The Ratio of GrzB+ − FoxP3+ over CD3+ T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Samples
2.2. Immunohistochemistry (IHC)
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Features of Melanoma
3.2. Quantification of Single TIL Biomarkers and PD-L1 in Primary Tumors and Unmatched In-Transit Metastatic Melanoma Tissues and Its Correlation with the Pathological Features
3.3. Correlation between Biomarkers
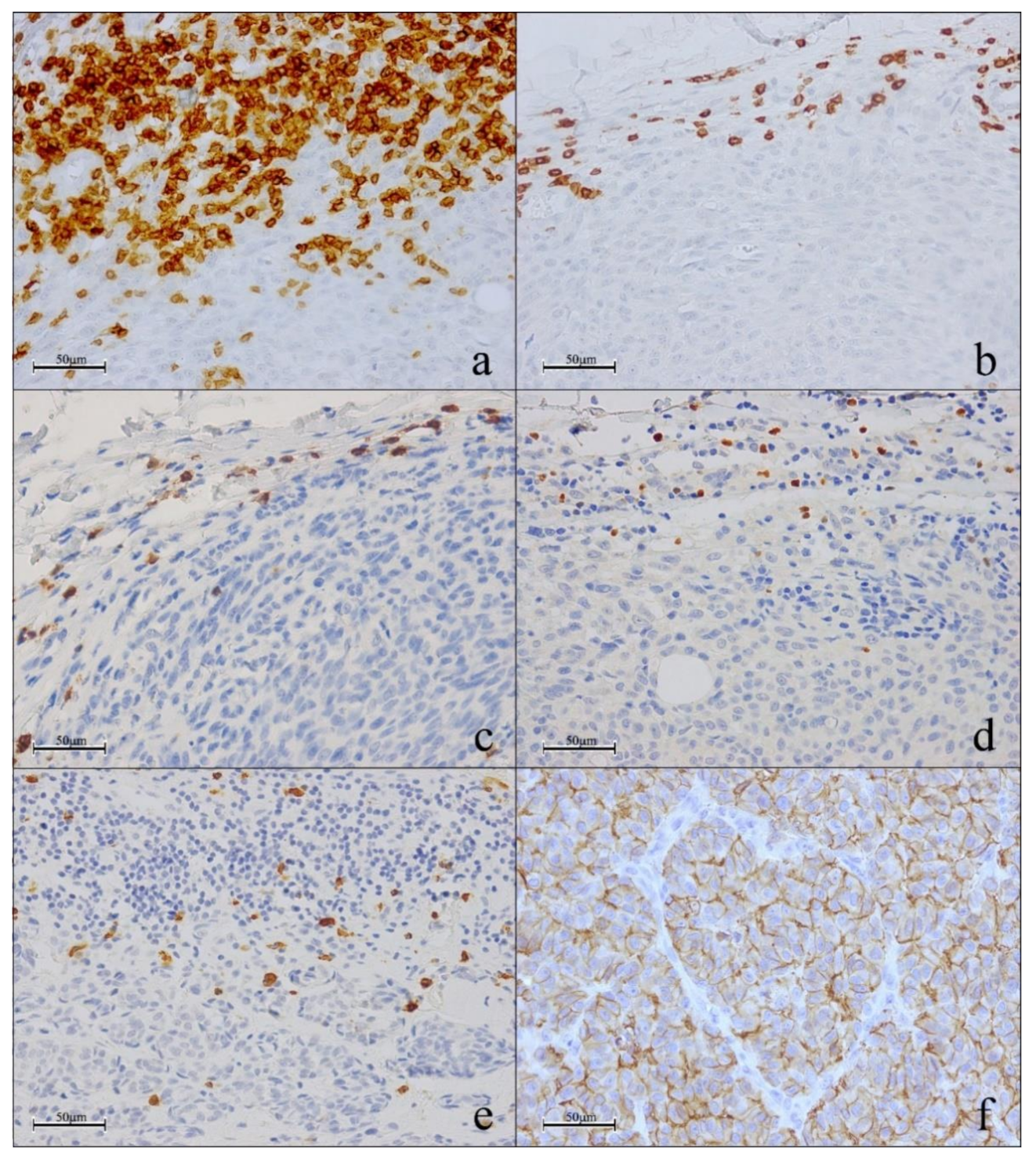
3.4. Simultaneous Evaluation of CD3, FOXP3 and GRZB through Triple Immunostaining
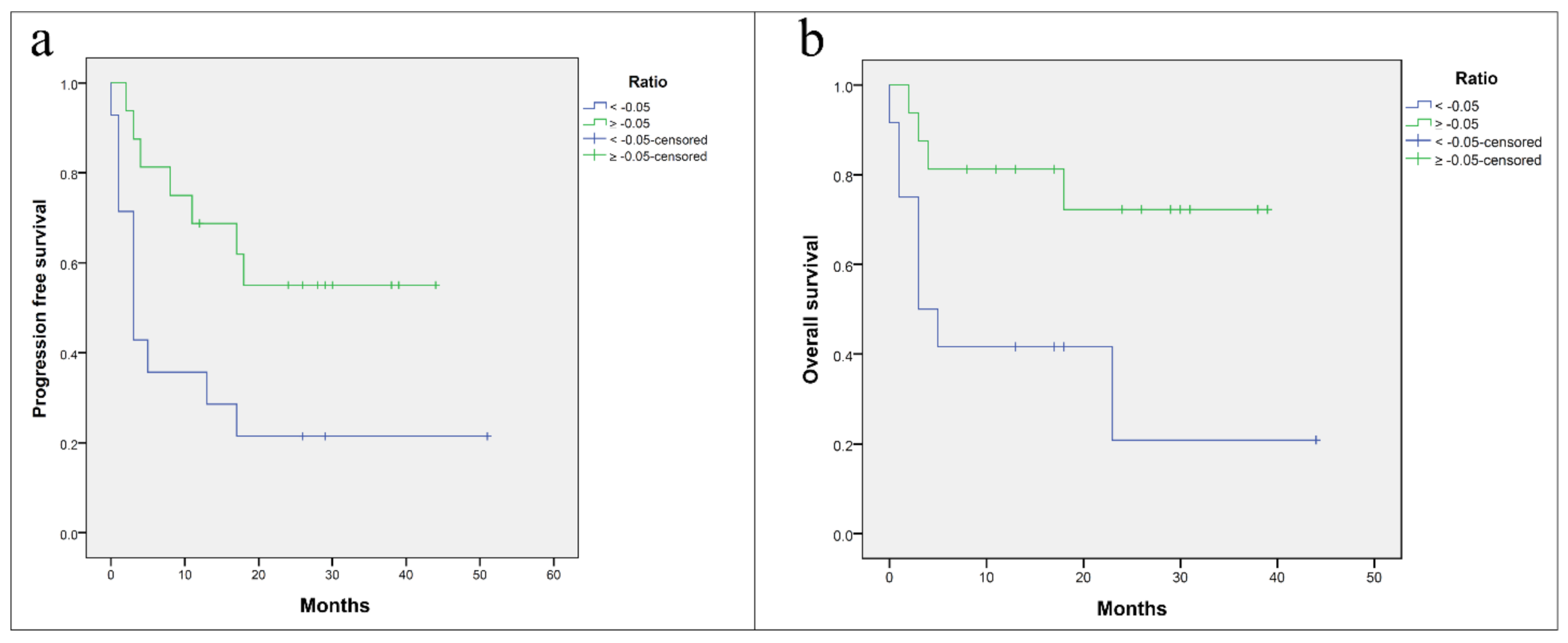
3.5. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keung, E.Z.; Gershenwald, J.E. Clinicopathological Features, Staging, and Current Approaches to Treatment in High-Risk Resectable Melanoma. J. Natl. Cancer Inst. 2020, 112, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Ribas, A. The evolution of checkpoint blockade as a cancer therapy: What’s here, what’s next? Curr. Opin. Immunol. 2015, 33, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Hodi, F.S. Melanoma in 2015: Immune-checkpoint blockade-durable cancer control. Nat. Rev. Clin. Oncol. 2016, 13, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Schmidt, H.; Nissan, A.; Ridolfi, L.; Aamdal, S.; Hansson, J.; Guida, M.; Hyams, D.M.; Gómez, H.; Bastholt, L.; et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 2011, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Zito, C.R.; Barr, M.L.; Baine, M.K.; Chiang, V.L.; Sznol, M.; Rimm, D.L.; Chen, L.; Jilaveanu, L.B. Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Clin. Cancer Res. 2015, 21, 3052–3060. [Google Scholar] [PubMed]
- Madore, J.; Vilain, R.E.; Menzies, A.M.; Kakavand, H.; Wilmott, J.S.; Hyman, J.; Yearley, J.H.; Kefford, R.F.; Thompson, J.F.; Long, G.V.; et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: Implications for anti-PD-1/PD-L1 clinical trials. Pigment. Cell Melanoma Res. 2015, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Wilson, C.; Palmer, B.; Richter, D.; Banerjee, A.; McCarter, M. Melanoma induces immunosuppression by up-regulating FOXP3+ regulatory T cells. J. Surg. Res. 2007, 141, 72–77. [Google Scholar] [CrossRef]
- Knol, A.C.; Nguyen, J.M.; Quéreux, G.; Brocard, A.; Khammari, A.; Dréno, B. Prognostic value of tumor-infiltrating Foxp3+ T-cell subpopulations in metastatic melanoma. Exp. Dermatol. 2011, 20, 430–434. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Dunstone, M.A.; Baran, K.; Whisstock, J.C.; Trapani, J.A. Perforin: Structure, function, and role in human immunopathology. Immunol. Rev. 2010, 235, 35–54. [Google Scholar] [CrossRef]
- van Houdt, I.S.; Sluijter, B.J.; Moesbergen, L.M.; Vos, W.M.; de Gruijl, T.D.; Molenkamp, B.G.; van den Eertwegh, A.J.; Hooijberg, E.; van Leeuwen, P.A.; Meijer, C.J.; et al. Favorable outcome in clinically stage II melanoma patients is associated with the presence of activated tumor infiltrating T-lymphocytes and preserved MHC class I antigen expression. Int. J. Cancer 2008, 123, 609–615. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 68–571. [Google Scholar] [CrossRef]
- Madonna, G.; Ballesteros-Merino, C.; Feng, Z.; Bifulco, C.; Capone, M.; Giannarelli, D.; Mallardo, D.; Simeone, E.; Grimaldi, A.M.; Caracò, C.; et al. PD-L1 expression with immune-infiltrate evaluation and outcome prediction in melanoma patients treated with ipilimumab. Oncoimmunology 2018, 7, e1405206. [Google Scholar] [CrossRef]
- Nie, R.C.; Yuan, S.Q.; Wang, Y.; Chen, Y.B.; Cai, Y.Y.; Chen, S.; Li, S.M.; Zhou, J.; Chen, G.M.; Luo, T.Q.; et al. Robust immunoscore model to predict the response to anti-PD1 therapy in melanoma. Aging 2019, 11, 11576–11590. [Google Scholar] [CrossRef]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Galon, J.; Lanzi, A. Immunoscore and its introduction in clinical practice. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 152–161. [Google Scholar] [CrossRef]
- Scognamiglio, G.; Capone, M.; Mallardo, D.; Botti, G.; Ascierto, P.A.; Madonna, G. Multiplex immunohistochemistry assay to evaluate the melanoma tumor microenvironment. Methods Enzymol. 2020, 635, 21–31. [Google Scholar]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Uhara, H. Recent advances in therapeutic strategies for unresectable or metastatic melanoma and real-world data in Japan. Int. J. Clin. Oncol. 2019, 24, 1508–1514. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Johnson, D.B.; Balko, J.M. Emerging biomarkers for cancer immunotherapy in melanoma. Semin. Cancer Biol. 2018, 52, 207–215. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Halse, H.; Colebatch, A.J.; Petrone, P.; Henderson, M.A.; Mills, J.K.; Snow, H.; Westwood, J.A.; Sandhu, S.; Raleigh, J.M.; Behren, A.; et al. Multiplex immunohistochemistry accurately defines the immune context of metastatic melanoma. Sci. Rep. 2018, 8, 11158. [Google Scholar] [CrossRef]
- Boni, A.; Cogdill, A.P.; Dang, P.; Udayakumar, D.; Njauw, C.N.; Sloss, C.M.; Ferrone, C.R.; Flaherty, K.T.; Lawrence, D.P.; Fisher, D.E.; et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010, 70, 5213–5219. [Google Scholar] [CrossRef]
- Antohe, M.; Nedelcu, R.I.; Nichita, L.; Popp, C.G.; Cioplea, M.; Brinzea, A.; Hodorogea, A.; Calinescu, A.; Balaban, M.; Ion, D.A.; et al. Tumor infiltrating lymphocytes: The regulator of melanoma evolution (Review). Oncol. Lett. 2019, 17, 4155–4161. [Google Scholar] [CrossRef]
- Chen, S.; Crabill, G.A.; Pritchard, T.S.; McMiller, T.L.; Wei, P.; Pardoll, D.M.; Pan, F.; Topalian, S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 2019, 7, 305. [Google Scholar] [CrossRef]
- Giavina-Bianchi, M.; Giavina-Bianchi, P.; Sotto, M.N. Increased NY-ESO-1 expression and reduced infiltrating CD3+ T cells in cutaneous melanoma. J. Immunol. Res. 2015, 2015, 761378. [Google Scholar] [CrossRef]
- Capone, M.; Madonna, G.; Sebastiao, N.; Bird, J.; Ayala, F.; Caracò, C.; Ciliberto, G.; La Fleur, B.; Mozzillo, N.; Botti, G.; et al. Immunoscore as new possible approach for the classification of melanoma. ASCO Annual Meeting 2014, Chicago. J. Clin. Oncol. 2014, 32, e20020. [Google Scholar] [CrossRef]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | No. (%) | |
|---|---|---|
| Age, years | ≤67 | 15 (50) |
| >67 | 15 (50) | |
| Gender | Female | 14 (46.7) |
| Male | 16 (53.3) | |
| Disease stage | IIIC | 1 (3.3) |
| IV | 29 (96.7) | |
| M Category | M0 | 1 (3.3) |
| M1A | 8 (26.7) | |
| M1B | 2 (6.6) | |
| M1C | 14 (46.7) | |
| M1D | 5 (16.7) | |
| Primary vs metastases | PC | 14 (46.7) |
| MC | 16 (53.3) | |
| BRAF status | Wild-type | 21 (70) |
| Mutant | 9 (30) | |
| Line of treatment | 1° | 12 (40) |
| 2° | 12 * (40) | |
| 3° | 5 ** (16.7) | |
| 4° | 1 *** (3.3) | |
| Lactate dehydrogenase | Normal | 21 (70) |
| Elevated | 6 (20) | |
| NA | 3 (10) | |
| Response | Complete response | 2 (6.6) |
| Partial response | 8 (26.7) | |
| Stable disease | 9 (30) | |
| Progressive disease | 11(36.7) | |
| Characteristic | No. | (%) | |
|---|---|---|---|
| Ulceration | Absent | 4 | (28.6) |
| Present | 10 | (71.4) | |
| Mitoses | <1/mm2 | 2 | (14.3) |
| >1/mm2 | 12 | (85.7) | |
| Tumor size | pT1 | 2 | (14.3) |
| pT2 | 2 | (14.3) | |
| pT3 | 6 | (42.9) | |
| pT4 | 4 | (28.6) | |
| Biomarker | Gender | Tumor | BRAF | Best Overall Response Rate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F (n = 14) | M (n = 16) | p-Value | Primary (n = 14) | Metastatic (n = 16) | p-Value | V600 (n = 9) | WT (n = 21) | p-Value | PD (n = 11) | SD (n = 9) | PR (n = 8) | CR (n = 2) | p-Value | |
| CD3 | 17.79 | 13.5 | 0.193 | 15.75 | 15.28 | 0.886 | 13.5 | 16.36 | 0.422 | 14.27 | 19.24 | 13.38 | 10.75 | 0.313 |
| CD8 | 16.64 | 14.5 | 0.525 | 15.18 | 15.78 | 0.854 | 14.17 | 16.07 | 0.594 | 17.09 | 16.06 | 13.25 | 13.25 | 0.79 |
| FOXP3 | 16.93 | 14.25 | 0.423 | 16.75 | 14.41 | 0.473 | 17.67 | 14.57 | 0.397 | 18.18 | 15.33 | 12.56 | 13.75 | 0.57 |
| GRZB | 16.14 | 14.94 | 0.728 | 16.43 | 14.69 | 0.608 | 15.72 | 15.4 | 0.929 | 12.86 | 14.5 | 19.44 | 18.75 | 0.389 |
| PD-L1 | 13.25 | 17.47 | 0.193 | 13.61 | 17.16 | 0.275 | 15.77 | 15.43 | 0.695 | 14.45 | 15.67 | 14.38 | 25 | 0.292 |
| Biomarker | CD3 | CD8 | FOXP3 | GRZB | |
|---|---|---|---|---|---|
| CD8 | Correlation | 0.785 | |||
| p-value | <0.001 | ||||
| FOXP3 | Correlation | 0.315 | 0.547 | ||
| p-value | 0.09 | 0.002 | |||
| GRZB | Correlation | 0.470 | 0.380 | 0.157 | |
| p-value | 0.009 | 0.038 | 0.407 | ||
| PD-L1 | Correlation | 0.057 | 0.184 | 0.106 | 0.214 |
| p-value | 0.764 | 0.329 | 0.576 | 0.256 | |
| Biomarker | Gender | Tumor | BRAF | Best Overall Response Rate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F (n = 14) | M (n = 16) | p-Value | Primary (n = 14) | Metastatic (n = 16) | p-Value | V600 (n = 9) | WT (n = 21) | p-Value | PD (n = 11) | SD (n = 9) | PR (n = 8) | CR (n = 2) | p-Value | |
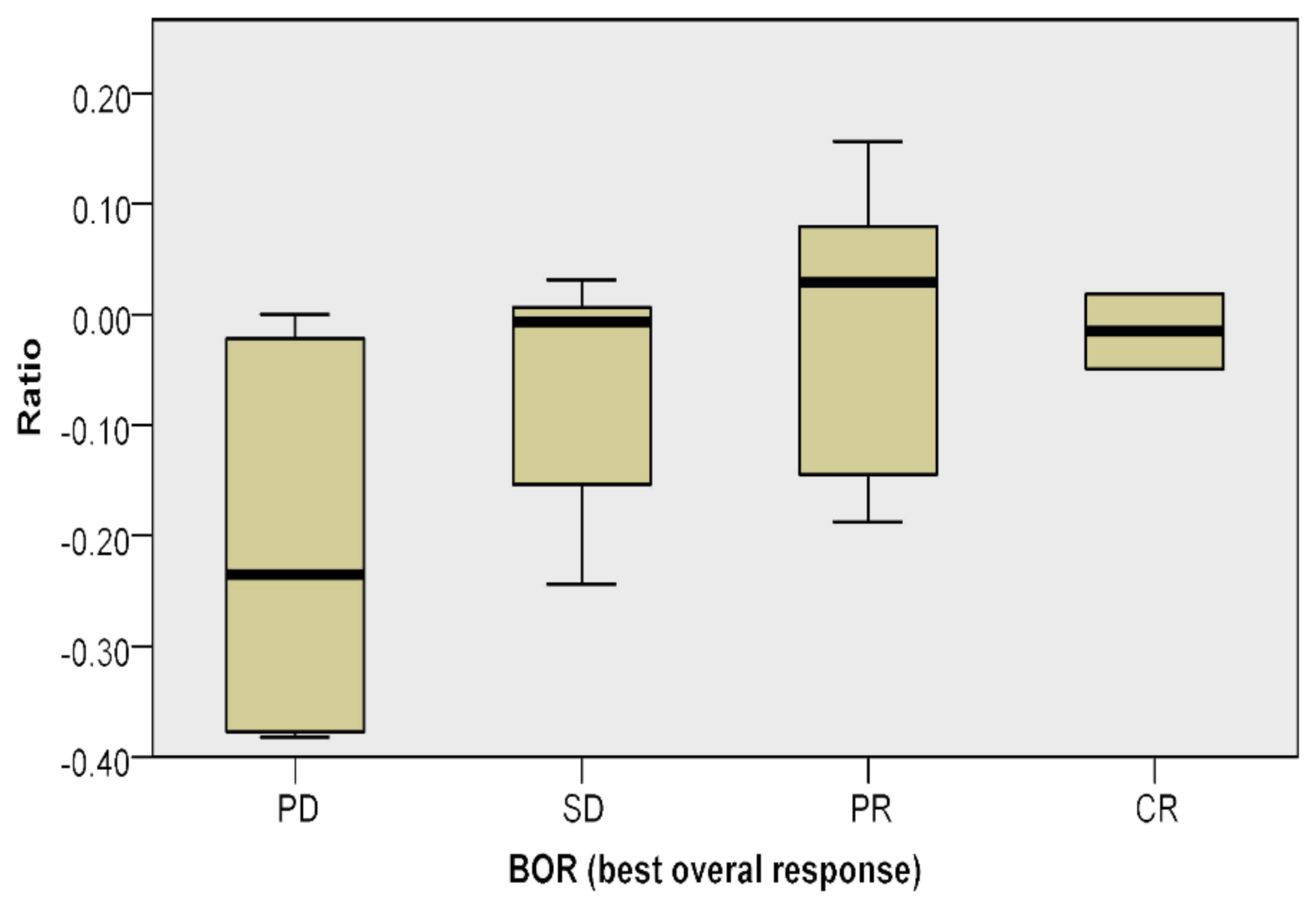
| Ratio | 16.21 | 14.88 | 0.697 | 13.43 | 17.31 | 0.24 | 13.67 | 16.29 | 0.476 | 9.55 | 16.56 | 21.25 | 20.5 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scognamiglio, G.; Capone, M.; Sabbatino, F.; Di Mauro, A.; Cantile, M.; Cerrone, M.; Madonna, G.; Grimaldi, A.M.; Mallardo, D.; Palla, M.; et al. The Ratio of GrzB+ − FoxP3+ over CD3+ T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma. Cancers 2021, 13, 2325. https://doi.org/10.3390/cancers13102325
Scognamiglio G, Capone M, Sabbatino F, Di Mauro A, Cantile M, Cerrone M, Madonna G, Grimaldi AM, Mallardo D, Palla M, et al. The Ratio of GrzB+ − FoxP3+ over CD3+ T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma. Cancers. 2021; 13(10):2325. https://doi.org/10.3390/cancers13102325
Chicago/Turabian StyleScognamiglio, Giosuè, Mariaelena Capone, Francesco Sabbatino, Annabella Di Mauro, Monica Cantile, Margherita Cerrone, Gabriele Madonna, Antonio Maria Grimaldi, Domenico Mallardo, Marco Palla, and et al. 2021. "The Ratio of GrzB+ − FoxP3+ over CD3+ T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma" Cancers 13, no. 10: 2325. https://doi.org/10.3390/cancers13102325
APA StyleScognamiglio, G., Capone, M., Sabbatino, F., Di Mauro, A., Cantile, M., Cerrone, M., Madonna, G., Grimaldi, A. M., Mallardo, D., Palla, M., Sarno, S., Anniciello, A. M., Di Bonito, M., Ascierto, P. A., & Botti, G. (2021). The Ratio of GrzB+ − FoxP3+ over CD3+ T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma. Cancers, 13(10), 2325. https://doi.org/10.3390/cancers13102325









