Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
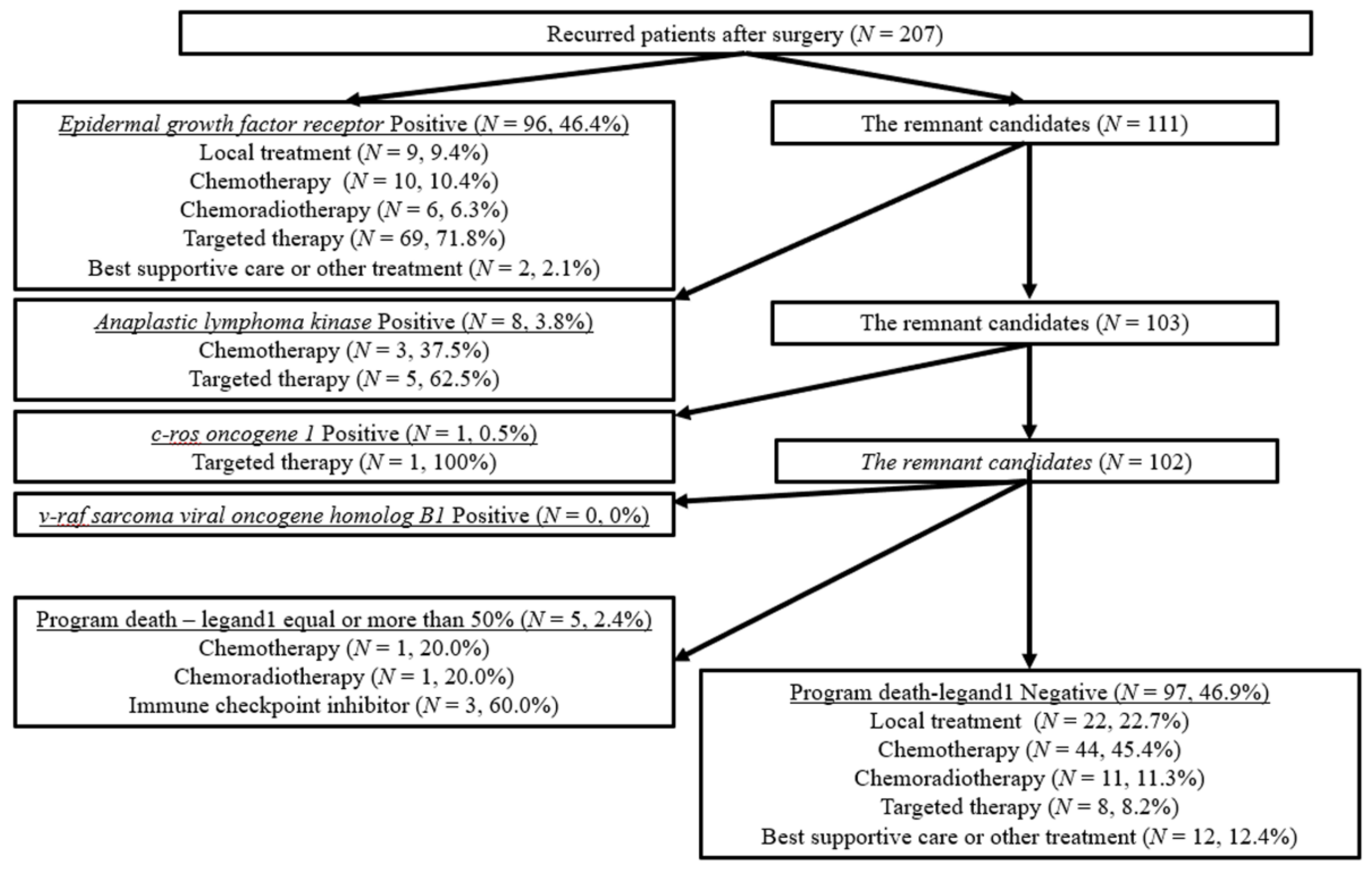
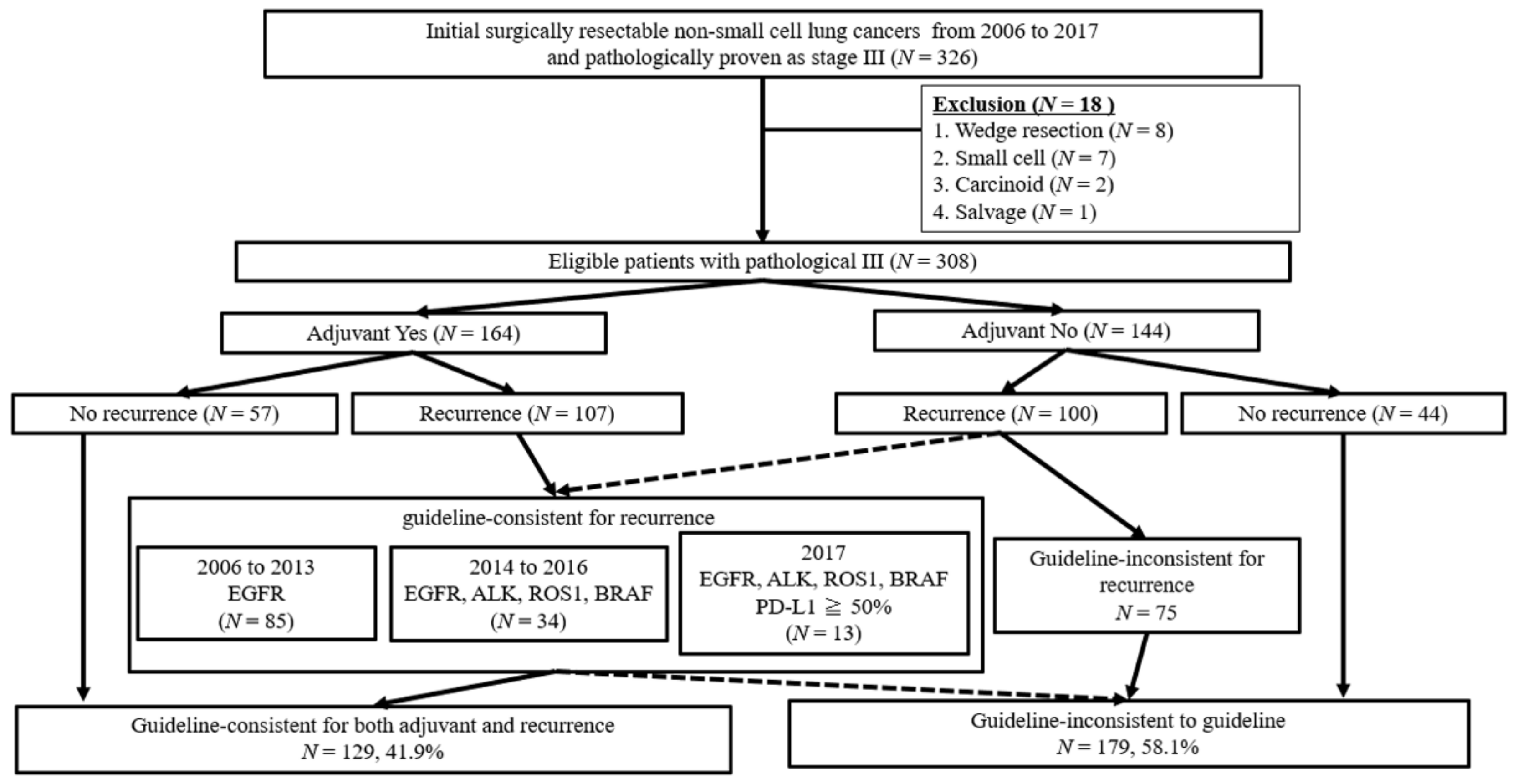
3.1. Patient Flow Algorism
3.2. Patient Characteristics
3.3. Surgical Outcomes and Therapeutic Efficacy in Recurred Patients
3.4. To Investigate the Prognostic Factor for OS
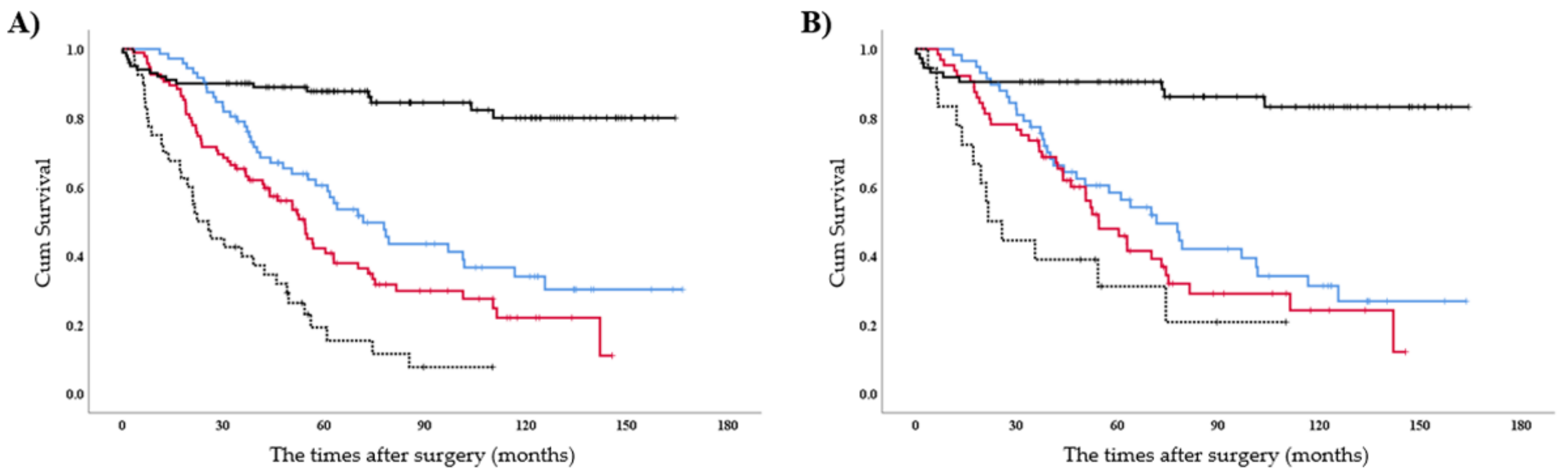
3.5. Subgroup Analyses for OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Spiro, S.G.; Silvestri, G.A. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 2005, 172, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, N.; Mizuno, T.; Kuroda, H.; Arimura, T.; Yatabe, Y.; Yoshimura, K.; Sakao, Y. The eighth TNM classification system for lung cancer: A consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer 2018, 118, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Le Chevalier, T.; Arriagada, R.; Pignon, J.; Scagliotti, G.V. Should adjuvant chemotherapy become standard treatment in all patients with resected non-small-cell lung cancer? Lancet Oncol. 2005, 6, 182–184. [Google Scholar] [CrossRef]
- Mori, S.; Usami, N.; Fukumoto, K.; Mizuno, T.; Kuroda, H.; Sakakura, N.; Yokoi, K.; Sakao, Y. The Significance of the Prognostic Nutritional Index in Patients with Completely Resected Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0136897. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1 2020. J. Natl. Compr. Canc. Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Haneda, M.; Masago, K.; Fujita, S.; Kato, S.; Sasaki, E.; Hosoda, W.; Murakami, Y.; Kuroda, H.; Horio, Y.; et al. Negative reactions of BRAF mutation-specific immunohistochemistry to non-V600E mutations of BRAF. Pathol. Int. 2020, 70, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Kuroda, H.; Nakada, T.; Takahashi, Y.; Sakakura, N.; Hida, T. Efficacy of Immune Checkpoint Inhibitor Monotherapy for Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. Int. J. Mol. Sci. 2020, 21, 2623. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Wu, Y.L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.S.; Sriuranpong, V.; Chao, T.Y.; Nakagawa, K.; Chu, D.T.; Saijo, N.; et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Yoshida, T.; Oya, Y.; Tanaka, K.; Shimizu, J.; Horio, Y.; Kuroda, H.; Sakao, Y.; Hida, T.; Yatabe, Y. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Sriuranpong, V.; Imamura, F.; Nogami, N.; Kurata, T.; Okamoto, I.; Zhou, C.; et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J. Thorac. Oncol. 2019, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.K.; Sriuranpong, V.; Ho, J.C.M.; Ong, C.K.; Tsai, C.M.; Chung, C.H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung. J. Thorac. Oncol. 2018, 13, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Kuroda, H.; Yoshida, T.; Sakata, S.; Mizuno, T.; Sakakura, N.; Hida, T.; Yatabe, Y.; Sakao, Y. Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag. Res. 2018, 10, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. KEYNOTE-042Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; de Castro, G., Jr.; Reck, M.; Wu, Y.L.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wilshire, C.L.; Rayburn, J.R.; Chang, S.C.; Gilbert, C.R.; Louie, B.E.; Aye, R.W.; Farivar, A.S.; Bograd, A.J.; Vallières, E.; Gorden, J.A. Not Following the Rules in Guideline Care for Lung Cancer Diagnosis and Staging Has Negative Impact. Ann. Thorac. Surg. 2020, 110, 1730–1738. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.I.; Nakagawa, K.; Suzuki, K.; Takamochi, K.; Ito, H.; Okami, J.; Aokage, K.; Saji, H.; Yoshioka, H.; Zenke, Y.; et al. Neoadjuvant and adjuvant therapy for Stage III non-small cell lung cancer. Lung Cancer Surgical Study Group (LCSSG) of the Japan Clinical Oncology Group (JCOG). JPN J. Clin. Oncol. 2017, 47, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-Analyses Collaborative Group; Arriagada, R.; Auperin, A.; Burdett, S.; Higgins, J.P.; Johnson, D.H.; Le Chevalier, T.; Le Pechoux, C.; Parmar, M.K.; Pignon, J.P.; et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Inconsistent | Consistent | p-Value | |
|---|---|---|---|---|
| n = 179 | n = 129 | |||
| Age (years old), median | 67 | 63 | <0.01 | |
| IQR | (61–73) | (58–67) | ||
| Gender, male (%) | 104 (58.1%) | 73 (56.6%) | 0.79 | |
| Era | 0.82 | |||
| 2006–2013 | 116 (64.8%) | 82 (63.6%) | ||
| 2014–2017 | 63 (35.2%) | 47 (36.4%) | ||
| Smoking history (pack-year), median | 34.0 | 16.0 | 0.08 | |
| IQR | (0–52.0) | (0–46.5) | ||
| Prognostic nutritional index, median | 49.8 | 52.4 | <0.01 | |
| IQR | (46.3–53.3) | (49.3–54.7) | ||
| Clinical stage N (number, %) | 0.31 | |||
| cN0 | 98 (54.7%) | 63 (48.8%) | ||
| cN1–2 | 81 (45.3%) | 66 (51.2%) | ||
| Clinical stage | 0.80 | |||
| cI | 68 (38.0%) | 51 (39.5%) | ||
| cII | 46 (25.7%) | 32 (24.8%) | ||
| cIII | 65 (36.3%) | 46 (35.7%) | ||
| Histology (number, %) | 0.05 | |||
| Adenocarcinoma | 117 (65.4%) | 98 (76.0%) | ||
| Others | 62 (34.6%) | 31 (24.0%) | ||
| Type of procedures (number, %) | 0.02 | |||
| Lobectomy | 149 (83.2%) | 119 (92.2%) | ||
| Pneumonectomy/Bilobectomy | 30 (16.8%) | 10 (7.8%) | ||
| ATCR (yes, %) | 35 (19.6%) | 129 (100%) | <0.01 | |
| Pathological-Stage | <0.01 | |||
| IIIA | 167 (93.2%) | 123 (95.3%) | ||
| IIIB | 12 (6.7%) | 6 (4.7%) | ||
| Single lymph node involvement | 0.35 | |||
| (yes, %) | 29 (16.2%) | 16 (12.4%) | ||
| Mutation status (yes/no/uninformative) | ||||
| EGFR | 68/111/0 | 55/74/0 | 0.41 | |
| ALK | 5/140/34 | 6/95/28 | 0.35 | |
| BRAF | 0/62/117 | 0/40/89 | NA | |
| ROS1 | 0/24/155 | 1/16/107 | NA | |
| Treatment after recurrence (yes, %) | 136 (76.0%) | 74 (57.4%) | 0.04 | |
| Local control | 26 (19.1%) | 7 (9.0%) | ||
| Chemotherapy ± Radiotherapy | 55 (40.4%) | 21 (28.4%) | ||
| Molecular target drug | 37 (27.2%) | 44 (59.5%) | ||
| Immune checkpoint inhibitor | 4 (3.0%) | 2 (2.7%) | ||
| Others | 14 (10.3%) | 0 (0%) | ||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p-Value | Hazard Ratio (95% CI 1) | p-Value | ||
| Patient characteristics | ||||
| Age | <0.01 * | 1.02 (1.01–1.04) | 0.02 * | |
| Male | <0.01 * | 0.73 (0.50–1.06) | 0.10 | |
| Pack-year | 0.11 | |||
| Prognostic nutritional index | ||||
| Score < 50 | 0.02 * | 0.74 (0.54–1.03) | 0.08 | |
| Era | ||||
| 2006–2013 | <0.01 * | 0.50 (0.33–0.76) | <0.01 * | |
| Clinical N stage | ||||
| N1–2 | 0.27 | |||
| Histology | ||||
| Adenocarcinoma | 0.57 | |||
| Procedures | ||||
| More than lobectomy | 0.81 | |||
| Guideline | ||||
| Inconsistent | <0.01 * | 0.49 (0.34–0.71) | <0.01 * | |
| Any mutation | ||||
| Yes | <0.01 * | 1.36 (0.94–1.97) | 0.10 | |
| Pathological N status | ||||
| Single involvement | 0.39 | |||
| Characteristics | Inconsistent | Consistent | p-Value | |
|---|---|---|---|---|
| n = 106 | n = 108 | |||
| Age (years old), median | 64 | 64 | 0.98 | |
| IQR | (60–68) | (58–69) | ||
| Gender, male (%) | 61 (57.5%) | 59 (54.6%) | 0.67 | |
| Era | 0.97 | |||
| 2006–2013 | 69 (65.1%) | 70 (64.8%) | ||
| 2014–2017 | 37 (34.9%) | 38 (35.2%) | ||
| Smoking history (pack-year), median | 20.0 | 32 | 0.49 | |
| IQR | (0–49.1) | (0–50.8) | ||
| Prognostic nutritional index, median | 0.81 | |||
| IQR | 36 (34.0%) | 35 (32.4%) | ||
| Any mutation (EGFR/ALK/BRAF/ROS1) | ||||
| (yes, %) | 50 (47.2) | 50 (46.3%) | 0.90 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, H.; Sugita, Y.; Masago, K.; Takahashi, Y.; Nakada, T.; Sasaki, E.; Sakakura, N.; Yamaguchi, R.; Matsushita, H.; Hida, T. Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study. Cancers 2021, 13, 2531. https://doi.org/10.3390/cancers13112531
Kuroda H, Sugita Y, Masago K, Takahashi Y, Nakada T, Sasaki E, Sakakura N, Yamaguchi R, Matsushita H, Hida T. Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study. Cancers. 2021; 13(11):2531. https://doi.org/10.3390/cancers13112531
Chicago/Turabian StyleKuroda, Hiroaki, Yusuke Sugita, Katsuhiro Masago, Yusuke Takahashi, Takeo Nakada, Eiichi Sasaki, Noriaki Sakakura, Rui Yamaguchi, Hirokazu Matsushita, and Toyoaki Hida. 2021. "Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study" Cancers 13, no. 11: 2531. https://doi.org/10.3390/cancers13112531
APA StyleKuroda, H., Sugita, Y., Masago, K., Takahashi, Y., Nakada, T., Sasaki, E., Sakakura, N., Yamaguchi, R., Matsushita, H., & Hida, T. (2021). Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study. Cancers, 13(11), 2531. https://doi.org/10.3390/cancers13112531






