A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics and Frequency of Complete Tumor Regression
2.2. Laboratory Parameters’ Associations with Complete Tumor Regression
2.3. Tumor and Nodal Stages’ Associations with Complete Tumor Regression
2.4. Size and Other MRI Characteristics’ Associations with Complete Tumor Regression
2.5. Factors Associated with Complete Tumor Regression
3. Discussion
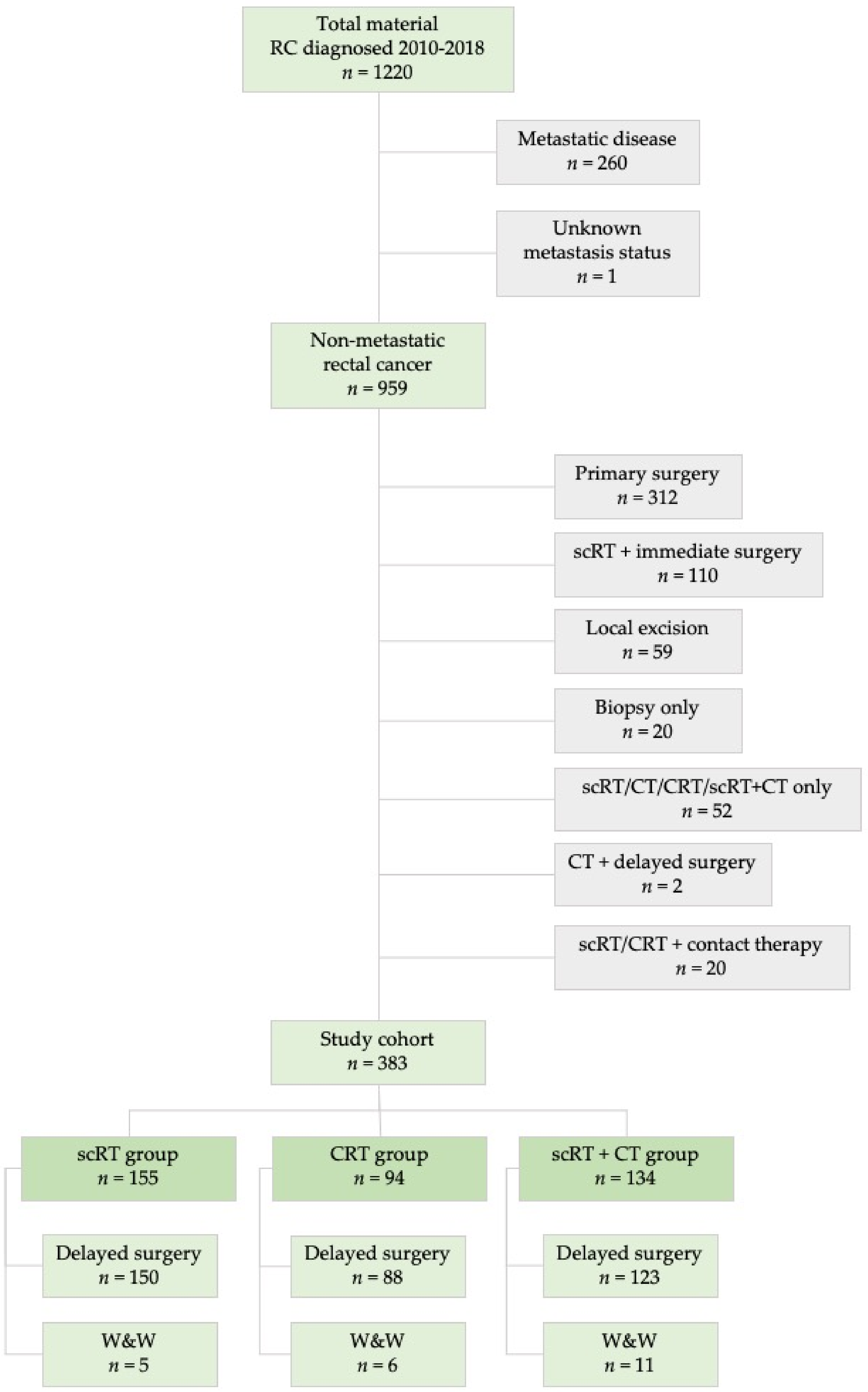
4. Materials and Methods
4.1. Clinical and Pathological Staging and Treatment
4.2. Statistical Methods
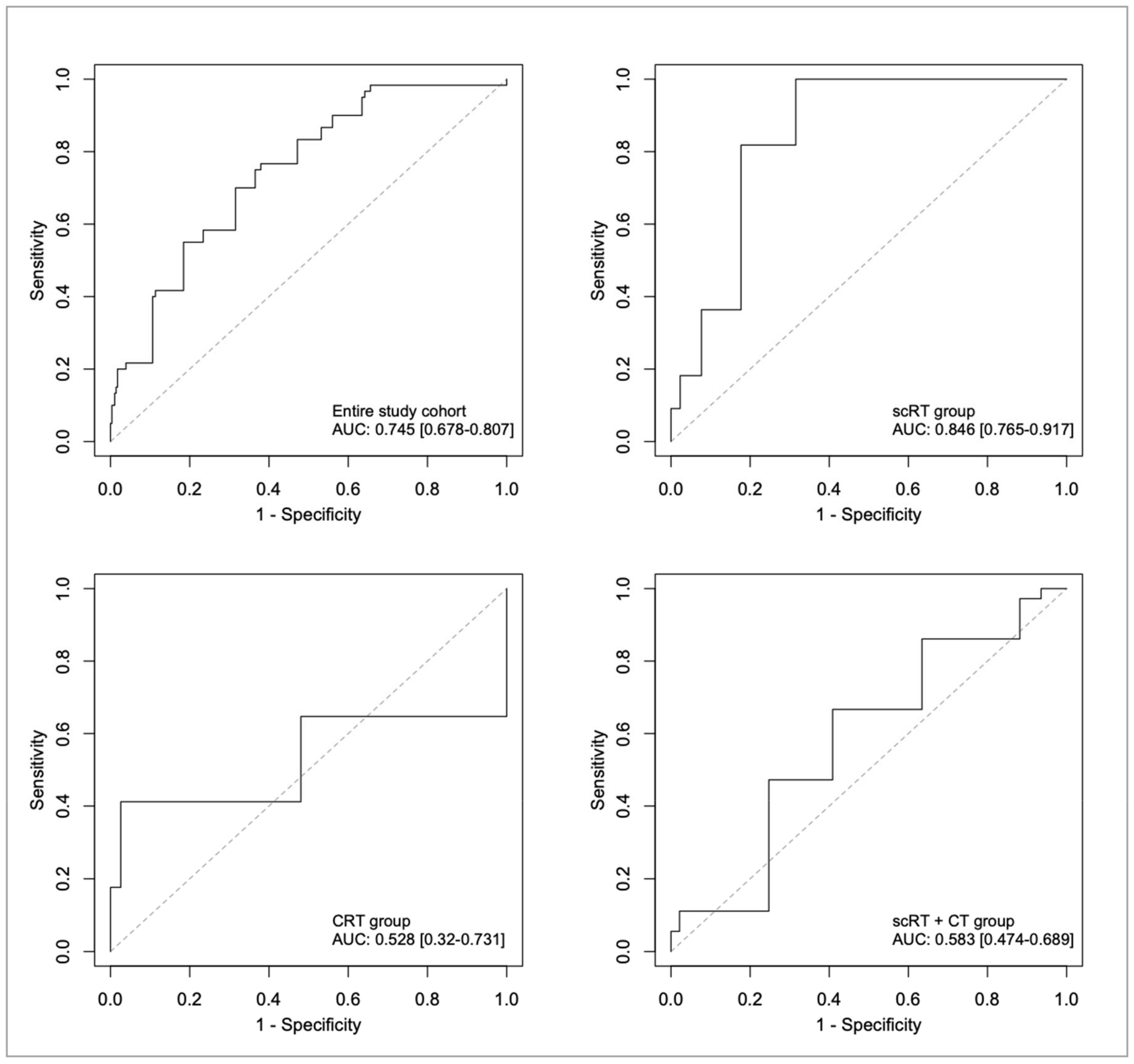
4.3. ROC Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glimelius, B.; Myklebust, T.Å.; Lundqvist, K.; Wibe, A.; Guren, M.G. Two countries—Two treatment strategies for rectal cancer. Radiother. Oncol. 2016, 121, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U.; Silva e Sousa, A.H., Jr.; Campos, F.G., Jr.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Warrier, S.K.; Lynch, A.C.; Heriot, A.G. Assessing pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Color. Dis. 2015, 17, 849–861. [Google Scholar] [CrossRef]
- The 2017 European Society of Coloproctology (ESCP) Collaborating Group. Evaluating the incidence of pathological complete response in current international rectal cancer practice: The barriers to widespread safe deferral of surgery. Colorectal Dis. 2018, 20 (Suppl. 6), 58–68. [Google Scholar] [CrossRef]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. BJS 2012, 99, 918–928. [Google Scholar] [CrossRef]
- Marijnen, C.A.M. Organ preservation in rectal cancer: Have all questions been answered? Lancet Oncol. 2015, 16, e13–e22. [Google Scholar] [CrossRef]
- Stijns, R.C.; Tromp, M.-S.R.; Hugen, N.; De Wilt, J.H. Advances in organ preserving strategies in rectal cancer patients. Eur. J. Surg. Oncol. 2018, 44, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.H.; Maas, M.; Heijnen, L.A.; Lambregts, D.M.; Leijtens, J.W.; Stassen, L.P.; Breukink, S.O.; Hoff, C.; Belgers, E.J.; Melenhorst, J.; et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Van Der Valk, M.J.M.; Hilling, E.D.; Bastiaannet, E.; Kranenbarg, E.M.-K.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, O.R.; Renehan, A.G.; Van De Velde, C.J.H.; et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection—A Matched-Controlled Study. Dis. Colon Rectum. 2017, 60, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.L.; White, A.D.; Osborne, E.M.; Shaw, A.M.; Smart, N.J.; Daniels, I.R. Predicting response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer with serum biomarkers. Ann. R. Coll. Surg. Engl. 2017, 99, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.L.; Vather, R.; Bunkley, N.; Pearse, M.; Bissett, I. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int. J. Color. Dis. 2014, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Matteucci, F.; Caroli, P.; Passardi, A. Biomarkers and Molecular Imaging as Predictors of Response to Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer. Clin. Color. Cancer 2015, 14, 227–238. [Google Scholar] [CrossRef]
- Pazdirek, F.; Minarik, M.; Benesova, L.; Halkova, T.; Belsanova, B.; Macek, M.; Stepanek, L.; Hoch, J. Monitoring of Early Changes of Circulating Tumor DNA in the Plasma of Rectal Cancer Patients Receiving Neoadjuvant Concomitant Chemoradiotherapy: Evaluation for Prognosis and Prediction of Therapeutic Response. Front. Oncol. 2020, 10, 1028. [Google Scholar] [CrossRef]
- Douglas, J.K.; Douglas, J.; Hothem, Z.; Cousineau, C.S.; Kawak, S.; Thibodeau, B.J.; Bergeron, S.; Li, W.; Peeples, C.E.; Wasvary, H.J. Genomic variation as a marker of response to neoadjuvant therapy in locally advanced rectal cancer. Mol. Cell. Oncol. 2020, 7, 1716618. [Google Scholar] [CrossRef]
- Hiyoshi, Y.; Akiyoshi, T.; Inoue, R.; Murofushi, K.; Yamamoto, N.; Fukunaga, Y.; Ueno, M.; Baba, H.; Mori, S.; Yamaguchi, T. Serum miR-143 levels predict the pathological response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Oncotarget 2017, 8, 79201–79211. [Google Scholar] [CrossRef]
- Wallin, U.G.; Rothenberger, D.; Lowry, A.; Luepker, R.; Mellgren, A. CEA—A Predictor for Pathologic Complete Response After Neoadjuvant Therapy for Rectal Cancer. Dis. Colon Rectum 2013, 56, 859–868. [Google Scholar] [CrossRef]
- Peng, H.; Wang, C.; Xiao, W.; Lin, X.; You, K.; Dong, J.; Wang, Z.; Yu, X.; Zeng, Z.; Zhou, T.; et al. Analysis of Clinical characteristics to predict pathologic complete response for patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. J. Cancer 2018, 9, 2687–2692. [Google Scholar] [CrossRef]
- Kim, E.; Kim, K.; Kim, S.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; Park, K.J.; Koh, J.; Kang, G.H.; Chie, E.K.; et al. Impact of Mucin Proportion in the Pretreatment MRI on the Outcomes of Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Cancer Res. Treat. 2018, 51, 1188–1197. [Google Scholar] [CrossRef]
- Tan, Y.; Fu, D.; Li, D.; Kong, X.; Jiang, K.; Chen, L.; Yuan, Y.; Ding, K. Predictors and Risk Factors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Population-Based Analysis. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.; Deroose, C.M.; Vandecaveye, V.; Haustermans, K. The role of diffusion-weighted MRI and 18F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: A systematic review. Radiother. Oncol. 2014, 113, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Warrier, S.K.; Lynch, A.C.; Ramsay, R.G.; Phillips, A.W.; Heriot, A.G. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Color. Dis. 2016, 18, 234–246. [Google Scholar] [CrossRef]
- Hammarström, K.; Imam, I.; Hult, N.K.; Ekström, J.; Sjöblom, T.; Glimelius, B. Determining the use of preoperative (chemo)radiotherapy in primary rectal cancer according to national and international guidelines. Radiother. Oncol. 2019, 136, 106–112. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practical Guidelines in Oncology. Breast Cancer, version. 2018. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 23 September 2020).
- Beets-Tan, R.; Beets, G.L.; Vliegen, R.; Kessels, A.; Van Boven, H.; De Bruine, A.; Von Meyenfeldt, M.F.; Baeten, C.; Van Engelshoven, J. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001, 357, 497–504. [Google Scholar] [CrossRef]
- Hammarström, K.; Mezheyeuski, A.; Hult, N.K.; Sjöblom, T.; Glimelius, B. Stage distribution utilizing magnetic resonance imaging in an unselected population of primary rectal cancers. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1858–1864. [Google Scholar] [CrossRef]
- Hughes, R.; Harrison, M.; Glynne-Jones, R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol. 2010, 49, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Chow, O.S.; Smith, D.D.; Marcet, E.J.; Cataldo, A.P.; Varma, M.G.; Kumar, A.S.; Oommen, S.; Coutsoftides, T.; Hunt, S.R.; et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 957–966. [Google Scholar] [CrossRef]
- Hoendervangers, S.; Couwenberg, A.M.; Intven, M.P.W.; Van Grevenstein, W.M.; Verkooijen, H.M. Comparison of pathological complete response rates after neoadjuvant short-course radiotherapy or chemoradiation followed by delayed surgery in locally advanced rectal cancer. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1013–1017. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, A.E.; Van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.-K.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2020. [Google Scholar] [CrossRef]
- Erlandsson, J.; Lörinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer—Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Verhoeven, R.H.; Elferink, M.; Poortmans, P.; Nagtegaal, I.D.; De Wilt, J.H.W. Tumor response after long interval comparing 5x5Gy radiation therapy with chemoradiation therapy in rectal cancer patients. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.; Debucquoy, A.; Fieuws, S.; Wolthuis, A.; Sagaert, X.; D’Hoore, A.; Haustermans, K. Can clinical factors be used as a selection tool for an organ-preserving strategy in rectal cancer? Acta Oncol. 2016, 55, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.M.; Oliva, K.; Koulis, C.; Yap, R.; McMurrick, P.J. Predictive factors of complete pathological response in patients with locally advanced rectal cancer. Int. J. Color. Dis. 2020, 35, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.J.; Korsgen, S.; Hill, J.; Speake, D.; Levy, B.; Steward, M.; Geh, J.I.; Robinson, J.; Sebag-Montefiore, D.; Bach, S.P. Multicentre study of short-course radiotherapy and transanal endoscopic microsurgery for early rectal cancer. BJS 2016, 103, 1069–1075. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Julião, G.P.S.; Gama-Rodrigues, J.; Vailati, B.B.; Ortega, C.; Fernandez, L.M.; Araújo, S.E.A.; Perez, R.O. Baseline T Classification Predicts Early Tumor Regrowth After Nonoperative Management in Distal Rectal Cancer After Extended Neoadjuvant Chemoradiation and Initial Complete Clinical Response. Dis. Colon Rectum 2017, 60, 586–594. [Google Scholar] [CrossRef]
- Wilkins, S.; Haydon, A.; Porter, I.; Oliva, K.; Staples, M.; Carne, P.; McMurrick, P.; Bell, S. Complete Pathological Response After Neoadjuvant Long-Course Chemoradiotherapy for Rectal Cancer and Its Relationship to the Degree of T3 Mesorectal Invasion. Dis. Colon Rectum 2016, 59, 361–368. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, D.; Young, C.J. Predictors for complete pathological response for stage II and III rectal cancer following neoadjuvant therapy—A systematic review and meta-analysis. Am. J. Surg. 2020, 220, 300–308. [Google Scholar] [CrossRef]
- Restivo, A.; Zorcolo, L.; Cocco, I.M.F.; Manunza, R.; Margiani, C.; Marongiu, L.; Casula, G. Elevated CEA Levels and Low Distance of the Tumor from the Anal Verge are Predictors of Incomplete Response to Chemoradiation in Patients with Rectal Cancer. Ann. Surg. Oncol. 2012, 20, 864–871. [Google Scholar] [CrossRef]
- Yasuda, K.; Sunami, E.; Kawai, K.; Nagawa, H.; Kitayama, J. Laboratory Blood Data Have a Significant Impact on Tumor Response and Outcome in Preoperative Chemoradiotherapy for Advanced Rectal Cancer. J. Gastrointest. Cancer 2011, 43, 236–243. [Google Scholar] [CrossRef]
- Wilson, M.J.; Van Haaren, M.; Harlaar, J.; Park, H.C.; Bonjer, H.; Jeekel, J.; Zwaginga, J.; Schipperus, M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kirat, H.T.; Ozturk, E.; Lavery, I.C.; Kiran, R.P. The predictive value of preoperative carcinoembryonic antigen level in the prognosis of colon cancer. Am. J. Surg. 2012, 204, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Colloca, G.A.; Venturino, A.; Vitucci, P. Pre-treatment carcinoembryonic antigen and outcome of patients with rectal cancer receiving neo-adjuvant chemo-radiation and surgical resection: A systematic review and meta-analysis. Med Oncol. 2017, 34. [Google Scholar] [CrossRef]
- Santos, M.D.; Silva, C.; Rocha, A.; Nogueira, C.; Castro-Poças, F.; Araujo, A.; Matos, E.; Pereira, C.; Medeiros, R.; Lopes, C. Predictive clinical model of tumor response after chemoradiation in rectal cancer. Oncotarget 2017, 8, 58133–58151. [Google Scholar] [CrossRef]
- Chand, M.; Siddiqui, M.R.S.; Swift, I.; Brown, G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World, J. Gastroenterol. 2016, 22, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.S.; Simillis, C.; Hunter, C.; Chand, M.; Bhoday, J.; Garant, A.; Vuong, T.; Artho, G.; Rasheed, S.; Tekkis, P.; et al. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br. J. Cancer 2017, 116, 1513–1519. [Google Scholar] [CrossRef]
- McCawley, N.; Clancy, C.; O’Neill, B.D.; Deasy, J.; McNamara, D.A.; Burke, J.P. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2016, 59, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Kodeda, K.; Johansson, R.S.; Zar, N.; Birgisson, H.; Dahlberg, M.; Skullman, S.; Lindmark, G.; Glimelius, B.; Pahlman, L.; Martling, A. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Color. Dis. 2015, 17, O168–O179. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.J.; Van Etten, B.; Hospers, G.A.P.; Påhlman, L.; Van De Velde, C.; Beets-Tan, R.G.H.; Blomqvist, L.; Beukema, J.C.; Kapiteijn, E.; Marijnen, C.A.M.; et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—The RAPIDO trial. BMC Cancer 2013, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, J.; Holm, T.; Pettersson, D.; Berglund, Å.; Cedermark, B.; Radu, C.; Johansson, H.; Machado, M.; Hjern, F.; Hallböök, O.; et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Radu, C.; Berglund, Å.; Påhlman, L.; Glimelius, B. Short-course preoperative radiotherapy with delayed surgery in rectal cancer—A retrospective study. Radiother. Oncol. 2008, 87, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Morris, E. Reporting colorectal cancer. Histopathology 2007, 50, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Melin, B.; Enblad, G.; Alafuzoff, I.; Beskow, A.; Ahlstrom, H.; Bill-Axelson, A.; Birgisson, H.; Bjor, O.; Edqvist, P.H.; et al. U-CAN: A prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta. Oncol. 2018, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
| CLINICAL CHARACTERISTICS | TOTAL POPULATION n = 383 | scRT n = 155 | CRT n = 94 | scRT+CT n = 134 | p-Value A |
|---|---|---|---|---|---|
| AGE AT DIAGNOSIS | |||||
| Median (range) years | 68 (31–91) | 74 (46–91) | 65 (32–80) | 65 (31–81) | |
| <65 | 138 (36) | 33 (21) | 43 (46) | 62 (46) | <0.001 |
| 65–79 | 194 (51) | 74 (47) | 49 (52) | 71 (53) | |
| >= 80 | 51 (14) | 48 (31) | 2 (2) | 1 (1) | |
| SEX | |||||
| Male | 204 (53) | 91 (59) | 49 (52) | 64 (48) | 0.172 |
| Female | 179 (47) | 64 (41) | 45 (48) | 70 (52) | |
| CLINICAL TN STAGE | |||||
| Stage I | 14 (4) | 9 (6) | 5 (5) | 0 | <0.001 |
| Stage II | 42 (11) | 31 (20) | 5 (5) | 6 (5) | |
| Stage III | 327 (85) | 115 (74) | 84 (90) | 128 (95) | |
| CLINICAL T STAGE B | |||||
| cT1 | 4 (1) | 3 (2) | 1 (1) | 0 | <0.001 |
| cT2 | 22 (6) | 15 (10) | 5 (5) | 2 (2) | |
| cT3 all | 208 (54) | 95 (61) | 44 (47) | 69 (52) | |
| cT3a | 26 (7) | 17 (11) | 3 (3) | 6 (5) | |
| cT3b | 66 (17) | 37 (24) | 9 (10) | 20 (15) | |
| cT3c | 87 (23) | 30 (19) | 21 (22) | 36 (27) | |
| cT3d | 24 (6) | 6 (4) | 11 (12) | 7 (5) | |
| cT3 unknown | 5 (1) | 5 (3) | 0 | 0 | |
| cT4 all | 148 (39) | 42 (27) | 44 (47) | 63 (47) | |
| cT4a | 41 (11) | 10 (7) | 9 (10) | 22 (17) | |
| cT4b | 107 (28) | 31 (20) | 35 (37) | 41 (30) | |
| Missing cT stage C | 1 | 1 | 0 | 0 | |
| CLINICAL N STAGE B | <0.001 | ||||
| cN0 | 56 (15) | 40 (26) | 10 (11) | 6 (5) | |
| cN1 | 143 (37) | 65 (42) | 24 (26) | 54 (40) | |
| cN2 | 184 (48) | 50 (32) | 60 (64) | 74 (55) | |
| TUMOR SIZE | |||||
| Median (range) cm | 5 (1–16) | 5 (1–16) | 5 (1–13) | 5 (1–13) | |
| <3 cm | 27 (7) | 18 (12) | 3 (3) | 6 (5) | 0.048 |
| 3–5 cm | 166 (44) | 59 (39) | 44 (47) | 63 (48) | |
| >5 cm | 184 (49) | 74 (49) | 47 (50) | 63 (48) | |
| Missing C | 6 | 4 | 0 | 2 | |
| TUMOR LEVEL | |||||
| 0–5 cm (low) | 150 (39) | 66 (43) | 42 (45) | 42 (31) | 0.083 |
| 6–10 cm (mid) | 143 (37) | 60 (39) | 32 (34) | 51 (38) | |
| 11–15 cm (high) | 90 (23) | 29 (19) | 20 (21) | 41 (31) | |
| MRI-IDENTIFIED EMVI | |||||
| EMVI− | 210 (57) | 99 (68) | 49 (53) | 62 (47) | 0.001 |
| EMVI+ | 160 (43) | 46 (32) | 44 (47) | 70 (53) | |
| Missing C | 13 | 10 | 1 | 2 | |
| MRI-IDENTIFIED MUCINOUS FEATURES | |||||
| Mucinous− | 257 (70) | 110 (76) | 66 (70) | 81 (62) | 0.029 |
| Mucinous+ | 112 (30) | 34 (23) | 28 (30) | 50 (38) | |
| Missing C | 14 | 11 | 0 | 3 | |
| MRI-DETECTED MRF INVOLVEMENT IN cT3 TUMORS (n = 208) | |||||
| MRF negative | 92 (45) | 54 (59) | 14 (32) | 24 (35) | 0.007 |
| MRF positive/threatened | 112 (55) | 37 (41) | 30 (68) | 45 (65) | |
| Missing C | 4 | 4 | 0 | 0 | |
| DELAY OF SURGERY AFTER RADIOTHERAPY D | |||||
| Median (range) weeks | 10 (4–36) | 7 (4–33) | 9 (5–28) | 19 (8–36) | <0.001 |
| <6 weeks | 5 (2) | 5 (4) | 0 | 0 | |
| 6–8 weeks | 95 (31) | 70 (55) | 25 (42) | 0 | |
| >8 weeks | 206 (67) | 51 (41) | 34 (58) | 121 (100) | |
| LABORATORY VALUES | |||||
| HEMOGLOBIN (Hb) | |||||
| Hb ≤ 110 g/L | 50 (13) | 25 (17) | 10 (11) | 15 (11) | 0.303 |
| Hb > 110 g/L | 327 (87) | 126 (83) | 83 (89) | 118 (89) | |
| Missing C | 6 | 4 | 1 | 1 | |
| LEUCOCYTES (LPK) | |||||
| LPK ≤ 9 × 109/L | 247 (75) | 89 (69) | 73 (85) | 85 (74) | 0.030 |
| LPK > 9 × 109/L | 83 (25) | 40 (31) | 13 (15) | 30 (26) | |
| Missing C | 53 | 26 | 8 | 19 | |
| C-REACTIVE PROTEIN (CRP) | |||||
| CRP ≤ 5 mg/L | 187 (61) | 65 (50) | 61 (76) | 61 (62) | 0.001 |
| CRP > 5 mg/L | 120 (39) | 64 (50) | 19 (24) | 37 (38) | |
| Missing C | 76 | 26 | 14 | 36 | |
| CARCINOEMBRYONIC ANTIGEN (CEA) | |||||
| CEA ≤ 3.8 μg/L | 176 (50) | 69 (50) | 40 (47) | 67 (51) | 0.861 |
| CEA > 3.8 μg/L | 179 (50) | 69 (50) | 45 (53) | 65 (49) | |
| Missing C | 28 | 17 | 9 | 2 | |
| TUMOR RESPONSE TO THERAPY | |||||
| COMPLETE REMISSION (CR) | 65 (17) | 12 (8) | 17 (18) | 36 (27) | <0.001 |
| NON-COMPLETE REMISSION (NON-CR) | 318 (83) | 143 (92) | 77 (82) | 98 (73) |
| Clinical Characteristics | scRT | CRT | scRT+CT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | NON-CR | p-Value A | CR | NON-CR | p-Value A | CR | NON-CR | p-Value A | ||
| HB | HB < 110 g/L | 3 (12) | 22 (88) | 0.420 | 0 | 2 (13) | 13 (87) | 0.354 | ||
| HB > 110 g/L | 9 (7) | 117 (93) | 17 (21) | 66 (79) | 34 (29) | 84 (71) | ||||
| MISSING B | 0 | 4 | 0 | 1 | 0 | 1 | ||||
| CEA | CEA < 3.8 μg/L | 7 (10) | 62 (90) | 0.165 | 11 (28) | 29 (73) | 0.103 | 24 (36) | 43 (64) | 0.025 |
| CEA > 3.8 μg/L | 2 (3) | 67 (97) | 6 (13) | 39 (87) | 12 (19) | 53 (81) | ||||
| MISSING B | 3 | 14 | 0 | 9 | 0 | 2 | ||||
| CRP | CRP < 5 mg/L | 5 (8) | 60 (92) | 0.980 | 14 (23) | 47 (77) | 0.333 | 22 (36) | 39 (64) | 0.009 |
| CRP > 5 mg/L | 5 (8) | 59 (92) | 2 (11) | 17 (89) | 4 (11) | 33 (89) | ||||
| MISSING B | 2 | 24 | 1 | 13 | 10 | 26 | ||||
| LPK | LPK < 9 × 109/L | 7 (8) | 82 (92) | 1.000 | 14 (19) | 59 (81) | 0.450 | 29 (34) | 56 (66) | 0.035 |
| LPK > 9 × 109/L | 3 (8) | 37 (92) | 1 (8) | 12 (92) | 4 (13) | 26 (87) | ||||
| MISSING B | 2 | 24 | 2 | 6 | 3 | 16 | ||||
| TUMOR STAGE C | cT1-3a | 7 (20) | 28 (80) | 0.004 | 6 (67) | 3 (33) | 0.001 | 3 (38) | 5 (62) | 0.446 |
| cT3b-4b | 4 (4) | 110 (96) | 11 (13) | 74 (87) | 33 (26) | 92 (74) | ||||
| MISSING B | 1 | 5 | 0 | 0 | 0 | 1 | ||||
| NODAL STAGE C | cN0 | 5 (12) | 35 (88) | 0.328 | 6 (60) | 4 (40) | 0.004 | 2 (33) | 4 (67) | 0.584 |
| cN1 | 5 (8) | 60 (92) | 4 (17) | 20 (83) | 12 (22) | 42 (78) | ||||
| cN2 | 2 (4) | 48 (96) | 7 (12) | 53 (88) | 22 (30) | 52 (70) | ||||
| TUMOR LEVEL | 0–5 cm (LOW) | 6 (9) | 60 (91) | 0.576 | 10 (24) | 32 (76) | 0.088 | 15 (36) | 27 (64) | 0.291 |
| 6–10 cm (MID) | 3 (5) | 57 (95) | 2 (6) | 30 (94) | 12 (24) | 39 (76) | ||||
| 11–15 cm (HIGH) | 3 (10) | 26 (90) | 5 (25) | 15 (75) | 9 (22) | 32 (78) | ||||
| TUMOR SIZE | <3 cm | 5 (28) | 13 (72) | 0.007 | 3 (100) | 0 | 0.006 | 4 (67) | 2 (33) | 0.023 |
| 3-5 cm | 2 (3) | 57 (97) | 8 (18) | 36 (82) | 20 (32) | 43 (68) | ||||
| >5 cm | 4 (5) | 70 (95) | 6 (13) | 41 (87) | 12 (19) | 51 (81) | ||||
| MISSING B | 1 | 3 | 0 | 0 | 0 | 2 | ||||
| EMVI STATUS | EMVI− | 11 (11) | 88 (89) | 0.017 | 8 (16) | 41 (84) | 0.813 | 18 (29) | 44 (71) | 0.418 |
| EMVI+ | 0 | 46 (100) | 8 (18) | 36 (82) | 16 (23) | 54 (77) | ||||
| MISSING B | 1 | 9 | 1 | 0 | 2 | 0 | ||||
| MUCINOUS STATUS | MUCIN− | 11 (10) | 99 (90) | 0.067 | 13 (20) | 53 (80) | 0.770 | 23 (28) | 58 (72) | 0.765 |
| MUCIN+ | 0 | 34 (100) | 4 (14) | 24 (86) | 13 (26) | 37 (74) | ||||
| MISSING B | 1 | 10 | 0 | 0 | 0 | 3 | ||||
| MRF STATUS D | MRF− | 5 (9) | 49 (91) | 0.696 | 4 (29) | 10 (71) | 0.184 | 7 (29) | 17 (71) | 0.670 |
| MRF + (<1 MM) | 2 (5) | 35 (95) | 3 (10) | 27 (90) | 11 (24) | 34 (76) | ||||
| MISSING B | 0 | 4 | 0 | 0 | 0 | 0 | ||||
| CLINICAL CHARACTERISTICS | TOTAL MATERIAL (N = 383) | |||
|---|---|---|---|---|
| CR | Non-CR | p-Value A | ||
| HB | Hb ≤ 110 g/L | 5 (10) | 45 (90) | 0.146 |
| Hb > 110 g/L | 60 (18) | 267 (82) | ||
| MISSING B | 0 | 6 | ||
| CEA | CEA ≤ 3.8 μg/L | 42 (24) | 134 (76) | 0.002 |
| CEA > 3.8 μg/L | 20 (11) | 159 (89) | ||
| MISSING B | 3 | 25 | ||
| CRP | CRP ≤ 5 mg/L | 41 (22) | 146 (78) | 0.004 |
| CRP > 5 mg/L | 11 (9) | 109 (91) | ||
| MISSING B | 13 | 63 | ||
| LPK | LPK ≤ 9 × 109/L | 50 (20) | 197 (80) | 0.028 |
| LPK > 9 × 109/L | 8 (10) | 75 (90) | ||
| MISSING B | 7 | 46 | ||
| TUMOR STAGE C | cT1-3a | 16 (31) | 36 (69) | 0.004 |
| cT3b-4b | 48 (15) | 276 (85) | ||
| MISSING B | 1 | 6 | ||
| NODAL STAGE | cN0 | 13 (23) | 43 (77) | 0.353 |
| cN1 | 21 (15) | 122 (85) | ||
| cN2 | 31 (17) | 153 (83) | ||
| TUMOR LEVEL | 0–5 cm (low) | 31 (21) | 119 (79) | 0.116 |
| 6–10 cm (mid) | 17 (12) | 126 (88) | ||
| 11–15 cm (high) | 17 (19) | 73 (81) | ||
| TUMOR SIZE | <3 cm | 12 (44) | 15 (56) | <0.001 |
| 3–5 cm | 30 (18) | 136 (82) | ||
| >5 cm | 22 (12) | 162 (88) | ||
| MISSING B | 1 | 5 | ||
| EMVI STATUS | EMVI– | 37 (18) | 173 (82) | 0.501 |
| EMVI+ | 24 (15) | 136 (85) | ||
| MISSING B | 4 | 9 | ||
| MUCINOUS STATUS | Mucin– | 47 (18) | 210 (82) | 0.468 |
| Mucin+ | 17 (15) | 95 (85) | ||
| MISSING B | 1 | 13 | ||
| MRF STATUS D | MRF− | 16 (17) | 76 (83) | 0.544 |
| MRF + (<1 mm) | 16 (14) | 96 (86) | ||
| MISSING B | 0 | 4 | ||
| TREATMENT | scRT | 12 (8) | 143 (92) | <0.001 |
| CRT | 17 (18) | 77 (82) | ||
| scRT+CT | 36 (27) | 98 (73) | ||
| CLINICAL CHARACTERISTICS | scRT GROUP | |||
|---|---|---|---|---|
| 95% CI | ||||
| PARAMETER A | CR, OR E | LOWER | UPPER | p-Value |
| cT STAGE | ||||
| cT1-3a | 1 | Ref | ||
| cT3b-4b | 0.087 | 0.014 | 0.551 | 0.010 |
| TUMOR LEVEL | ||||
| 0–5 cm (low) | 1 | Ref | ||
| 6–10 cm (mid) | 2.590 | 0.403 | 16.647 | 0.316 |
| 11–15 cm (high) | 14.534 | 1.456 | 145.088 | 0.023 |
| CRT GROUP | ||||
| 95% CI | ||||
| PARAMETER B | CR, OR D | LOWER | UPPER | P-VALUE |
| cT STAGE | ||||
| cT1-3a | 1 | Ref | ||
| cT3b-4b | 0.106 | 0.019 | 0.588 | 0.010 |
| ScRT+CT GROUP | ||||
| 95% CI | ||||
| PARAMETER C | CR, OR D | LOWER | UPPER | P-VALUE |
| TUMOR SIZE | ||||
| <3 cm | 1 | Ref | ||
| 3–5 cm | 0.157 | 0.023 | 1.065 | 0.058 |
| >5 cm | 0.097 | 0.014 | 0.663 | 0.017 |
| CEA VALUE | ||||
| CEA ≤ 3.8 μg/L | 1 | Ref | ||
| CEA > 3.8 μg/L | 0.392 | 0.166 | 0.924 | 0.032 |
| ENTIRE STUDY COHORT | ||||
| 95% CI | ||||
| PARAMETER D | CR, OR D | LOWER | UPPER | P-VALUE |
| STUDY TREATMENT | ||||
| scRT | 1 | Ref | ||
| CRT | 7.680 | 2.503 | 23.567 | <0.001 |
| ScRT+CT | 12.235 | 4.248 | 35.239 | <0.001 |
| TUMOR SIZE | ||||
| <3 cm | 1 | Ref | ||
| 3–5 cm | 0.209 | 0.063 | 0.690 | 0.010 |
| >5 cm | 0.134 | 0.039 | 0.462 | 0.001 |
| CEA VALUE | ||||
| CEA ≤ 3.8μg/L | 1 | Ref | ||
| CEA > 3.8 μg/L | 0.506 | 0.264 | 0.966 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammarström, K.; Imam, I.; Mezheyeuski, A.; Ekström, J.; Sjöblom, T.; Glimelius, B. A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer. Cancers 2021, 13, 16. https://doi.org/10.3390/cancers13010016
Hammarström K, Imam I, Mezheyeuski A, Ekström J, Sjöblom T, Glimelius B. A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer. Cancers. 2021; 13(1):16. https://doi.org/10.3390/cancers13010016
Chicago/Turabian StyleHammarström, Klara, Israa Imam, Artur Mezheyeuski, Joakim Ekström, Tobias Sjöblom, and Bengt Glimelius. 2021. "A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer" Cancers 13, no. 1: 16. https://doi.org/10.3390/cancers13010016
APA StyleHammarström, K., Imam, I., Mezheyeuski, A., Ekström, J., Sjöblom, T., & Glimelius, B. (2021). A Comprehensive Evaluation of Associations Between Routinely Collected Staging Information and The Response to (Chemo)Radiotherapy in Rectal Cancer. Cancers, 13(1), 16. https://doi.org/10.3390/cancers13010016






