Acquired Evolution of Mitochondrial Metabolism Regulated by HNF1B in Ovarian Clear Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
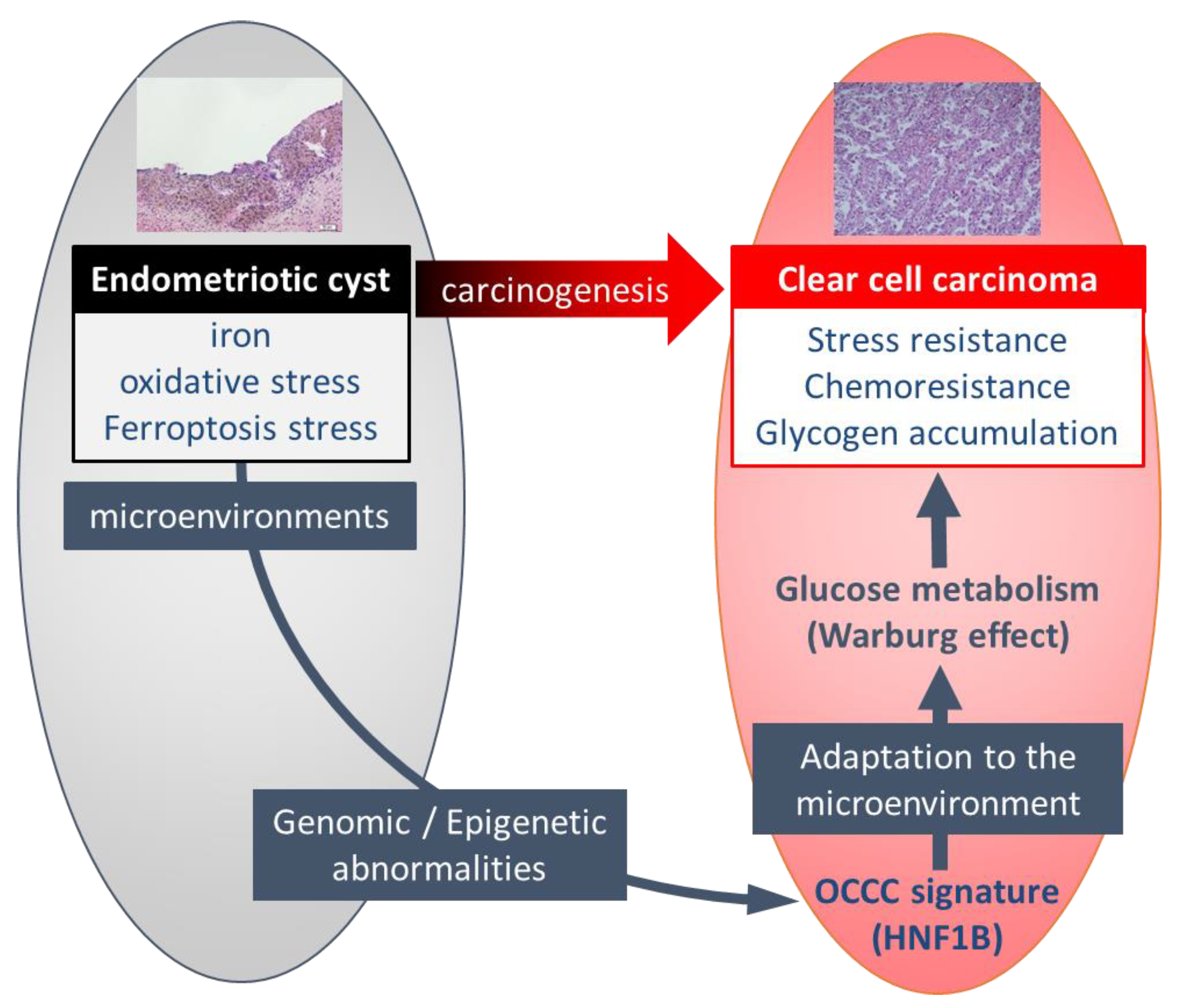
2. Endometriotic Cyst as a Carcinogenic Environment for Ovarian Cancer
3. Two Hypothetical Theories for Carcinogenesis Process among Endometriosis-Related Ovarian Cancer
4. Unique Characteristics of Ovarian CCC Induced in Carcinogenic Environments
5. Genomic and Epigenetic Alterations in Ovarian CCC
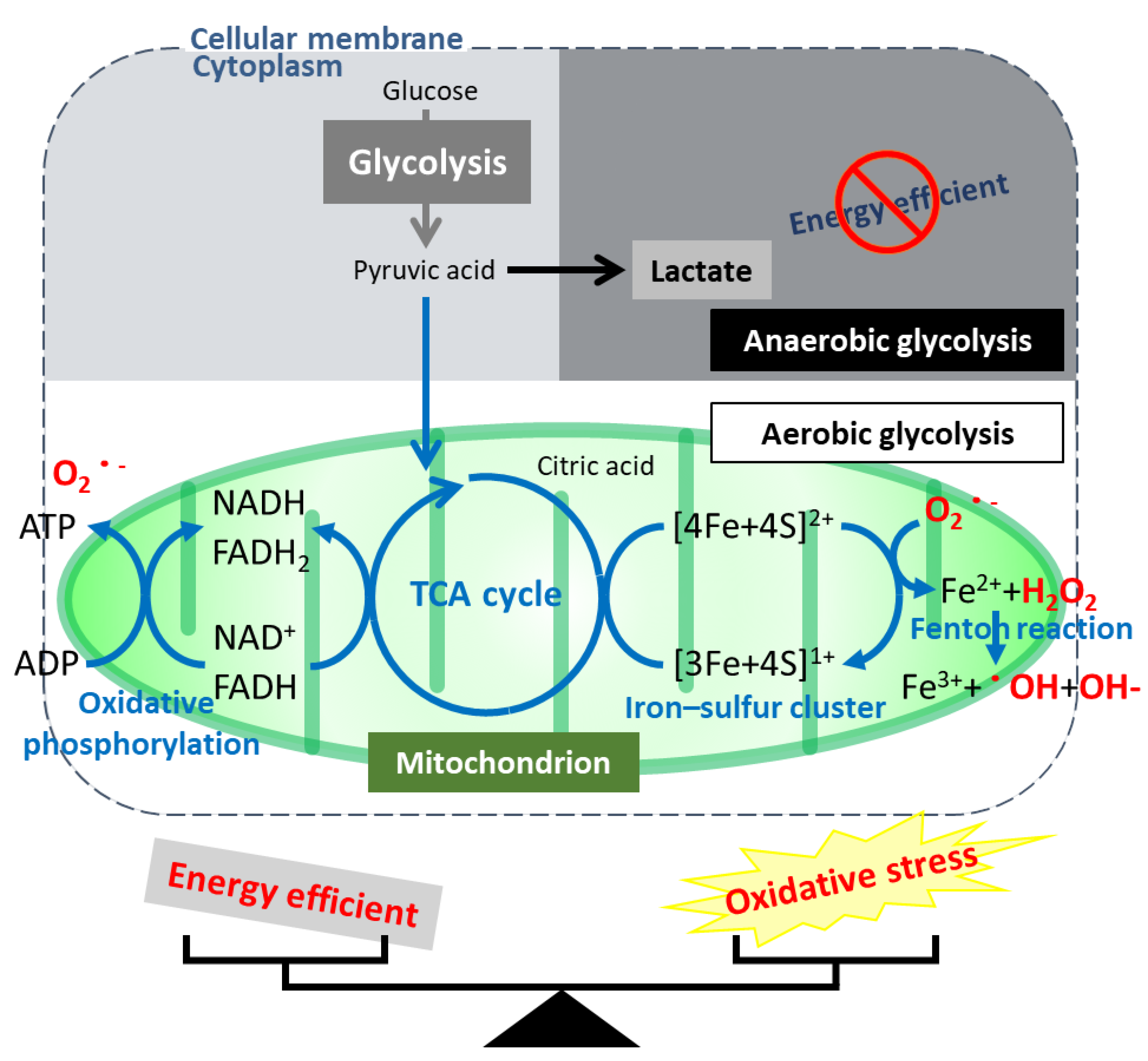
6. Regulation of the Unique Characteristics and Mitochondrial Metabolism by HNF1B in Ovarian CCC
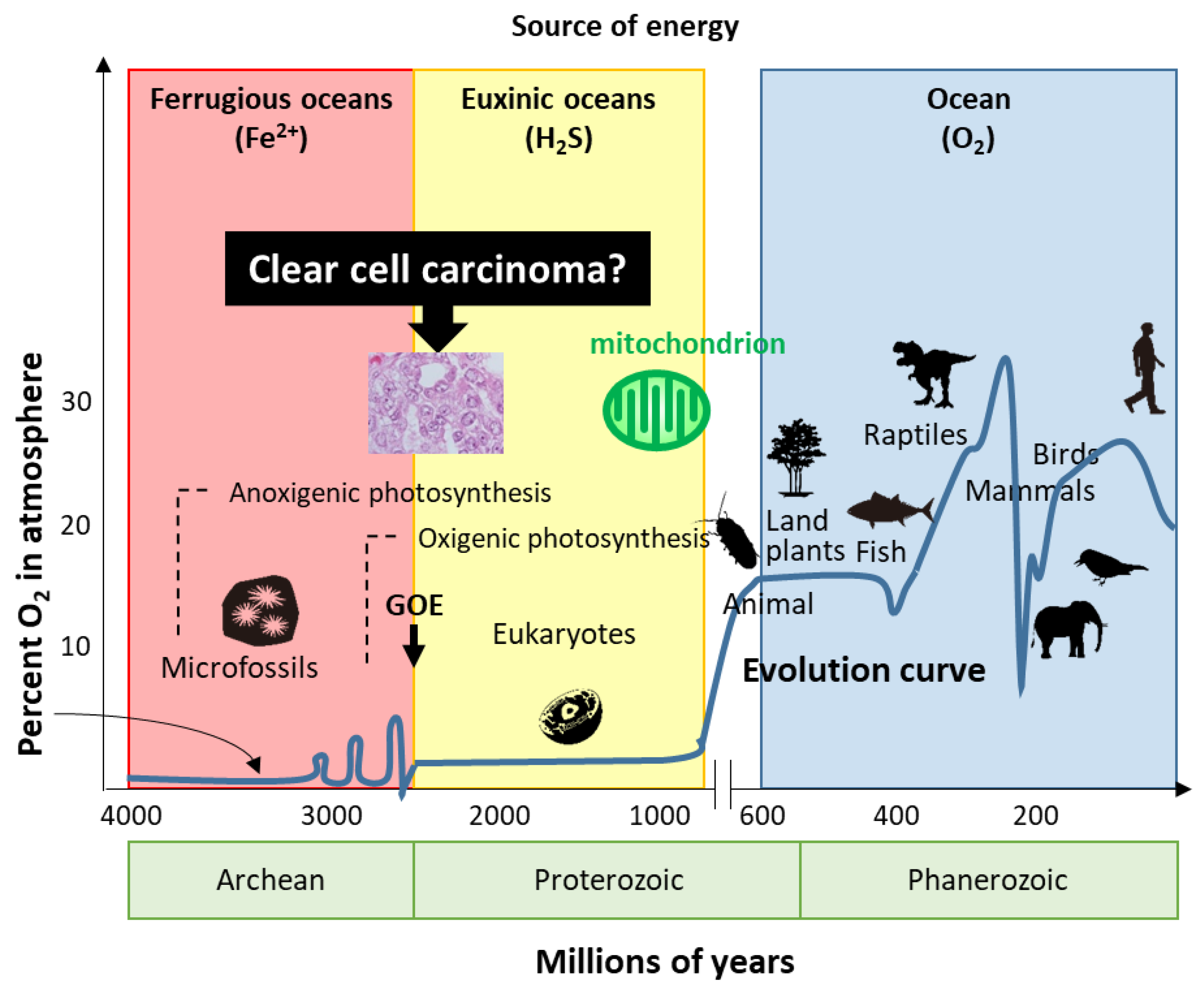
7. Hypothesis—Evolution or Reversion of Mitochondrial Metabolism in Ovarian CCC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Kalloger, S.E.; Lee, S.; Duggan, M.A.; Kelemen, L.E.; Prentice, L.; Kalli, K.R.; Fridley, B.L.; Visscher, D.W.; Keeney, G.L.; et al. Biomarker-based ovarian carcinoma typing: A histologic investigation in the ovarian tumor tissue analysis consortium. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kamura, T.; Kigawa, J.; Terakawa, N.; Kikuchi, Y.; Kita, T.; Suzuki, M.; Sato, I.; Taguchi, K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000, 88, 2584–2589. [Google Scholar] [CrossRef]
- Chan, J.K.; Teoh, D.; Hu, J.M.; Shin, J.Y.; Osann, K.; Kapp, D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol. Oncol. 2008, 109, 370–376. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Cho, K.R.; Gilks, C.B.; Pearce, C.L.; Huntsman, D.G. The disparate origins of ovarian cancers: Pathogenesis and prevention strategies. Nat. Rev. Cancer 2017, 17, 65–74. [Google Scholar] [CrossRef]
- Okamoto, A.; Glasspool, R.M.; Mabuchi, S.; Matsumura, N.; Nomura, H.; Itamochi, H.; Takano, M.; Takano, T.; Susumu, N.; Aoki, D.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int. J. Gynecol. Cancer 2014, 24, S20–S25. [Google Scholar] [CrossRef]
- Saito, T.; Takahashi, F.; Katabuchi, H.; 2016 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: Patient Annual Report for 2014 and Treatment Annual Report for 2009. J. Obstet Gynaecol. Res. 2017, 43, 1667–1677. [Google Scholar] [CrossRef]
- Yoonessi, M.; Weldon, D.; Satchidand, S.K.; Crickard, K. Clear cell ovarian adenocarcinoma. J. Surg. Oncol. 1984, 27, 289–297. [Google Scholar] [CrossRef]
- Matsuura, Y.; Robertson, G.; Marsden, D.E.; Kim, S.N.; Gebski, V.; Hacker, N.F. Thromboembolic complications in patients with clear cell carcinoma of the ovary. Gynecol. Oncol. 2007, 104, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Mao, T.L.; Jones, S.; Veras, E.; Ayhan, A.; Wang, T.L.; Glas, R.; Slamon, D.; Velculescu, V.E.; Kuman, R.J.; et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 2009, 174, 1597–1601. [Google Scholar] [CrossRef]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.L.; Shih Ie, M.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A., Jr.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Fridley, B.L.; Song, H.; Lawrenson, K.; Cunningham, J.M.; Ramus, S.J.; Cicek, M.S.; Tyrer, J.; Stram, D.; Larson, M.C.; et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nat. Commun. 2013, 4, 1628. [Google Scholar] [CrossRef] [PubMed]
- Burghaus, S.; Fasching, P.A.; Häberle, L.; Rübner, M.; Büchner, K.; Blum, S.; Engel, A.; Ekici, A.B.; Hartmann, A.; Hein, A.; et al. Genetic risk factors for ovarian cancer and their role for endometriosis risk. Gynecol. Oncol. 2017, 145, 142–147. [Google Scholar] [CrossRef]
- Yachida, N.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Ueda, H.; Sugino, K.; Yamaguchi, M.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. ARID1A protein expression is retained in ovarian endometriosis with ARID1A loss-of-function mutations: Implication for the two-hit hypothesis. Sci. Rep. 2020, 10, 14260. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Mitochondria at Work: New Insights into Regulation and Dysregulation of Cellular Energy Supply and Metabolism. Biomedicines 2020, 8, 526. [Google Scholar] [CrossRef]
- Sayasneh, A.; Tsivos, D.; Crawford, R. Endometriosis and ovarian cancer: A systematic review. ISRN Obstet. Gynecol. 2011, 2011, 140310. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Mitsopoulou, A.; Iliopoulou, S.M.; Daniilidis, A.; Samartzis, E.P.; Economopoulos, K.P. Association between endometriosis and gynecological cancers: A critical review of the literature. Arch. Gynecol. Obstet. 2020, 301, 355–367. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Horne, A.W.; Missmer, S.A. Informing women with endometriosis about ovarian cancer risk. Lancet 2017, 390, 2433–2434. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef]
- Toyokuni, S. Iron-induced carcinogenesis: The role of redox regulation. Free Radic. Biol. Med. 1996, 20, 553–566. [Google Scholar] [CrossRef]
- Fenton, H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Nelson, R.L. Dietary iron and colorectal cancer risk. Free Radic. Biol. Med. 1992, 12, 161–168. [Google Scholar] [CrossRef]
- Lee, D.H.; Anderson, K.E.; Folsom, A.R.; Jacobs, D.R. Heme iron, zinc and upper digestive tract cancer: The Iowa Women’s Health Study. Int. J. Cancer 2005, 117, 643–647. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef]
- Abou-Taleb, H.; Yamaguchi, K.; Matsumura, N.; Murakami, R.; Nakai, H.; Higasa, K.; Amano, Y.; Abiko, K.; Yoshioka, Y.; Hamanishi, J.; et al. Comprehensive assessment of the expression of the SWI/SNF complex defines two distinct prognostic subtypes of ovarian clear cell carcinoma. Oncotarget 2016, 7, 54758–54770. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Oura, T.; Matsumura, N.; Hamanishi, J.; Baba, T.; Matsui, S.; Murphy, S.K.; Konishi, I. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene 2010, 29, 1741–1752. [Google Scholar] [CrossRef]
- Stevens, R.G.; Jones, D.Y.; Micozzi, M.S.; Taylor, P.R. Body iron stores and the risk of cancer. N. Engl. J. Med. 1988, 319, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Cologne, J.B.; Nakachi, K.; Grant, E.J.; Neriishi, K. Body iron stores and breast cancer risk in female atomic bomb survivors. Cancer Sci. 2011, 102, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod 2020, 36, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Palte, M.J.; Deik, A.A.; Li, H.; Eaton, J.K.; Wang, W.; Tseng, Y.Y.; Deasy, R.; Kost-Alimova, M.; Dančík, V.; et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019, 10, 1617. [Google Scholar] [CrossRef]
- Mandai, M.; Yamaguchi, K.; Matsumura, N.; Baba, T.; Konishi, I. Ovarian cancer in endometriosis: Molecular biology, pathology, and clinical management. Int. J. Clin. Oncol. 2009, 14, 383–391. [Google Scholar] [CrossRef]
- Toyokuni, S. Iron and carcinogenesis: From Fenton reaction to target genes. Redox Rep. 2002, 7, 189–197. [Google Scholar] [CrossRef]
- Hiroyasu, M.; Ozeki, M.; Kohda, H.; Echizenya, M.; Tanaka, T.; Hiai, H.; Toyokuni, S. Specific allelic loss of p16 (INK4A) tumor suppressor gene after weeks of iron-mediated oxidative damage during rat renal carcinogenesis. Am. J. Pathol. 2002, 160, 419–424. [Google Scholar] [CrossRef]
- Tanaka, T.; Iwasa, Y.; Kondo, S.; Hiai, H.; Toyokuni, S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene 1999, 18, 3793–3797. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsuda, H.; Aida, S.; Shimazaki, H.; Tamai, S.; Matsubara, O. Immunohistochemical detection of hepatocyte nuclear factor 1beta in ovarian and endometrial clear-cell adenocarcinomas and nonneoplastic endometrium. Hum. Pathol. 2007, 38, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Cuff, J.; Salari, K.; Clarke, N.; Esheba, G.E.; Forster, A.D.; Huang, S.; West, R.B.; Higgins, J.P.; Longacre, T.A.; Pollack, J.R. Integrative bioinformatics links HNF1B with clear cell carcinoma and tumor-associated thrombosis. PLoS ONE 2013, 8, e74562. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Huang, Z.; Matsumura, N.; Mandai, M.; Okamoto, T.; Baba, T.; Konishi, I.; Berchuck, A.; Murphy, S.K. Epigenetic determinants of ovarian clear cell carcinoma biology. Int. J. Cancer 2014, 135, 585–597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murakami, R.; Matsumura, N.; Brown, J.B.; Higasa, K.; Tsutsumi, T.; Kamada, M.; Abou-Taleb, H.; Hosoe, Y.; Kitamura, S.; Yamaguchi, K.; et al. Exome Sequencing Landscape Analysis in Ovarian Clear Cell Carcinoma Shed Light on Key Chromosomal Regions and Mutation Gene Networks. Am. J. Pathol. 2017, 187, 2246–2258. [Google Scholar] [CrossRef]
- Itamochi, H.; Oishi, T.; Oumi, N.; Takeuchi, S.; Yoshihara, K.; Mikami, M.; Yaegashi, N.; Terao, Y.; Takehara, K.; Ushijima, K.; et al. Whole-genome sequencing revealed novel prognostic biomarkers and promising targets for therapy of ovarian clear cell carcinoma. Br. J. Cancer 2017, 117, 717–724. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.W.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Seo, J.S. Genomic landscape of ovarian clear cell carcinoma via whole exome sequencing. Gynecol. Oncol. 2018, 148, 375–382. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Ohira, M.; Itami, M.; Hippo, Y.; Nagase, H. Identification of novel mutations in Japanese ovarian clear cell carcinoma patients using optimized targeted NGS for clinical diagnosis. Gynecol. Oncol. 2017, 144, 377–383. [Google Scholar] [CrossRef]
- Shibuya, Y.; Tokunaga, H.; Saito, S.; Shimokawa, K.; Katsuoka, F.; Bin, L.; Kojima, K.; Nagasaki, M.; Yamamoto, M.; Yaegashi, N.; et al. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosomes Cancer 2018, 57, 51–60. [Google Scholar] [CrossRef]
- Uehara, Y.; Oda, K.; Ikeda, Y.; Koso, T.; Tsuji, S.; Yamamoto, S.; Asada, K.; Sone, K.; Kurikawa, R.; Makii, C.; et al. Integrated copy number and expression analysis identifies profiles of whole-arm chromosomal alterations and subgroups with favorable outcome in ovarian clear cell carcinomas. PLoS ONE 2015, 10, e0128066. [Google Scholar] [CrossRef]
- Okamoto, A.; Sehouli, J.; Yanaihara, N.; Hirata, Y.; Braicu, I.; Kim, B.G.; Takakura, S.; Saito, M.; Yanagida, S.; Takenaka, M.; et al. Somatic copy number alterations associated with Japanese or endometriosis in ovarian clear cell adenocarcinoma. PLoS ONE 2015, 10, e0116977. [Google Scholar] [CrossRef]
- Yamashita, Y.; Akatsuka, S.; Shinjo, K.; Yatabe, Y.; Kobayashi, H.; Seko, H.; Kajiyama, H.; Kikkawa, F.; Takahashi, T.; Toyokuni, S. Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis. PLoS ONE 2013, 8, e57724. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Mao, T.L.; Chen, X.; Feng, Y.; Nakayama, K.; Wang, Y.; Glas, R.; Ma, M.J.; Kurman, R.J.; Shih, I.M.; et al. DNA copy numbers profiles in affinity-purified ovarian clear cell carcinoma. Clin. Cancer Res. 2010, 16, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Sasou, S.; Motoyama, T. Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod. Pathol. 2006, 19, 83–89. [Google Scholar] [CrossRef]
- Horikawa, Y.; Enya, M.; Fushimi, N.; Fushimi, Y.; Takeda, J. Screening of diabetes of youth for hepatocyte nuclear factor 1 mutations: Clinical phenotype of HNF1β-related maturity-onset diabetes of the young and HNF1α-related maturity-onset diabetes of the young in Japanese. Diabet Med. 2014, 31, 721–727. [Google Scholar] [CrossRef]
- Okamoto, T.; Mandai, M.; Matsumura, N.; Yamaguchi, K.; Kondoh, H.; Amano, Y.; Baba, T.; Hamanishi, J.; Abiko, K.; Kosaka, K.; et al. Hepatocyte nuclear factor-1β (HNF-1β) promotes glucose uptake and glycolytic activity in ovarian clear cell carcinoma. Mol. Carcinog. 2015, 54, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Mandai, M.; Yamaguchi, K.; Matsumura, N.; Kharma, B.; Baba, T.; Abiko, K.; Hamanishi, J.; Yoshioka, Y.; Konishi, I. Metabolic alterations caused by HNF1β expression in ovarian clear cell carcinoma contribute to cell survival. Oncotarget 2015, 6, 26002–26017. [Google Scholar] [CrossRef]
- WARBURG, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Clairmont, A.; Ebert, T.; Weber, H.; Zoidl, C.; Eickelmann, P.; Schulz, W.A.; Sies, H.; Ryffel, G.U. Lowered amounts of the tissue-specific transcription factor LFB1 (HNF1) correlate with decreased levels of glutathione S-transferase alpha messenger RNA in human renal cell carcinoma. Cancer Res. 1994, 54, 1319–1323. [Google Scholar]
- Ogiwara, H.; Takahashi, K.; Sasaki, M.; Kuroda, T.; Yoshida, H.; Watanabe, R.; Maruyama, A.; Makinoshima, H.; Chiwaki, F.; Sasaki, H.; et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell 2019, 35, 177–190.e8. [Google Scholar] [CrossRef]
- Lu, W.; Sun, J.; Zhou, H.; Wang, F.; Zhao, C.; Li, K.; Fan, C.; Ding, G.; Wang, J. HNF1B inhibits cell proliferation via repression of SMAD6 expression in prostate cancer. J. Cell Mol. Med. 2020, 24, 14539–14548. [Google Scholar] [CrossRef]
- Shigetomi, H.; Sudo, T.; Shimada, K.; Uekuri, C.; Tsuji, Y.; Kanayama, S.; Naruse, K.; Yamada, Y.; Konishi, N.; Kobayashi, H. Inhibition of cell death and induction of G2 arrest accumulation in human ovarian clear cells by HNF-1β transcription factor: Chemosensitivity is regulated by checkpoint kinase CHK1. Int. J. Gynecol. Cancer 2014, 24, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.L. The role of iron-sulfur clusters in in vivo hydroxyl radical production. Free Radic. Res. 1996, 25, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gentric, G.; Kieffer, Y.; Mieulet, V.; Goundiam, O.; Bonneau, C.; Nemati, F.; Hurbain, I.; Raposo, G.; Popova, T.; Stern, M.H.; et al. PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab. 2019, 29, 156–173.e10. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Torrealba, N.; Tomkova, V.; Jadhav, S.B.; Blazkova, K.; Merta, L.; Lettlova, S.; Adamcová, M.K.; Rosel, D.; Brábek, J.; et al. Targeting mitochondrial iron metabolism suppresses tumor growth and metastasis by inducing mitochondrial dysfunction and mitophagy. Cancer Res. 2021, 81, 2289–2303. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Kutschera, U. Symbiogenesis, natural selection, and the dynamic Earth. Theory Biosci. 2009, 128, 191–203. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, K.; Kitamura, S.; Furutake, Y.; Murakami, R.; Yamanoi, K.; Taki, M.; Ukita, M.; Hamanishi, J.; Mandai, M. Acquired Evolution of Mitochondrial Metabolism Regulated by HNF1B in Ovarian Clear Cell Carcinoma. Cancers 2021, 13, 2413. https://doi.org/10.3390/cancers13102413
Yamaguchi K, Kitamura S, Furutake Y, Murakami R, Yamanoi K, Taki M, Ukita M, Hamanishi J, Mandai M. Acquired Evolution of Mitochondrial Metabolism Regulated by HNF1B in Ovarian Clear Cell Carcinoma. Cancers. 2021; 13(10):2413. https://doi.org/10.3390/cancers13102413
Chicago/Turabian StyleYamaguchi, Ken, Sachiko Kitamura, Yoko Furutake, Ryusuke Murakami, Koji Yamanoi, Mana Taki, Masayo Ukita, Junzo Hamanishi, and Masaki Mandai. 2021. "Acquired Evolution of Mitochondrial Metabolism Regulated by HNF1B in Ovarian Clear Cell Carcinoma" Cancers 13, no. 10: 2413. https://doi.org/10.3390/cancers13102413
APA StyleYamaguchi, K., Kitamura, S., Furutake, Y., Murakami, R., Yamanoi, K., Taki, M., Ukita, M., Hamanishi, J., & Mandai, M. (2021). Acquired Evolution of Mitochondrial Metabolism Regulated by HNF1B in Ovarian Clear Cell Carcinoma. Cancers, 13(10), 2413. https://doi.org/10.3390/cancers13102413





