Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
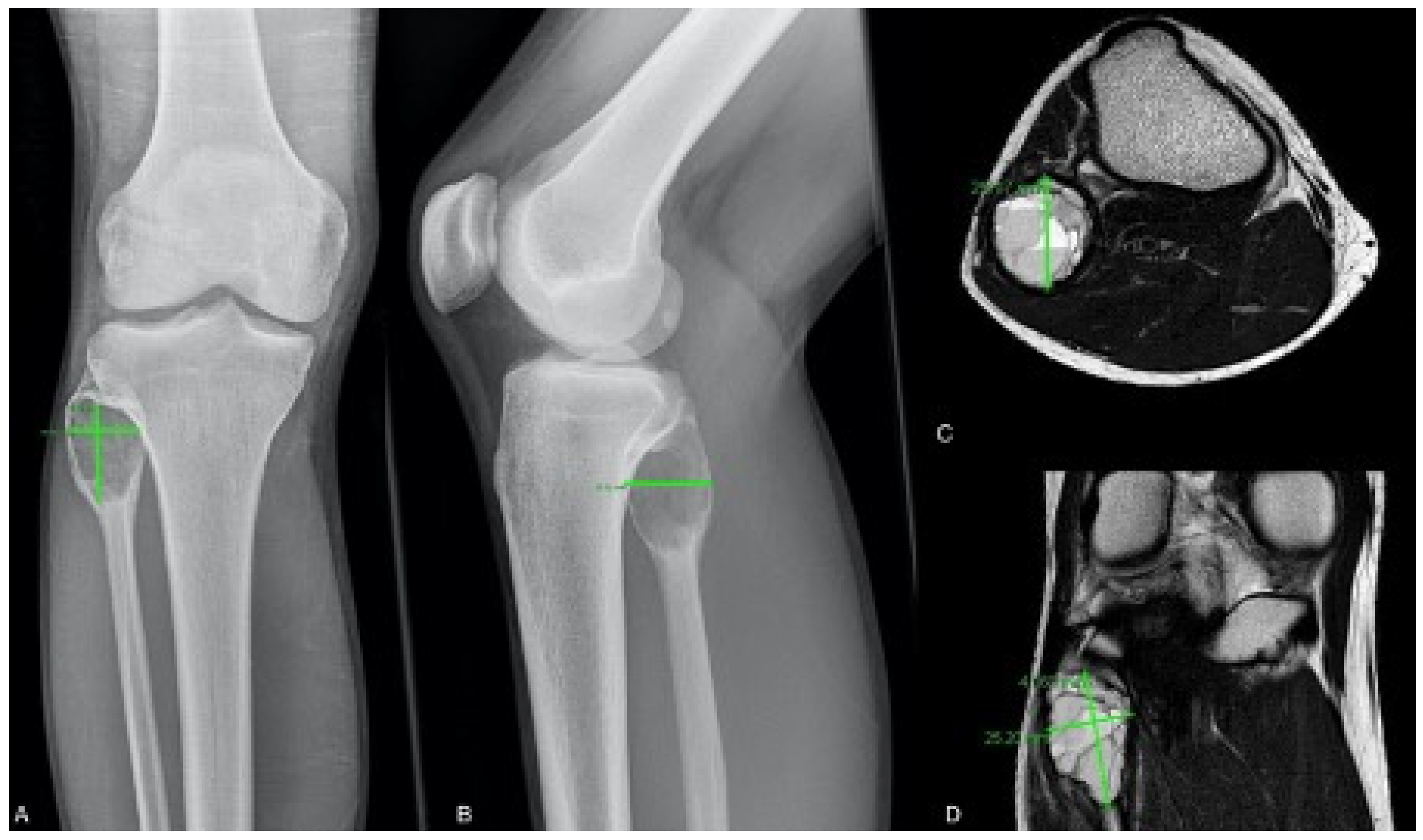
2.1. Radiological Classification and Measurement
2.2. Curettage Group
2.3. Instillation Group
2.4. Resection Group
2.5. Follow-Up
2.6. Statistics
3. Results
3.1. Cyst Volume Measurement
3.2. Curettage Group
3.3. Instillation Group
3.4. Resection Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaffe, H.L.; Lichtenstein, L. Solitary unicameral bone cyst: With emphasis on the roentgen picture, the pathologic appearance and the pathogenesis. Arch. Surg. 1942, 44, 1004–1025. [Google Scholar] [CrossRef]
- Kaiser, C.L.; Yeung, C.M.; Raskin, K.A.; Lozano-Calderon, S.A. Aneurysmal bone cyst of the clavicle: A series of 13 cases. J. Shoulder Elb. Surg. 2019, 28, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tsagozis, P.; Brosjo, O. Current Strategies for the Treatment of Aneurysmal Bone Cysts. Orthop. Rev. 2015, 7, 6182. [Google Scholar] [CrossRef] [PubMed]
- Dormans, J.P.; Hanna, B.G.; Johnston, D.R.; Khurana, J.S. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin. Orthop. Relat. Res. 2004, 421, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Noordin, S.; Ahmad, T.; Umer, M.; Allana, S.; Hilal, K.; Uddin, N.; Hashmi, P. Aneurysmal bone cyst of the pelvis and extremities. Int. J. Surg. Oncol. 2019, 4, e71. [Google Scholar] [CrossRef]
- Mascard, E.; Gomez-Brouchet, A.; Lambot, K. Bone cysts: Unicameral and aneurysmal bone cyst. Orthop. Traumatol. Surg. Res. 2015, 101, S119–S127. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, P.R.; Davila, J.I.; Jackson, R.A.; Fadra, N.; Atiq, M.A.; Pitel, B.A.; Nair, A.A.; VanDeWalker, T.J.; Hessler, M.G.; Hovel, S.K.; et al. RNA sequencing identifies a novel USP9X-USP6 promoter swap gene fusion in a primary aneurysmal bone cyst. Genes Chromosomes Cancer 2019, 58, 589–594. [Google Scholar] [CrossRef]
- Grahneis, F.; Klein, A.; Baur-Melnyk, A.; Knosel, T.; Birkenmaier, C.; Jansson, V.; Durr, H.R. Aneurysmal bone cyst: A review of 65 patients. J. Bone Oncol. 2019, 18, 100255. [Google Scholar] [CrossRef]
- Bazzocchi, A.; Spinnato, P.; Mercatelli, D.; Aparisi, G.M.P. Fluid-Fluid Levels in Aneurysmal Bone Cysts. J. Pediatr. 2019, 204, 317. [Google Scholar] [CrossRef]
- Deventer, N.; Deventer, N.; Gosheger, G.; Budny, T.; de Vaal, M.; Riegel, A.; Heitkoetter, B.; Kessler, T.; Poeppelmann, M.; Rossig, C.; et al. Aneurysmal bone cyst inadvertently treated with chemotherapy-A series of three cases. Pediatr. Blood Cancer 2020, 67, e28638. [Google Scholar] [CrossRef]
- Borowski, A.; Drobniewski, M.; Skrzypek, M.; Pstrągowski, K.; Synder, M.A.; Krasińska, M.; Synder, M. Giant aneurysmal bone cyst mimicking malignant tumor. Nowotw. J. Oncol. 2019, 69, 67–70. [Google Scholar] [CrossRef]
- Docquier, P.L.; Delloye, C.; Galant, C. Histology can be predictive of the clinical course of a primary aneurysmal bone cyst. Arch. Orthop. Trauma Surg. 2010, 130, 481–487. [Google Scholar] [CrossRef]
- Panoutsakopoulos, G.; Pandis, N.; Kyriazoglou, I.; Gustafson, P.; Mertens, F.; Mandahl, N. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer 1999, 26, 265–266. [Google Scholar] [CrossRef]
- Zhang, L.; Hwang, S.; Benayed, R.; Zhu, G.G.; Mullaney, K.A.; Rios, K.M.; Sukhadia, P.Y.; Agaram, N.; Zhang, Y.; Bridge, J.A.; et al. Myositis ossificans-like soft tissue aneurysmal bone cyst: A clinical, radiological, and pathological study of seven cases with COL1A1-USP6 fusion and a novel ANGPTL2-USP6 fusion. Mod. Pathol. 2020, 33, 1492–1504. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Perez-Atayde, A.R.; Inwards, C.Y.; Medeiros, F.; Derr, V.; Hsi, B.L.; Gebhardt, M.C.; Rosenberg, A.E.; Fletcher, J.A. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am. J. Pathol. 2004, 165, 1773–1780. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Hsi, B.L.; Weremowicz, S.; Rosenberg, A.E.; Dal Cin, P.; Joseph, N.; Bridge, J.A.; Perez-Atayde, A.R.; Fletcher, J.A. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004, 64, 1920–1923. [Google Scholar] [CrossRef]
- Park, H.Y.; Yang, S.K.; Sheppard, W.L.; Hegde, V.; Zoller, S.D.; Nelson, S.D.; Federman, N.; Bernthal, N.M. Current management of aneurysmal bone cysts. Curr. Rev. Musculoskelet. Med. 2016, 9, 435–444. [Google Scholar] [CrossRef]
- Guseva, N.V.; Jaber, O.; Tanas, M.R.; Stence, A.A.; Sompallae, R.; Schade, J.; Fillman, A.N.; Miller, B.J.; Bossler, A.D.; Ma, D. Anchored multiplex PCR for targeted next-generation sequencing reveals recurrent and novel USP6 fusions and upregulation of USP6 expression in aneurysmal bone cyst. Genes Chromosomes Cancer 2017, 56, 266–277. [Google Scholar] [CrossRef]
- Sekoranja, D.; Zupan, A.; Mavcic, B.; Martincic, D.; Salapura, V.; Snoj, Z.; Limpel, N.A.K.; Pizem, J. Novel ASAP1-USP6, FAT1-USP6, SAR1A-USP6, and TNC-USP6 fusions in primary aneurysmal bone cyst. Genes Chromosomes Cancer 2020, 59, 357–365. [Google Scholar] [CrossRef]
- Li, L.; Bui, M.M.; Zhang, M.; Sun, X.; Han, G.; Zhang, T.; Huang, X.; Ding, Y. Validation of Fluorescence in situ Hybridization Testing of USP6 Gene Rearrangement for Diagnosis of Primary Aneurysmal Bone Cyst. Ann. Clin. Lab. Sci. 2019, 49, 590–597. [Google Scholar]
- Kieser, D.C.; Mazas, S.; Cawley, D.T.; Fujishiro, T.; Tavolaro, C.; Boissiere, L.; Obeid, I.; Pointillart, V.; Vital, J.M.; Gille, O. Bisphosphonate therapy for spinal aneurysmal bone cysts. Eur. Spine J. 2018, 27, 851–858. [Google Scholar] [CrossRef]
- Woon, J.T.K.; Hoon, D.; Graydon, A.; Flint, M.; Doyle, A.J. Aneurysmal bone cyst treated with percutaneous doxycycline: Is a single treatment sufficient? Skelet. Radiol. 2019, 48, 765–771. [Google Scholar] [CrossRef]
- Flont, P.; Kolacinska-Flont, M.; Niedzielski, K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J. Surg. Oncol. 2013, 11, 109. [Google Scholar] [CrossRef]
- Brosjo, O.; Pechon, P.; Hesla, A.; Tsagozis, P.; Bauer, H. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop. 2013, 84, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Varshney, M.K.; Trikha, V.; Khan, S.A.; Choudhury, B.; Safaya, R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J. Bone Jt. Surg. Br. 2006, 88, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Varshney, M.K.; Rastogi, S.; Khan, S.A.; Trikha, V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2010, 468, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Hegde, P.; Gulia, A.; Parikh, M. Primary aneurysmal bone cysts. Bone Jt. J. 2020, 102, 186–190. [Google Scholar] [CrossRef]
- Capanna, R.; Bettelli, G.; Biagini, R.; Ruggieri, P.; Bertoni, F.; Campanacci, M. Aneurysmal cysts of long bones. Ital. J. Orthop. Traumatol. 1985, 11, 409–417. [Google Scholar] [PubMed]
- Enneking, W.F. A system of staging musculoskeletal neoplasms. Clin. Orthop. Relat. Res. 1986, 204, 9–24. [Google Scholar] [CrossRef]
- Gobel, V.; Jurgens, H.; Etspuler, G.; Kemperdick, H.; Jungblut, R.M.; Stienen, U.; Gobel, U. Prognostic significance of tumor volume in localized Ewing’s sarcoma of bone in children and adolescents. J. Cancer Res. Clin. Oncol. 1987, 113, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, I.; Nicolas, N.; Rizkallah, M.; Slaba, S. Sclerotherapy using Surgiflo and alcohol: A new alternative for the treatment of aneurysmal bone cysts. J. Child. Orthop. 2017, 11, 448–454. [Google Scholar] [CrossRef]
- Docquier, P.L.; Paul, L.; Menten, R.; Cartiaux, O.; Francq, B.; Banse, X. Measurement of bone cyst fluid volume using k-means clustering. Magn. Reson. Imaging 2009, 27, 1430–1439. [Google Scholar] [CrossRef]
- Peeters, S.P.; Van der Geest, I.C.; de Rooy, J.W.; Veth, R.P.; Schreuder, H.W. Aneurysmal bone cyst: The role of cryosurgery as local adjuvant treatment. J. Surg. Oncol. 2009, 100, 719–724. [Google Scholar] [CrossRef]
- Garg, S.; Mehta, S.; Dormans, J.P. Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J. Pediatr. Orthop. 2005, 25, 387–392. [Google Scholar] [CrossRef]
- Balke, M.; Schremper, L.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Koehler, G.; Hardes, J.; Gosheger, G. Giant cell tumor of bone: Treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008, 134, 969–978. [Google Scholar] [CrossRef]
- Deventer, N.; Deventer, N.; Gosheger, G.; de Vaal, M.; Budny, T.; Laufer, A.; Heitkoetter, B.; Luebben, T. Chondroblastoma: Is intralesional curettage with the use of adjuvants a sufficient way of therapy? J. Bone Oncol. 2021, 26, 100342. [Google Scholar] [CrossRef]
- Guibaud, L.; Herbreteau, D.; Dubois, J.; Stempfle, N.; Berard, J.; Pracros, J.P.; Merland, J.J. Aneurysmal bone cysts: Percutaneous embolization with an alcoholic solution of zein--series of 18 cases. Radiology 1998, 208, 369–373. [Google Scholar] [CrossRef]
- Adamsbaum, C.; Mascard, E.; Guinebretiere, J.M.; Kalifa, G.; Dubousset, J. Intralesional Ethibloc injections in primary aneurysmal bone cysts: An efficient and safe treatment. Skelet. Radiol. 2003, 32, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Batisse, F.; Schmitt, A.; Vendeuvre, T.; Herbreteau, D.; Bonnard, C. Aneurysmal bone cyst: A 19-case series managed by percutaneous sclerotherapy. Orthop. Traumatol. Surg. Res. 2016, 102, 213–216. [Google Scholar] [CrossRef]
- Bavan, L.; Wijendra, A.; Kothari, A. Efficacy of treatment interventions for primary aneurysmal bone cysts: A systematic review. Bone Jt. Open 2021, 2, 125–133. [Google Scholar] [CrossRef]

| Radiological Classification | Instillation Group | Curettage Group | En Bloc Resection Group | Over All | |
|---|---|---|---|---|---|
| Enneking classification | still | 1 | 1 | 0 | 2 |
| active | 8 | 15 | 0 | 23 | |
| aggressive | 23 | 18 | 8 | 49 | |
| Capanna classification | I | 1 | 1 | 0 | 2 |
| II | 5 | 4 | 0 | 9 | |
| III | 7 | 8 | 1 | 16 | |
| IV | 1 | 0 | 0 | 1 | |
| V | 2 | 6 | 0 | 8 | |
| not applicable | 16 | 15 | 7 | 38 | |
| Rastogi classification | I | 20 | - | - | 20 |
| II | 3 | - | - | 3 | |
| II | 3 | - | - | 3 | |
| IV | 6 | - | - | 6 | |
| Parameter | Instillation Group | Curettage Group | En Bloc Resection Group | |
|---|---|---|---|---|
| Number | 32 | 34 | 8 | |
| Gender (male:female) | 13:19 | 17:17 | 3:5 | |
| Mean age (years) | 16.88 | 17.15 | 15.5 | |
| Age groups | ≤10 years | 5 | 5 | 1 |
| >10 years | 27 | 29 | 7 | |
| Mean Pre-interventional cyst volume (cm3) | 46.72 | 23.66 | 48.01 | |
| Mean number of instillations | 5.74 | - | - | |
| Mean volume of instillation (mL) | 6.02 | - | - | |
| Mean residual cyst volume (cm3) | 24.3 | - | - | |
| Additional therapy | embolization | 8 | 2 | 2 |
| intralesional cortisone injection | 0 | 1 | 0 | |
| Denosumab therapy | 0 | 0 | 1 | |
| Local recurrence | no need for treatment | 0 | 3 | 1 |
| in need of treatment | 0 | 13 | 1 | |
| over all | 0 | 16 | 2 | |
| Persistent disease | no need for treatment | 19 | 0 | 2 |
| in need of treatment | 10 | 2 | 0 | |
| over all | 29 | 2 | 2 | |
| Complications | pre-interventional | 5 | 3 | 3 |
| post-interventional | 2 | 2 | 3 | |
| Mean follow-up (months) | 36.12 | 41.36 | 54.73 | |
| Localization | Instillation Group | Curettage Group | En Bloc Resection Group | Overall |
|---|---|---|---|---|
| Humerus | 5 | 4 | 0 | 9 |
| Ulna | 2 | 0 | 0 | 2 |
| Hand | 1 | 0 | 0 | 1 |
| Femur | 2 | 8 | 1 | 11 |
| Tibia | 7 | 6 | 0 | 13 |
| Fibula | 0 | 1 | 0 | 1 |
| Foot | 4 | 5 | 0 | 9 |
| Clavicula | 3 | 2 | 0 | 5 |
| Scapula | 1 | 1 | 0 | 2 |
| Spine | 0 | 1 | 5 | 6 |
| Pelvis | 7 | 6 | 2 | 15 |
| Total | 32 | 34 | 8 | 74 |
| Patient ID | MRI Volume (cm3) | Radiographic Volume (cm3) | Difference | Localization |
|---|---|---|---|---|
| 1. | 18.5 | 8.55 | 9.95 | Tibia |
| 2. | 38.67 | 22.45 | 16.22 | Humerus |
| 3. | 4.96 | 3.26 | 1.7 | Tibia |
| 10. | 17.64 | 4.10 | 13.57 | Foot |
| 11. | 77.28 | 63.46 | 13.82 | Tibia |
| 12. | 34.35 | 10.64 | 23.71 | Femur |
| 13. | 31.85 | 6.20 | 25.65 | Foot |
| 16. | 54.83 | 38.61 | 16.22 | Humerus |
| 19. | 13.87 | 9.59 | 4.28 | Ulna |
| 21. | 105.33 | 55.53 | 49.80 | Humerus |
| 26. | 29.41 | 17.96 | 11.53 | Tibia |
| 27. | 11.24 | 6.27 | 4.97 | Foot |
| 31. | 6.67 | 1.25 | 5.42 | Ulna |
| 32. | 263.71 | 184.58 | 79.13 | Femur |
| 39. | 21.59 | 3.86 | 17.73 | Humerus |
| 43. | 2.60 | 1.49 | 1.11 | Femur |
| 45. | 31.77 | 17.43 | 14.34 | Humerus |
| 50. | 1.12 | 0.93 | 0.17 | Foot |
| 51. | 16.47 | 10.45 | 6.02 | Fibula |
| 52. | 10.82 | 17.09 | −6.27 | Humerus |
| 54. | 56.50 | 26.80 | 29.70 | Femur |
| 55. | 35.08 | 8.25 | 26.83 | Tibia |
| 67. | 16.56 | 17.47 | −0.91 | Tibia |
| 74. | 11.87 | 4.13 | 7.74 | Spine |
| 75. | 5.42 | 1.48 | 3.94 | Spine |
| 76. | 227.64 | 167.19 | 60.45 | Femur |
| Mean | 44.07 | 27.27 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deventer, N.; Schulze, M.; Gosheger, G.; de Vaal, M.; Deventer, N. Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers 2021, 13, 2362. https://doi.org/10.3390/cancers13102362
Deventer N, Schulze M, Gosheger G, de Vaal M, Deventer N. Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers. 2021; 13(10):2362. https://doi.org/10.3390/cancers13102362
Chicago/Turabian StyleDeventer, Nils, Martin Schulze, Georg Gosheger, Marieke de Vaal, and Niklas Deventer. 2021. "Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases" Cancers 13, no. 10: 2362. https://doi.org/10.3390/cancers13102362
APA StyleDeventer, N., Schulze, M., Gosheger, G., de Vaal, M., & Deventer, N. (2021). Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers, 13(10), 2362. https://doi.org/10.3390/cancers13102362






