C.; Kim, J.H. Weekly docetaxel in patients with platinum-refractory metastatic or recurrent squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 2009, 65, 27–32. [Google Scholar] [CrossRef]
- Price, K.A.R.; Cohen, E.E. Current Treatment Options for Metastatic Head and Neck Cancer. Curr. Treat. Options Oncol. 2012, 13, 35–46. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Kang, E.J.; Keam, B.; Choi, J.-H.; Kim, J.-S.; Park, K.U.; Lee, K.E.; Kwon, J.H.; Kim, M.K.; Ahn, H.K.; et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: A nationwide retrospective cohort study (KCSG HN13–01). BMC Cancer 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Tang, W.-H.; Sun, W.; Long, G.-X. Concurrent cisplatin or cetuximab with radiotherapy in patients with locally advanced head and neck squamous cell carcinoma. Medicine 2020, 99, e21785. [Google Scholar] [CrossRef] [PubMed]
- Mollnar, S.; Pondorfer, P.; Kasparek, A.-K.; Reinisch, S.; Moik, F.; Stotz, M.; Halm, M.; Szkandera, J.; Terbuch, A.; Eisner, F.; et al. Decrease in treatment intensity predicts worse outcome in patients with locally advanced head and neck squamous cell carcinoma undergoing radiochemotherapy. Clin. Transl. Oncol. 2021, 23, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.; Gupta, V.; Ghiassi-Nejad, Z.; Miles, B.; Scarborough, B.; Misiukiewicz, K.J.; Reckson, B.; Sheu, R.-D.; Bakst, R.L. Premature discontinuation of curative radiation therapy: Insights from head and neck irradiation. Adv. Radiat. Oncol. 2018, 3, 62–69. [Google Scholar] [CrossRef]
- Lazzari, G.; De Cillis, M.A.; Buccoliero, G.; Silvano, G. Competing Morbidities in Advanced Head and Neck Squamous Cell Carcinoma Concurrent Chemoradiotherapy: A Strong Implication of a Multidisciplinary Team Approach. Cancer Manag. Res. 2019, 11, 9771–9782. [Google Scholar] [CrossRef]
- Strojan, P.; Vermorken, J.B.; Beitler, J.J.; Saba, N.F.; Haigentz, M.; Bossi, P.; Worden, F.P.; Langendijk, J.A.; Eisbruch, A.; Mendenhall, W.M.; et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2015, 38, E2151–E2158. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Lawrence, T.S.; Rosenberg, S.A. Dose intensity and combination chemotherapy. In Cancer—Principles & Practice of Oncology, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 315. [Google Scholar]
- Noronha, V.; Joshi, A.; Patil, V.M.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Murthy, V.; Gupta, T.; D’Cruz, A.K.; Banavali, S.; et al. Once-a-Week Versus Once-Every-3-Weeks Cisplatin Chemoradiation for Locally Advanced Head and Neck Cancer: A Phase III Randomized Noninferiority Trial. J. Clin. Oncol. 2018, 36, 1064–1072. [Google Scholar] [CrossRef]
- van Bokhorst-de van der Schueren, M.A.; von Blomberg-van der Flier, B.M.E.; Kuik, D.J.; Scholten, P.E.T.; Siroen, M.P.C.; Snow, G.B.; Quak, J.J.; Van Leeuwen, P.A.M. Survival of Malnourished Head and Neck Cancer Patients Can Be Predicted by Human Leukocyte Antigen-DR Expression and Interleukin-6/Tumor Necrosis Factor-α Response of the Monocyte. J. Parenter. Enter. Nutr. 2000, 24, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Jordan, R.C.K.; Zhao, W.; Sturgis, E.M.; Burtness, B.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Lees Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur. J. Cancer Care 1999, 8, 133–136. [CrossRef] [PubMed]
- Linn, B.S.; Robinson, D.S.; Klimas, N.G. Effects of Age and Nutritional Status on Surgical Outcomes in Head and Neck Cancer. Ann. Surg. 1988, 207, 267–273. [Google Scholar] [CrossRef]
- Ravasco, P.; Monteiro-Grillo, I.; Vidal, P.M.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.N.; Norman, A.R.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Barrios, R.; Tsakos, G.; García-Medina, B.; Martínez-Lara, I.; Bravo, M. Oral health-related quality of life and malnutrition in patients treated for oral cancer. Support. Care Cancer 2014, 22, 2927–2933. [Google Scholar] [CrossRef]
- Capuano, G.; Gentile, P.C.; Bianciardi, F.; Tosti, M.; Palladino, A.; Di Palma, M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support. Care Cancer 2009, 18, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.T.D. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.I.S.D.S.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E.R. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- van Bokhorst-de van der Schueren, M.A.; van Leeuwen, P.A.; Sauerwein, H.P.; Kuik, D.J.; Snow, G.B.; Quak, J.J. Assessment of malnutrition parameters in head and neck cancer and their relation to postoperative complications. Head Neck. 1997, 19, 419–425. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S51–S63. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Ling, Y.; Zhang, L.; Wan, H. Comparative effects of different enteral feeding methods in head and neck cancer patients receiving radiotherapy or chemoradiotherapy: A network meta-analysis. OncoTargets Ther. 2016, 9, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.; Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Hammerlid, E. Effect of prophylactic percutaneous endoscopic gastrostomy tube on swallowing in advanced head and neck cancer: A randomized controlled study. Head Neck 2017, 39, 908–915. [Google Scholar] [CrossRef]
- Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Larsson, S.; Hammerlid, E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer—A randomized study. Head Neck 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Pignon, J.-P.; le Maître, A.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Oosting, S.F.; Haddad, R.I. Best Practice in Systemic Therapy for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 815. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef]
- Kapała, A. Nutritional therapy during the treatment of head and neck cancer. Oncol. Clin. Pract. 2018, 14, 79–85. [Google Scholar]
- Coca-Pelaz, A.; Halmos, G.B.; Strojan, P.; Bree, R.; Bossi, P.; Bradford, C.R.; Rinaldo, A.; Poorten, V.V.; Sanabria, A.; Takes, R.P.; et al. The role of age in treatment-related adverse events in patients with head and neck cancer: A systematic review. Head Neck 2019, 41, 2410–2429. [Google Scholar] [CrossRef] [PubMed]
- Kapała, A. Analysis of the nutritional value of a liquid hospital diet. Adv. Clin. Nutr. 2012, 4, 28–33. (In Polish) [Google Scholar]
- Haehl, E.; Rühle, A.; David, H.; Kalckreuth, T.; Sprave, T.; Stoian, R.; Becker, C.; Knopf, A.; Grosu, A.-L.; Nicolay, N.H. Radiotherapy for geriatric head-and-neck cancer patients: What is the value of standard treatment in the elderly? Radiat. Oncol. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G.B. Nutritional Status-a Prognostic Indicator in Head and Neck Cancer. Otolaryngol. Neck Surg. 1985, 93, 69–74. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Stokes, W.A.; Sumner, W.A.; Breggren, K.L.; Rathbun, J.T.; Raben, D.; McDermott, J.D.; Gan, G.; Karam, S.D. A comparison of concurrent cisplatin versus cetuximab with radiotherapy in locally-advanced head and neck cancer: A bi-institutional analysis. Rep. Pr. Oncol. Radiother. 2017, 22, 389–395. [Google Scholar] [CrossRef] [PubMed]

| Variable | Study Population | Control Population | p |
|---|---|---|---|
| Total number of patients | 153 | 72 | |
| Sex | |||
| Male | 79.8% (122) | 86.1% (62) | 0.248 |
| Female | 20.2% (31) | 13.9% (10) | |
| Mean age (years) | 58 | 61.7 | 0.007 |
| ≤70 years old | 92.8% (142) | 84.7% (61) | |
| >70 years old | 7.2% (11) | 15.3% (11) | |
| BMI M (SD) | 25.60 (4.32) | 26.23 (5.24) | 0.341 |
| PEG | 31.4% (48) | 41.7% (30) | 0.130 |
| TF | 17.2% (26) | 21.1% (15) | 0.609 |
| Undergone surgery | |||
| Yes | 37.9% (58) | 34.7% (25) | 0.644 |
| No | 62.1% (95) | 65.3% (47) | |
| Neoadjuvant CTH | |||
| Yes | 28.8% (41) | 18.1% (13) | 0.152 |
| No | 71.2% (112) | 81.9% (59) | |
| The agent used in CRT | |||
| Cisplatin/Carboplatin | 91.4% (140) | 100% (72) | 0.011 |
| Cetuximab | 8.6% (13) | 0% (0) | |
| Tumor site | |||
| Nasopharynx | 11.1% (17) | 9.7% (7) | 0.933 |
| Oropharynx | 30.1% (46) | 37.5% (27) | 0.338 |
| Laryngopharynx | 13.1% (20) | 2.8% (2) | 0.029 |
| Tongue | 14.4% (23) | 8.3% (6) | 0.236 |
| Floor of the mouth | 3.9% (6) | 1.4% (1) | 0.434 |
| Larynx | 18.3% (28) | 31.9% (23) | 0.035 |
| Other | 3.9% (6) | 2.8% (2) | 1.000 |
| Unknown | 4.6% (7) | 5.6% (4) | 0.748 |
| Tumor | |||
| Tx | 5.2% (8) | 5.6% (4) | 1.000 |
| T1 | 15.7% (25) | 9.7% (7) | 0.262 |
| T2 | 19.0% (29) | 30.6% (22) | 0.077 |
| T3 | 28.0% (43) | 27.8% (20) | 1.000 |
| T4 | 31.4% (48) | 26.4% (19) | 0.544 |
| Nodules | |||
| Nx | 1.3% (2) | 1.4% (1) | 1.000 |
| N0 | 9.8% (15) | 8.3% (6) | 0.914 |
| N1 | 21.6% (34) | 19.4% (14) | 0.764 |
| N2a | 11.8% (18) | 11.1% (8) | 1.000 |
| N2b | 18.3% (28) | 33.3% (24) | 0.020 |
| N2c | 23.5% (36) | 18.1% (13) | 0.450 |
| N3 | 13.1% (20) | 8.3% (6) | 0.416 |
| Variable | Study Population | Control | Statistical Significance | ||
|---|---|---|---|---|---|
| WBC (G/L) | 7.66 | SD = 8.08 | 4.75 | SD = 4.26 | p < 0.001 |
| NEUT (G/L) | 6.28 | SD =7.78 | 3.4 | SD = 3.83 | p < 0.001 |
| HGB (G/dL) | 11.91 | SD = 1.47 | 12.01 | SD = 1.48 | p = 0.631 |
| ALB (G/L) | 37.77 | SD = 3.68 | 33.86 | SD = 4.57 | p < 0.001 |
| Variable | I Weight Loss (kg) | II Used Percentage of the Planned Dose of Drugs (%) | III The Incidence of Adverse Events (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Control | Study | Control | Study | Control | ||||
| A General | |||||||||
| −5.26 SD = 3.68 | −7.54 SD = 5.46 | p = 0.006 | 85.90 SD = 19.28 | 73.87 SD = 18.33 | p < 0.001 | 16.3 | 25 | p = 0.123 | |
| B Depending on the age | |||||||||
| ≤70 years old | −5.20 SD = 3.70 | −8.05 SD = 5.61 | p < 0.001 | 86.69 SD = 19.13 | 76.48 SD = 16.54 | p < 0.001 | 14.9 | 27.9 | p = 0.03 |
| >70 years old | −5.76 SD = 3.52 | −4.40 SD = 3.17 | p = 0.493 | 70.57 SD = 18.09 | 58.0 SD = 21.49 | p = 0.493 | 33.3 | 10 | p = 0.30 |
| C Depending on primary surgical treatment | |||||||||
| Surgery | −5.15 SD = 3.57 | −6.00 SD = 5.40 | p = 0.414 | 88.38 SD = 17.86 | 66.00 SD = 20.92 | p < 0.001 | 7 | 28 | p = 0.029 |
| No surgery | −5.33 SD = 3.77 | −8.37 SD = 5.37 | p < 0.001 | 84.21 SD = 20.13 | 78.15 SD = 15.36 | p = 0.080 | 22.3 | 23.9 | p = 0.83 |
| D Depending on initial weight loss | |||||||||
| ≤10% | −5.73 SD = 3.71 | −7.91 SD = 5.51 | p = 0.001 | 86.43 SD = 18.96 | 74.05 SD = 18.81 | p < 0.001 | 17.2 | 25.4 | p = 0.187 |
| >10% | −3.26 SD = 2.85 | −4.63 SD = 4.31 | p = 0.425 | 84.04 SD = 21.12 | 72.50 SD = 14.88 | p = 0.136 | 14.3 | 25.0 | p = 0.597 |
| E Depending on PEG | |||||||||
| No | −5.91 SD = 3.80 | −7.96 SD = 4.94 | p = 0.030 | 86.53 SD = 19.16 | 73.05 SD = 18.16 | p = 0.065 | 17.1 | 28.6 | p = 0.120 |
| Yes | −3.87 SD = 3.00 | −6.97 SD = 6.16 | p = 0.043 | 84.55 SD = 19.70 | 75.00 SD = 18.80 | p = 0.035 | 14.6 | 20.0 | p = 0.532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapała, A.; Surwiłło-Snarska, A.; Jodkiewicz, M.; Kawecki, A. Nutritional Care in Patients with Head and Neck Cancer during Chemoradiotherapy (CRT) and Bioradiotherapy (BRT) Provides Better Compliance with the Treatment Plan. Cancers 2021, 13, 2532. https://doi.org/10.3390/cancers13112532
Kapała A, Surwiłło-Snarska A, Jodkiewicz M, Kawecki A. Nutritional Care in Patients with Head and Neck Cancer during Chemoradiotherapy (CRT) and Bioradiotherapy (BRT) Provides Better Compliance with the Treatment Plan. Cancers. 2021; 13(11):2532. https://doi.org/10.3390/cancers13112532
Chicago/Turabian StyleKapała, Aleksandra, Agnieszka Surwiłło-Snarska, Magdalena Jodkiewicz, and Andrzej Kawecki. 2021. "Nutritional Care in Patients with Head and Neck Cancer during Chemoradiotherapy (CRT) and Bioradiotherapy (BRT) Provides Better Compliance with the Treatment Plan" Cancers 13, no. 11: 2532. https://doi.org/10.3390/cancers13112532
APA StyleKapała, A., Surwiłło-Snarska, A., Jodkiewicz, M., & Kawecki, A. (2021). Nutritional Care in Patients with Head and Neck Cancer during Chemoradiotherapy (CRT) and Bioradiotherapy (BRT) Provides Better Compliance with the Treatment Plan. Cancers, 13(11), 2532. https://doi.org/10.3390/cancers13112532







