Volumetric Modulated Arc Therapy Improves Outcomes in Definitive Radiochemotherapy for Anal Cancer Whilst Reducing Acute Toxicities and Increasing Treatment Compliance
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Eligibility, Staging Procedures, Treatment Strategies, and Ethical Approval
2.2. Chemoradiotherapy
2.3. Toxicity Scoring and Patient Follow-Up
2.4. Statistical Procedures
3. Results
3.1. Baseline Patient Characteristics and Follow-Up
3.2. Chemoradiotherapy Characteristics and Treatment Compliance
3.3. Acute Toxicities, Hematologic Toxicities, and Late Toxicities
3.4. Overall Treatment Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lum, C.; Prenen, H.; Body, A.; Lam, M.; Segelov, E. A 2020 update of anal cancer: The increasing problem in women and expanding treatment landscape. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 665–680. [Google Scholar] [CrossRef]
- Deshmukh, A.; Suk, R.; Shiels, M.S.; Sonawane, K.; Nyitray, A.G.; Liu, Y.; Gaisa, M.M.; Palefsky, J.M.; Sigel, K. Recent Trends in Squamous Cell Carcinoma of the Anus Incidence and Mortality in the United States, 2001–2015. J. Natl. Cancer Inst. 2020, 112, 829–838. [Google Scholar] [CrossRef]
- Martin, D.; Rödel, C.; Fokas, E. Chemoradiotherapy for anal cancer: Are we as good as we think? Strahlenther. Onkol. 2019, 195, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Meadows, H.; Lopes, A.; Muirhead, R.; Sebag-Montefiore, D.; Adams, R. Impact of compliance to chemoradiation on long-term outcomes in squamous cell carcinoma of the anus: Results of a post hoc analysis from the randomised phase III ACT II trial. Ann. Oncol. 2020, 31, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Shakir, R.; Adams, R.; Cooper, R.; Downing, A.; Geh, I.; Gilbert, D.; Jacobs, C.; Jones, C.; Lorimer, C.; Namelo, W.C.; et al. Patterns and Predictors of Relapse Following Radical Chemoradiation Therapy Delivered Using Intensity Modulated Radiation Therapy With a Simultaneous Integrated Boost in Anal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2020, 106, 329–339. [Google Scholar] [CrossRef]
- Nilsson, M.P.; Nilsson, E.D.; Johnsson, A.; Leon, O.; Gunnlaugsson, A.; Scherman, J. Patterns of recurrence in anal cancer: A detailed analysis. Radiat. Oncol. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ludmir, E.B.; Kachnic, L.A.; Czito, B.G. Evolution and Management of Treatment-Related Toxicity in Anal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 91–113. [Google Scholar] [CrossRef]
- Sterner, A.; Derwinger, K.; Staff, C.; Nilsson, H.; Angenete, E. Quality of life in patients treated for anal carcinoma—A systematic literature review. Int. J. Color. Dis. 2019, 34, 1517–1528. [Google Scholar] [CrossRef]
- Koerber, S.A.; Seither, B.; Slynko, A.; Haefner, M.F.; Krug, D.; Liermann, J.; Adeberg, S.; Herfarth, K.; Debus, J.; Sterzing, F. Chemoradiation in female patients with anal cancer: Patient-reported outcome of acute and chronic side effects. Tumori J. 2019, 105, 174–180. [Google Scholar] [CrossRef]
- Mehta, S.; Ramey, S.J.; Kwon, D.; Rich, B.J.; Ahmed, A.A.; Wolfson, A.; Yechieli, R.; Portelance, L.; Mellon, E.A. Impact of radiotherapy duration on overall survival in squamous cell carcinoma of the anus. J. Gastrointest. Oncol. 2020, 11, 277–290. [Google Scholar] [CrossRef]
- Koerber, S.A.; Slynko, A.; Haefner, M.F.; Krug, D.; Schoneweg, C.; Kessel, K.; Kopp-Schneider, A.; Herfarth, K.; Debus, J.; Sterzing, F. Efficacy and toxicity of chemoradiation in patients with anal cancer—A retrospective analysis. Radiat. Oncol. 2014, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Michaud, A.L.; Harandi, N.K.; Kim, E.J.; Semrad, T.; Khatri, V.; Mayadev, J.; Perks, J.; Monjazeb, A.M. The Role of Image-guided Radiotherapy in the Treatment of Anorectal Cancer Using Prone Belly-board Positioning. Anticancer. Res. 2016, 36, 3013–3017. [Google Scholar] [PubMed]
- Vaios, E.J.; Wo, J.Y. Proton beam radiotherapy for anal and rectal cancers. J. Gastrointest. Oncol. 2020, 11, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A Phase 2 Evaluation of Dose-Painted Intensity Modulated Radiation Therapy in Combination With 5-Fluorouracil and Mitomycin-C for the Reduction of Acute Morbidity in Carcinoma of the Anal Canal. Int. J. Radiat. Oncol. 2013, 86, 27–33. [Google Scholar] [CrossRef]
- Arcadipane, F.; Silvetti, P.; Olivero, F.; Gastino, A.; De Luca, V.; Mistrangelo, M.; Cassoni, P.; Racca, P.; Gallio, E.; Lesca, A.; et al. Bone Marrow-Sparing IMRT in Anal Cancer Patients Undergoing Concurrent Chemo-Radiation: Results of the First Phase of a Prospective Phase II Trial. Cancers 2020, 12, 3306. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Tan, D.; Hughes, R.; Hoskin, P. Squamous-cell carcinoma of the anus: Progress in radiotherapy treatment. Nat. Rev. Clin. Oncol. 2016, 13, 447–459. [Google Scholar] [CrossRef]
- Fredman, E.T.; Abdel-Wahab, M.; Kumar, A.M. Influence of radiation treatment technique on outcome and toxicity in anal cancer. J. Radiat. Oncol. 2017, 6, 413–421. [Google Scholar] [CrossRef]
- Bazan, J.G.; Hara, W.; Hsu, A.; Kunz, P.A.; Ford, J.; Fisher, G.A.; Welton, M.L.; Shelton, A.; Kapp, D.S.; Koong, A.C.; et al. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer 2011, 117, 3342–3351. [Google Scholar] [CrossRef]
- Chuong, M.D.; Freilich, J.M.; Hoffe, S.E.; Fulp, W.; Weber, J.M.; Almhanna, K.; Dinwoodie, W.; Rao, N.; Meredith, K.L.; Shridhar, R. Intensity-Modulated Radiation Therapy vs. 3D Conformal Radiation Therapy for Squamous Cell Car-cinoma of the Anal Canal. Gastrointest. Cancer Res. 2013, 6, 39–45. [Google Scholar]
- Dasgupta, T.; Rothenstein, D.; Chou, J.F.; Zhang, Z.; Wright, J.L.; Saltz, L.B.; Temple, L.K.; Paty, P.B.; Weiser, M.R.; Guillem, J.G.; et al. Intensity-modulated radiotherapy vs. conventional radiotherapy in the treatment of anal squamous cell carcinoma: A propensity score analysis. Radiother. Oncol. 2013, 107, 189–194. [Google Scholar] [CrossRef]
- Cendales, R.; Vásquez, J.; Arbelaez, J.; Bobadilla, I.; Torres, F.; Gaitan, A. IMRT, RapidArc® and conformal radiotherapy in the treatment of tumours of the anal canal. Ecancermedicalscience 2014, 8, 469. [Google Scholar] [CrossRef]
- Clivio, A.; Fogliata, A.; Franzetti-Pellanda, A.; Nicolini, G.; Vanetti, E.; Wyttenbach, R.; Cozzi, L. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiother. Oncol. 2009, 92, 118–124. [Google Scholar] [CrossRef]
- Vieillot, S.; Azria, D.; Lemanski, C.; Moscardo, C.L.; Gourgou, S.; Dubois, J.-B.; Ailleres, N.; Fenoglietto, P. Plan comparison of volumetric-modulated arc therapy (RapidArc) and conventional intensity-modulated radiation therapy (IMRT) in anal canal cancer. Radiat. Oncol. 2010, 5, 92. [Google Scholar] [CrossRef]
- Yucel, S.; Kadioglu, H.; Gural, Z.; Akgun, Z.; Saglam, E. Outcomes of patients with anal cancer treated with volumetric-modulated arc therapy or intensity-modulated radiotherapy and concurrent chemotherapy. J. Cancer Res. Ther. 2021, 17, 51–55. [Google Scholar] [CrossRef]
- Franco, P.; Arcadipane, F.; Ragona, R.; Mistrangelo, M.; Cassoni, P.; Munoz, F.; Rondi, N.; Morino, M.; Racca, P.; Ricardi, U. Volumetric modulated arc therapy (VMAT) in the combined modality treatment of anal cancer patients. Br. J. Radiol. 2016, 89, 20150832. [Google Scholar] [CrossRef]
- Yordanov, K.; Cima, S.; Richetti, A.; Pesce, G.; Martucci, F.; Azinwi, N.C.; Valli, M.C. Concurrent chemoradiation with volumetric modulated Arc therapy of patients treated for anal cancer—acute toxicity and treatment outcome. J. Gastrointest. Oncol. 2017, 8, 361–367. [Google Scholar] [CrossRef]
- Weber, M.H.E.; Dröge, M.L.H.; Hennies, M.S.; Herrmann, M.M.K.; Gaedcke, P.D.M.J.; Wolff, P.D.M.H.A. Volumetric intensity-modulated arc therapy vs. 3-dimensional conformal radiotherapy for primary chemoradiotherapy of anal carcinoma. Strahlenther. Onkol. 2015, 191, 827–834. [Google Scholar] [CrossRef]
- Tozzi, A.; Cozzi, L.; Iftode, C.; Ascolese, A.; Campisi, M.C.; Clerici, E.; Comito, T.; De Rose, F.; Fogliata, A.; Franzese, C.; et al. Radiation therapy of anal canal cancer: From conformal therapy to volumetric modulated arc therapy. BMC Cancer 2014, 14, 833. [Google Scholar] [CrossRef][Green Version]
- Aigner, F.; Analkarzinom, D.L.; Werner, R.N.; Koswig, S.; Gaskins, M.; Rödel, C.; Kahlke, V.; Raab, H.R.; Siegel, R. Zusammenfassung und Kommentar zur S3-Leitlinie Analkarzinom; Diagnostik, Therapie und Nachsorge von Analkanal- und Analrandkarzinomen. Der Chir. 2021, 92, 244–247. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D. Anal cancer: ESMO–ESSO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother. Oncol. 2014, 111, 330–339. [Google Scholar] [CrossRef]
- Myerson, R.J.; Garofalo, M.C.; El Naqa, I.; Abrams, R.A.; Apte, A.; Bosch, W.R.; Das, P.; Gunderson, L.L.; Hong, T.S.; Kim, J.J.; et al. Elective Clinical Target Volumes for Conformal Therapy in Anorectal Cancer: A Radiation Therapy Oncology Group Consensus Panel Contouring Atlas. Int. J. Radiat. Oncol. 2009, 74, 824–830. [Google Scholar] [CrossRef]
- Wolff, H.A.; Wagner, D.M.; Conradi, L.-C.; Hennies, S.; Ghadimi, M.; Hess, C.F.; Christiansen, H. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: A planning study with clinical implications. Radiother. Oncol. 2012, 102, 30–37. [Google Scholar] [CrossRef]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, Mitomycin, and Radiotherapy vs. Fluorouracil, Cisplatin, and Radiotherapy for Carcinoma of the Anal Canal. JAMA 2008, 299, 1914–1921. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Winter, K.A.; Ajani, J.A.; Pedersen, J.E.; Moughan, J.; Benson, A.B.; Jr, C.R.T.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; et al. Long-Term Update of US GI Intergroup RTOG 98-11 Phase III Trial for Anal Carcinoma: Survival, Relapse, and Colostomy Failure With Concurrent Chemoradiation Involving Fluorouracil/Mitomycin Versus Fluorouracil/Cisplatin. J. Clin. Oncol. 2012, 30, 4344–4351. [Google Scholar] [CrossRef]
- Eng, C.; Chang, G.J.; You, Y.N.; Das, P.; Xing, Y.; Delclos, M.; Wolff, R.A.; Rodriguez-Bigas, M.A.; Skibber, J.; Ohinata, A.; et al. Long-term results of weekly/daily cisplatin-based chemoradiation for locally advanced squamous cell carcinoma of the anal canal. Cancer 2013, 119, 3769–3775. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Evens (CTCAE), Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf (accessed on 22 March 2021).
- Rubin, P.; Constine, L.S.; Fajardo, L.F.; Phillips, T.L.; Wasserman, T.H. Overview: Late effects of normal tissues (LENT) scoring system. Int. J. Radiat. Oncol. 1995, 31, 1041–1042. [Google Scholar] [CrossRef]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A Convenient Tool for Graphical Presentation of Results from Kaplan-Meier Survival Time Analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Albert, A.; Sheth, N.; Adedoyin, P.; Rowley, J.; Schreiber, D. Patterns of care and outcomes of intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for anal cancer. J. Gastrointest. Oncol. 2019, 10, 623–631. [Google Scholar] [CrossRef]
- Martin, D.; Von Der Grün, J.; Rödel, C.; Fokas, E. Management of anal cancer patients—A pattern of care analysis in German-speaking countries. Radiat. Oncol. 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pepek, J.M.; Willett, C.G.; Wu, Q.J.; Yoo, S.; Clough, R.W.; Czito, B.G. Intensity-Modulated Radiation Therapy for Anal Malignancies: A Preliminary Toxicity and Disease Outcomes Analysis. Int. J. Radiat. Oncol. 2010, 78, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Salama, J.K.; Mell, L.K.; Schomas, D.A.; Miller, R.C.; Devisetty, K.; Jani, A.B.; Mundt, A.J.; Roeske, J.C.; Liauw, S.L.; Chmura, S.J. Concurrent Chemotherapy and Intensity-Modulated Radiation Therapy for Anal Canal Cancer Patients: A Multicenter Experience. J. Clin. Oncol. 2007, 25, 4581–4586. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Zu Eissen, J.M.; Karstens, J.H.; Bremer, M. Chemoradiotherapy in patients with anal cancer: Impact of length of unplanned treatment interruption on outcome. Acta Oncol. 2006, 45, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Scher, E.D.; Ahmed, I.; Yue, N.J.; Jabbour, S.K. Technical aspects of radiation therapy for anal cancer. J. Gastrointest. Oncol. 2014, 5, 198–211. [Google Scholar]
- Rich, T.A.; Shepard, R.C.; Mosley, S.T. Four Decades of Continuing Innovation with Fluorouracil: Current and Future Approaches to Fluorouracil Chemoradiation Therapy. J. Clin. Oncol. 2004, 22, 2214–2232. [Google Scholar] [CrossRef]
- Alrubai, A.M.M.; Al-Naqqash, M.A.; Alshewered, A.S. Epidemiological and prognostic single center study of anal carcinoma. J. Coloproctology 2020, 40, 202–208. [Google Scholar] [CrossRef]
- Weiss, E.; Hirnle, P.; Arnold-Bofinger, H.; Hess, C.F.; Bamberg, M. Therapeutic outcome and relation of acute and late side effects in the adjuvant radiotherapy of endometrial carcinoma stage I and II. Radiother. Oncol. 1999, 53, 37–44. [Google Scholar] [CrossRef]
- Dörr, W.; Hendry, J.H. Consequential late effects in normal tissues. Radiother. Oncol. 2001, 61, 223–231. [Google Scholar] [CrossRef]
- Goodman, K.A.; Julie, D.; Cercek, A.; Cambridge, L.; Woo, K.M.; Zhang, Z.; Wu, A.J.; Reidy, D.L.; Segal, N.H.; Stadler, Z.K.; et al. Capecitabine With Mitomycin Reduces Acute Hematologic Toxicity and Treatment Delays in Patients Undergoing Definitive Chemoradiation Using Intensity Modulated Radiation Therapy for Anal Cancer. Int. J. Radiat. Oncol. 2017, 98, 1087–1095. [Google Scholar] [CrossRef]
- Rattan, R.; Kapoor, R.; Bahl, A.; Gupta, R.; Oinam, A.S.; Kaur, S. Comparison of bone marrow sparing intensity modulated radiotherapy (IMRT) and three-dimensional conformal radiotherapy (3DCRT) in carcinoma of anal canal: A prospective study. Ann. Transl. Med. 2016, 4, 70. [Google Scholar]
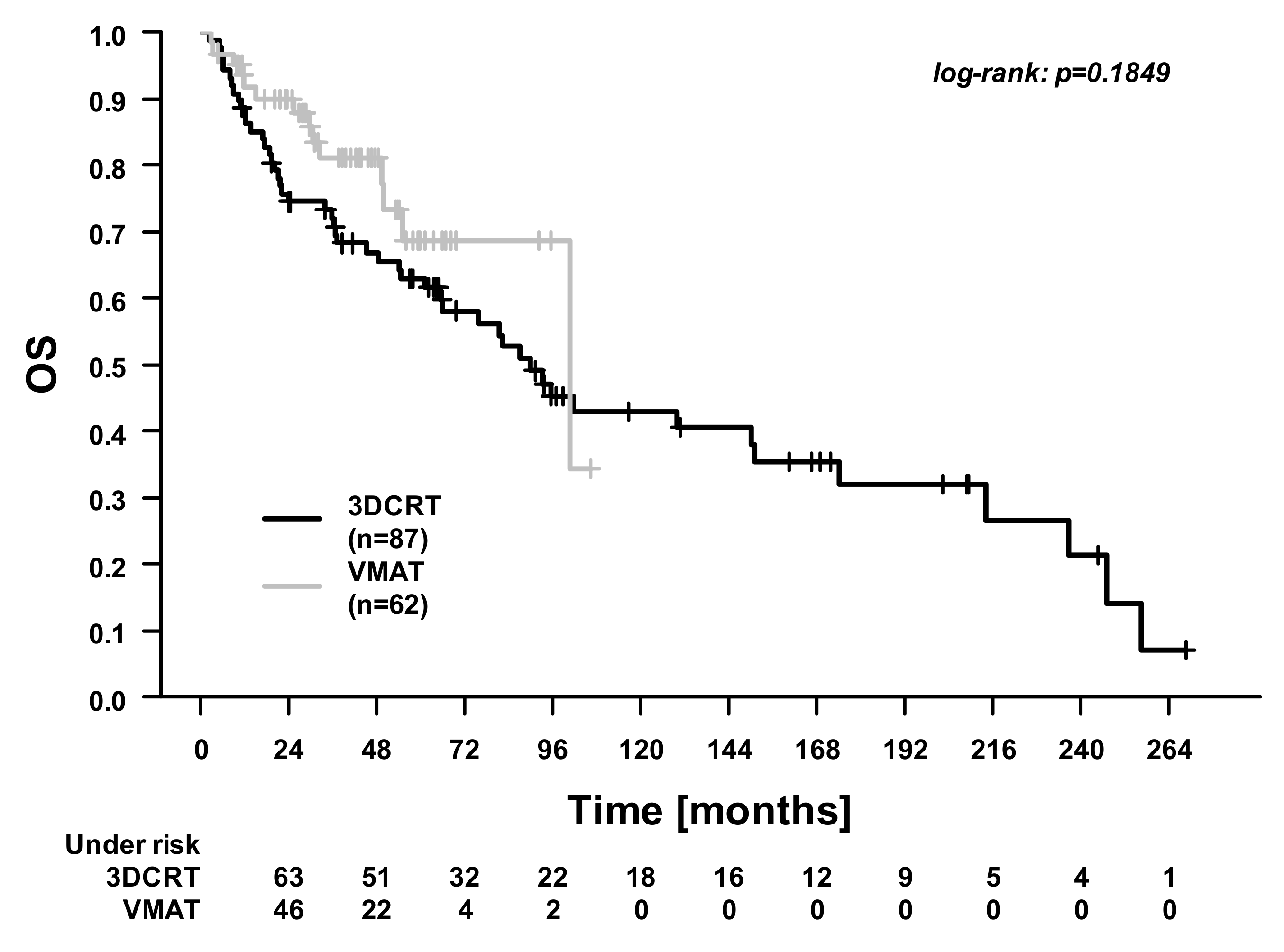
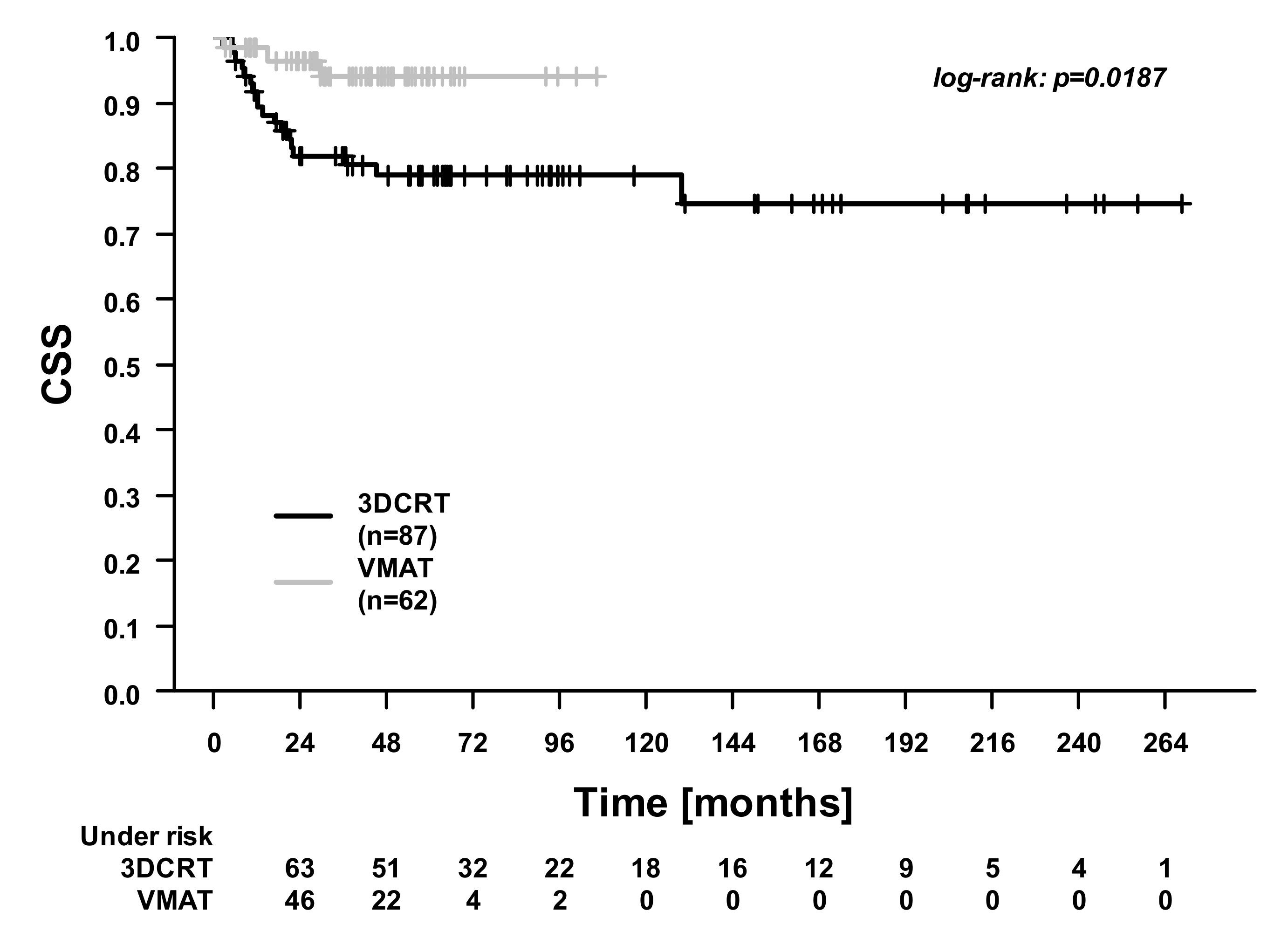
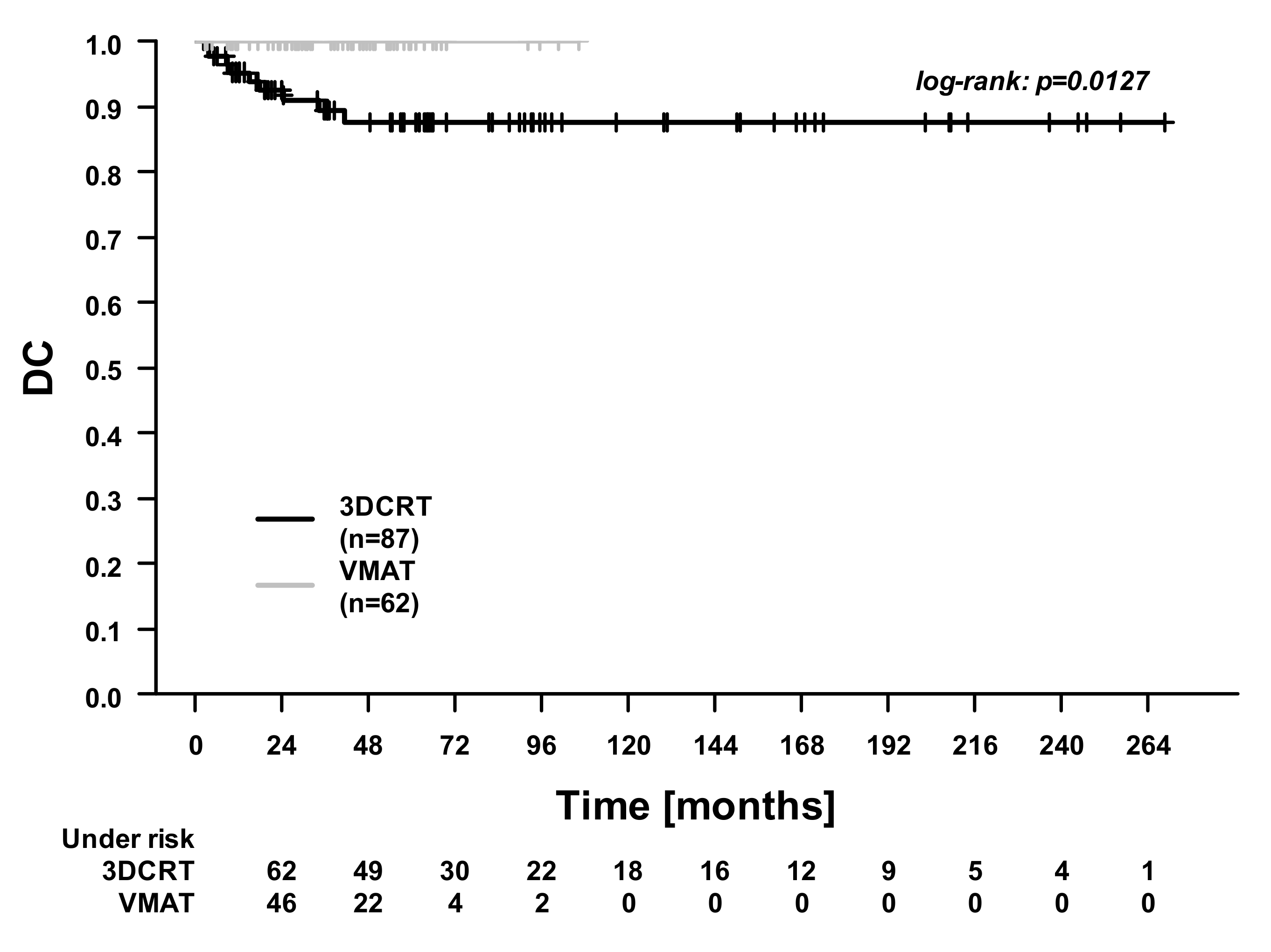
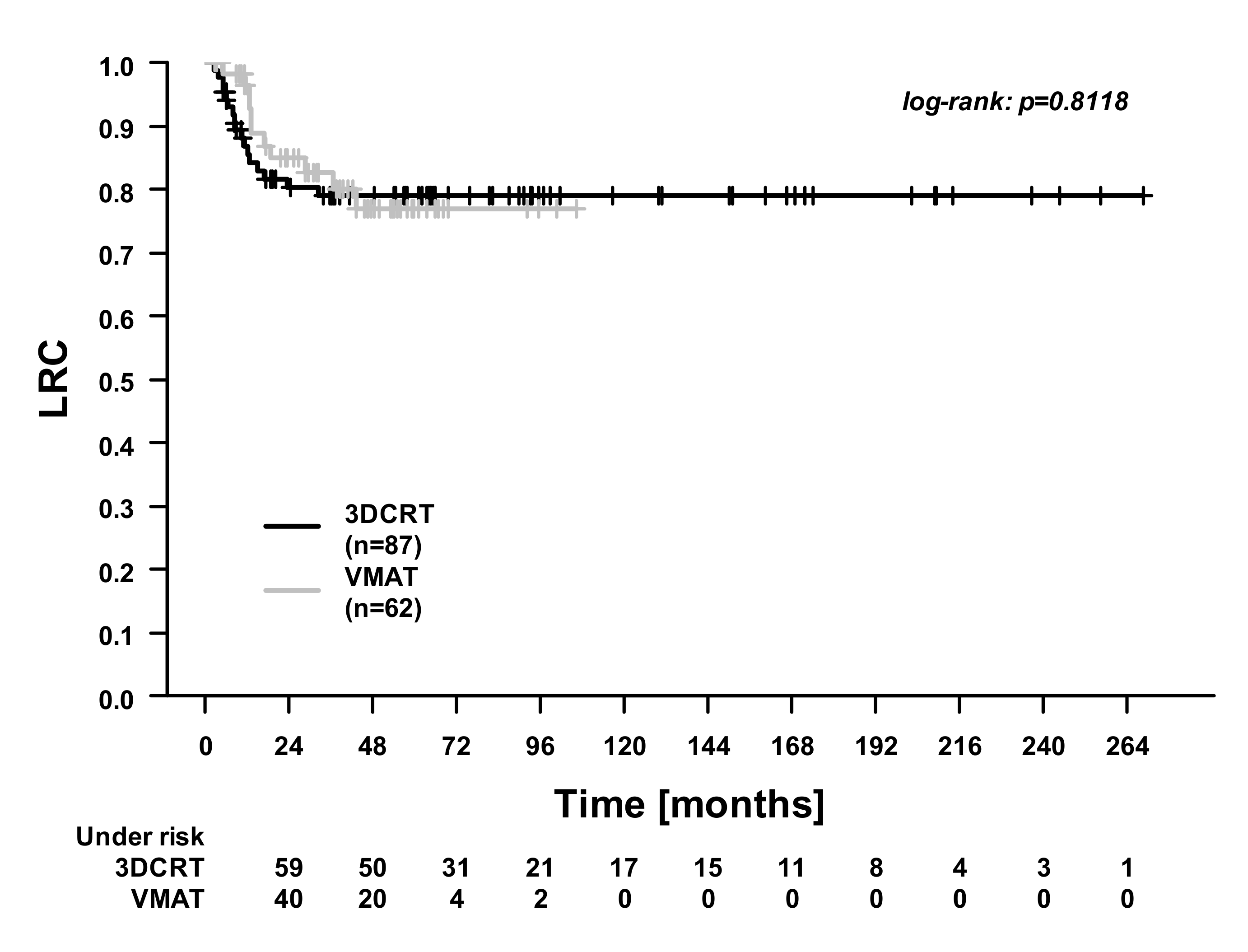
| Characteristics | 3DCRT (n = 87) | VMAT (n = 62) | p-Value |
|---|---|---|---|
| Age (years) | 64.3 (29.5–90.9) | 60.2 (33.3–84.5) | 0.11 # |
| Sex | 0.92 * | ||
| Female | 61 (70.1) | 43 (69.1) | |
| Male | 26 (29.9) | 19 (30.6) | |
| Body Mass Index (kg/m2) | 25.6 (14.1–44.6) | 25.8 (17.4–38.5) | 0.83 # |
| Body Surface Area (DuBois method, m2) | 1.76 (1.31–2.22) | 1.8 (1.45–2.46) | 0.21 # |
| Charlson Comorbidity Index | 0.54 * | ||
| 1–3 | 27 (31.0) | 23 (37.1) | |
| 4–6 | 52 (59.8) | 35 (56.5) | |
| 7–10 | 8 (9.2) | 4 (6.4) | |
| Grading | <0.001 * | ||
| G1 | 18 (20.7) | 0 | |
| G2 | 56 (64.4) | 42 (67.7) | |
| G3 | 12 (13.8) | 18 (29.0) | |
| Undetermined | 1 (1.1) | 2 (3.2) | |
| cT status | 0.19 * | ||
| T1 | 14 (16.1) | 12 (19.4) | |
| T2 | 40 (46.0) | 20 (32.3) | |
| T3 | 26 (29.9) | 19 (30.6) | |
| T4 | 7 (8.0) | 11 (17.7) | |
| cN status | 0.05 * | ||
| N0 | 60 (69.0) | 33 (53.2) | |
| N1 | 27 (31.0) | 29 (46.8) | |
| AJCC classification (8th edition, 2017) | 0.06 * | ||
| I | 11 (12.6) | 10 (16.1) | |
| IIA | 35 (40.2) | 12 (19.4) | |
| IIB | 11 (12.6) | 6 (9.7) | |
| IIIA | 7 (8.0) | 10 (16.1) | |
| IIIB | 3 (3.4) | 6 (9.7) | |
| IIIC | 20 (23.0) | 18 (29.0) |
| Parameters | 3DCRT (n = 87) | VMAT (n = 62) | p-Value |
|---|---|---|---|
| Radiotherapy | |||
| Planned dose | 50.4 (40.0–61.0) | 50.4 (50.4–60.4) | 0.22 # |
| Administered dose | 50.4 (40.0–61.0) | 50.4 (41.4–59.4) | 0.06 # |
| Received 100% of planned dose | 73 (83.9) | 57 (91.9) | 0.15 * |
| Received >80% of planned dose | 87 (100.0) | 62 (100.0) | - |
| Interruptions or delays (patients) | 37 (42.5) | 4 (6.5) | <0.001 * |
| Interruptions or delays (days, mean, range) 1 | 2.65 (0.0–27.0) | 0.26 (0.0–6.0) | <0.001 # |
| Chemotherapy | |||
| Received concomitant chemotherapy | 80 (92.0) | 59 (95.2) | 0.44 * |
| Chemotherapy regimen | 0.06 * | ||
| 5-fluorouracil/mitomycin c | 78 (97.5) | 53 (85.5) | |
| Other regimen | 2 (2.5) | 6 (14.5) | |
| Chemotherapy compliance and dose | |||
| Interruptions or delays (patients) | 5 (6.3) | 1 (1.7) | 0.19 * |
| Received <100% of planned dose | 12 (15.0) | 9 (15.3) | 0.97 * |
| 5-fluorouracil: absolute dose applied (mg) 2 | 13,700 (6000–15,000) | 14,400 (6240–18,000) | 0.001 # |
| Mitomycin c: absolute dose applied (mg) 2 | 34.6 (10.0–41.0) | 35.6 (15.6–40.0) | 0.78 # |
| Cisplatin: absolute dose applied (mg) | 400 (1 patient) | 255.75 (131.0–393.0) | - |
| Chemoradiotherapy | |||
| Overall treatment time (days) | 41 (28–74) | 38 (31–49) | 0.02 # |
| Toxicities | 3DCRT (n = 87) | VMAT (n = 62) | p-Value |
|---|---|---|---|
| Acute organ toxicity | |||
| Overall acute organ toxicity, ≥grade 3 | 48 (55.2) | 11 (17.7) | <0.001 |
| Dermatitis, ≥grade 3 | 39 (44.8) | 9 (14.5) | <0.001 |
| Enteritis, ≥grade 3 | 9 (10.3) | 1 (1.6) | 0.04 |
| Proctitis, ≥grade 3 | 4 (4.6) | 1 (1.6) | 0.32 |
| Cystitis, ≥grade 3 | 3 (3.4) | 2 (3.2) | 0.94 |
| Hematologic toxicity | |||
| Overall hematologic toxicity, ≥grade 3 | 15 (17.2) | 17 (27.4) | 0.14 |
| Anemia, ≥grade 3 | 2 (2.3) | 1 (1.6) | 0.77 |
| Leukopenia, ≥grade 3 | 11 (12.6) | 12 (19.4) | 0.26 |
| Thrombopenia, ≥grade 3 | 2 (2.3) | 9 (14.5) | 0.01 |
| Late toxicity | |||
| GI and urinary, ≥grade 3 | 11 (12.6) | 2 (3.2) | 0.05 |
| Vagina, grades 1–2 1 | 7 (13.0) | 5 (12.2) | 0.91 |
| Pelvic bone fractures, ≥grade 3 2 | 6 (6.9) | 1 (1.6) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possiel, J.; Ammon, H.E.; Guhlich, M.; Conradi, L.-C.; Ghadimi, M.; Wolff, H.A.; Schirmer, M.A.; Samel, S.; Mügge, M.; Rieken, S.; et al. Volumetric Modulated Arc Therapy Improves Outcomes in Definitive Radiochemotherapy for Anal Cancer Whilst Reducing Acute Toxicities and Increasing Treatment Compliance. Cancers 2021, 13, 2533. https://doi.org/10.3390/cancers13112533
Possiel J, Ammon HE, Guhlich M, Conradi L-C, Ghadimi M, Wolff HA, Schirmer MA, Samel S, Mügge M, Rieken S, et al. Volumetric Modulated Arc Therapy Improves Outcomes in Definitive Radiochemotherapy for Anal Cancer Whilst Reducing Acute Toxicities and Increasing Treatment Compliance. Cancers. 2021; 13(11):2533. https://doi.org/10.3390/cancers13112533
Chicago/Turabian StylePossiel, Jacqueline, Hanne Elisabeth Ammon, Manuel Guhlich, Lena-Christin Conradi, Michael Ghadimi, Hendrik Andreas Wolff, Markus Anton Schirmer, Stephan Samel, Michael Mügge, Stefan Rieken, and et al. 2021. "Volumetric Modulated Arc Therapy Improves Outcomes in Definitive Radiochemotherapy for Anal Cancer Whilst Reducing Acute Toxicities and Increasing Treatment Compliance" Cancers 13, no. 11: 2533. https://doi.org/10.3390/cancers13112533
APA StylePossiel, J., Ammon, H. E., Guhlich, M., Conradi, L.-C., Ghadimi, M., Wolff, H. A., Schirmer, M. A., Samel, S., Mügge, M., Rieken, S., Leu, M., & Dröge, L. H. (2021). Volumetric Modulated Arc Therapy Improves Outcomes in Definitive Radiochemotherapy for Anal Cancer Whilst Reducing Acute Toxicities and Increasing Treatment Compliance. Cancers, 13(11), 2533. https://doi.org/10.3390/cancers13112533









