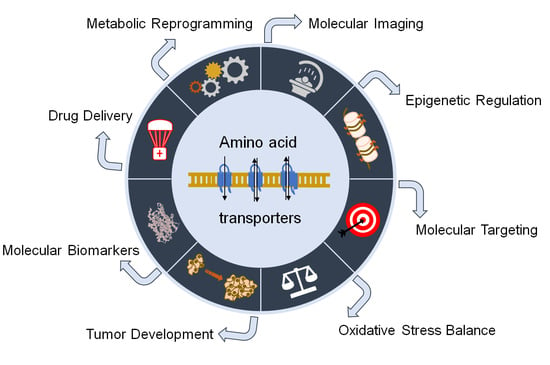
Amino Acid Transporters on the Guard of Cell Genome and Epigenome
Simple Summary
Abstract
1. Introduction
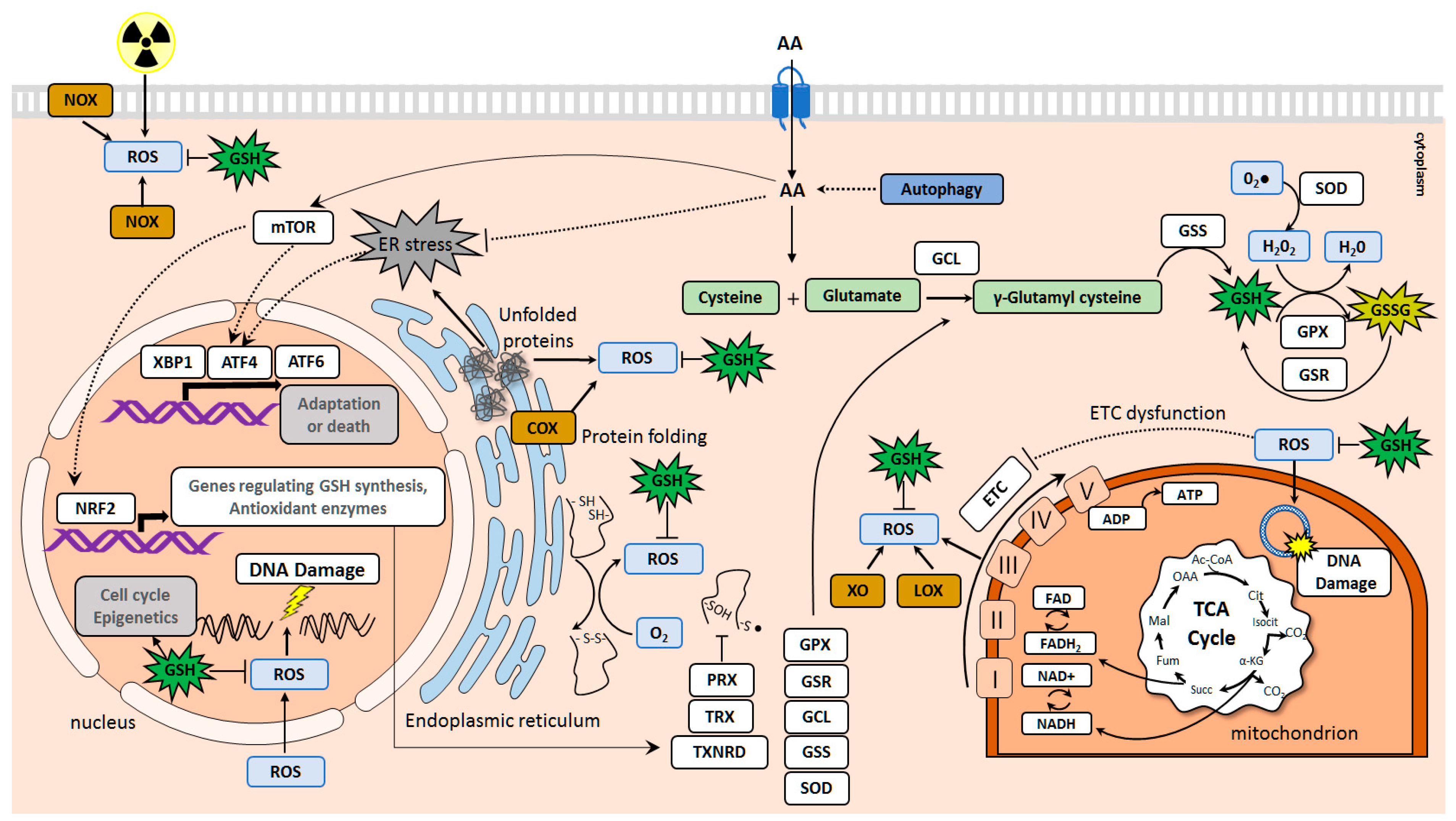
2. Amino Acid Transporters Upregulated in Tumors and Their Role in Cell Protection against Oxidative Damage and DNA Injury
2.1. SLC1 Family of Amino Acid Transporters
2.2. SLC3 and SLC7 Families of Heteromeric Amino Acid Transporters
2.3. SLC38 Family of Sodium-Dependent Neutral Amino Acid Transporters
2.4. SLC25 Family of Mitochondrial Transporters
3. The Role of Amino Acid Transporters in the Epigenetic Regulation
3.1. DNA and Histone Methylation and Demethylation Processes
3.2. Histone Acetylation and Deacetylation Mechanisms
3.3. Other Chromatin Modifications and Epigenetic Regulations
4. Amino Acid Transporters as Targets for Tumor Treatment
4.1. Targeting SLC1 Transporter Family
4.2. Targeting SLC7 Transporter Family
5. Molecular Imaging by Using Amino Acid Transporters
5.1. SLC1A5/ASCT2-Dependent Tumor Imaging
5.2. SLC3 and SLC7-Dependent Tumor Imaging
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 4EBP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| 5mC | 5-methylcytosine |
| 18F-Ala-BF3 | 18F labeled alanine trifluoroborate |
| 18F-FBPA | 18F-fluoro-phenylalaine |
| 18F-FDG PET | (18F)-Fluorodeoxyglucose Positron Emission Tomography |
| αKG | α-ketoglutarate |
| AA | Amino acid |
| AAA | Aromatic amino acid |
| AABA | 2-amino-4-bis(aryloxybenzyl)aminobutanoic acid |
| AARE | Amino acid response element |
| Acetyl-CoA | Acetyl-Coenzyme A |
| Ac | Acetylation |
| AKT | AKT Serine/Threonine Kinase |
| ALDH | Aldehyde dehydrogenase |
| ALL | Acute lymphoblastic leukemia |
| AMPK | AMP-activated protein kinase |
| AR | Androgen Receptor |
| ARE | Antioxidant response element |
| Arg | Arginine |
| ASCT2/ SLC1A5 | Alanine, serine, cysteine-preferring transporter 2 |
| ASNase | Asparaginase |
| ASNS | asparagine synthase |
| Asp | Aspartate |
| ATC | Anaplastic thyroid cancer |
| ATF4 | Activating Transcription Factor 4 |
| ATF6 | Activating Transcription Factor 6 |
| ATG5 | Autophagy Related 5 |
| BCAA | Branched-chain amino acid |
| BenSer | Benzylserine |
| BSO | Buthionine sulphoximine |
| BTA | 1,2,3-benzene-tricarboxylic acid |
| CAT | Catalase |
| CD44 | Cluster of differentiation 44 |
| CD44v | Cluster of differentiation 44 variant |
| CDK1 | Cycling-Dependent Kinase 1 |
| CDK7 | Cyclin-Dependent Kinase 7 |
| CIC | Citrate carrier |
| circRNA | Circular RNA |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CSC | Cancer stem cells |
| Cys | Cysteine |
| δT | Delta-tocotrienol |
| D-loop | Displacement loop |
| DNA | Deoxyribonucleic Acid |
| DNMT | DNA Methyltransferase |
| EAA | Essential amino acid |
| EAAC1 | Excitatory Amino Acid Carrier 1 |
| EGFR | Epidermal Growth Factor Receptor |
| eIF2α | Eukaryotic Translation Initiation Factor 2A |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| Erα | Estrogen receptor alpha |
| ETC | Electron transport chain |
| EZH2 | Enhancer of zeste homolog 2 |
| GCL | Glutamate-cysteine ligase |
| GCN2 | General amino acid control non-derepressible 2 |
| Gln | Glutamine |
| GLN-CD | Glutamine-β-cyclodextrin |
| GSL | Glutaminase |
| Gly | Glycine |
| GPNA | l-g-glutamyl-p-nitroanilide |
| GPXs | Glutathione Peroxidases |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GSS | Glutathione synthetase |
| GSSG | Glutathione disulfide |
| H2O2 | Hydrogen Peroxide |
| HAT | Histone Acetyltransferase |
| HATC | Heterodimeric transmembrane amino acid transporter complex |
| HCC | Hepatocellular Carcinoma |
| HDAC | Histone Deacetylase |
| HIF1α | Hypoxia Inducible Factor 1 Subunit Alpha |
| HIF2α | Hypoxia Inducible Factor 2 Subunit Alpha |
| His | Histidine |
| HMT | Histone Methyltransferase |
| HNSCC | Head and neck squamous cell carcinoma |
| Ile | Isoleucine |
| IMCA | 2-imino-6-methoxy-2H-chromene-3-carbothioamide |
| IR | Ionizing radiation |
| ISR | Integrated Stress Response |
| JDP2 | Jun Dimerization Protein 2 |
| KDM3B | Lysine Demethylase 3B |
| KO | Knockout |
| KRAS | KRAS Proto-Oncogene, GTPase |
| LAT1/SLC7A5 | L-type Amino Acid Transporter 1 |
| Leu | Leucine |
| lncRNA-XIST | Long Non-Coding RNA (lncRNA) X-Inactive Specific Transcript (XIST) |
| LOX | Lipoxygenase |
| LUAD | Lung adenocarcinoma |
| Lys | Lysine |
| mAB | monoclonal antibody |
| MAPK | Mitogen-activated protein kinase |
| MAT | Methionine Adenosyltransferase |
| MDR1 | Multidrug resistance protein 1 |
| Met | Methionine |
| miRNA | MicroRNA |
| MS | Methionine Synthase |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| mTORC2 | Mammalian target of rapamycin complex 2 |
| NAC | N-acetyl-l-Cysteine |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NF-kB | Nuclear Factor kappa B |
| NOX | NADPH Oxidase |
| Nrf2 | Nuclear factor-erythroid 2 -related factor 2 |
| OXPHOS | Oxidative Phosphorylation |
| OXY | Oxyfedrine |
| PEL | Primary Effusion Lymphoma |
| P-gp | P-glycoprotein |
| p70S6k | The 70-kDa ribosomal protein S6 kinase |
| PCa | Prostate Cancer |
| PDH | Pyruvate dehydogenase |
| PDX | Patient-derived xenograft |
| PET | Positron emission tomography |
| PGS | Polyglutamine |
| PP2Ac | Protein Phosphatase 2 Catalytic Subunit Alpha |
| PPARδ | Peroxisome Proliferator Activated Receptor Delta |
| PPM1F | Protein phosphatase, Mg2+/Mn2+ dependent 1F |
| Pro | Proline |
| PRX | Peroxiredoxin |
| RB | Retinoblastoma protein |
| RNA | Ribonucleic acid |
| RCC | Renal Cell Carcinoma |
| RONS | Reactive oxygen and nitrogen species |
| ROS | Reactive oxygen species |
| S6K1 | The mTOR Substrate S6 Kinase 1 |
| SAM | S-Adenosylmethionine |
| Ser26 | Serine26 |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| SLC | Solute carrier |
| SLC1A1 | Solute Carrier Family 1 Member 1 |
| SLC1A2 | Solute Carrier Family 1 Member 2 |
| SLC1A3 | Solute Carrier Family 1 Member 3 |
| SLC1A4 | Solute Carrier Family 1 Member 4 |
| SLC1A5 | Solute Carrier Family 1 Member 5 |
| SLC1A6 | Solute Carrier Family 1 Member 6 |
| SLC1A7 | Solute Carrier Family 1 Member 7 |
| SLC25A1 | Solute Carrier Family 25 Member 1 |
| SLC25A4 | Solute Carrier Family 25 Member 4 |
| SLC25A5 | Solute Carrier Family 25 Member 5 |
| SLC25A8 | Solute Carrier Family 25 Member 8 |
| SLC25A10 | Solute Carrier Family 25 Member 10 |
| SLC25A23 | Solute Carrier Family 25 Member 23 |
| SLC25A26 | Solute Carrier Family 25 Member 26 |
| SLC25A32 | Solute Carrier Family 25 Member 32 |
| SLC38A1 | Solute Carrier Family 38 Member 1 |
| SLC38A2 | Solute Carrier Family 38 Member 2 |
| SLC38A3 | Solute Carrier Family 38 Member 3 |
| SLC3A1 | Solute Carrier Family 3 Member 1 |
| SLC3A2 | Solute Carrier Family 3 Member 2 |
| SLC6A1 | Solute Carrier Family 6 Member 1 |
| SLC6A14 | Solute Carrier Family 6 Member 14 |
| SLC7A1 | Solute Carrier Family 7 Member 1 |
| SLC7A2 | Solute Carrier Family 7 Member 2 |
| SLC7A3 | Solute Carrier Family 7 Member 3 |
| SLC7A4 | Solute Carrier Family 7 Member 4 |
| SLC7A5 | Solute Carrier Family 7 Member 5 |
| SLC7A6 | Solute Carrier Family 7 Member 6 |
| SLC7A7 | Solute Carrier Family 7 Member 7 |
| SLC7A8 | Solute Carrier Family 7 Member 8 |
| SLC7A9 | Solute Carrier Family 7 Member 9 |
| SLC7A10 | Solute Carrier Family 7 Member 10 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| SLC7A12 | Solute Carrier Family 7 Member 12 |
| SLC7A13 | Solute Carrier Family 7 Member 13 |
| SLC7A14 | Solute Carrier Family 7 Member 14 |
| SLC7A15 | Solute Carrier Family 7 Member 15 |
| SNAT1/ SLC38A1 | Sodium-coupled neutral amino acid transporter 1 |
| SNAT2/SLC38A2 | Sodium-coupled neutral amino acid transporter 2 |
| SOD | superoxide dismutase |
| SorCS2 | Sortilin Related VPS10 Domain Containing Receptor 2 |
| SSZ | Sulfasalazine |
| T3 | Triiodothyronine |
| TCA cycle | Tricarboxylic acid cycle |
| TET | Ten-Eleven Translocation |
| TMZ | Temozolomide |
| TNBC | Triple-negative breast cancer |
| Trp | Tryptophan |
| TRX | Thioredoxin |
| TXN2 | Thioredoxin 2 |
| TXNRD | Thioredoxin Reductase |
| UBE2C | Ubiquitin Conjugating Enzyme E2 C |
| UPR | Unfolded protein response |
| UPS | Ubiquitin-Proteasome System |
| Val | Valine |
| VPS10 | Vacuolar protein sorting 10 |
| VSMC | Vascular smooth muscle cells |
| WT | Wild type |
| XO | Xanthine oxidase |
References
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Coller, H.A. Is cancer a metabolic disease? Am. J. Pathol. 2014, 184, 4–17. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H. Nutrition needs of mammalian cells in tissue culture. Science 1955, 122, 501–514. [Google Scholar] [CrossRef]
- Kekuda, R.; Prasad, P.D.; Fei, Y.J.; Torres-Zamorano, V.; Sinha, S.; Yang-Feng, T.L.; Leibach, F.H.; Ganapathy, V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J. Biol. Chem. 1996, 271, 18657–18661. [Google Scholar] [CrossRef]
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef]
- Peitzsch, C.; Gorodetska, I.; Klusa, D.; Shi, Q.; Alves, T.C.; Pantel, K.; Dubrovska, A. Metabolic regulation of prostate cancer heterogeneity and plasticity. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Nunes, S.C.; Serpa, J. Glutathione in Ovarian Cancer: A Double-Edged Sword. Int. J. Mol. Sci. 2018, 19, 1182. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Manig, F.; Lehmann, L.; Sorour, N.; Lock, S.; Yu, Z.; Dubrovska, A.; Baumann, M.; Kessler, B.M.; Stasyk, O.; et al. Dual role of ER stress in response to metabolic co-targeting and radiosensitivity in head and neck cancer cells. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. mTOR-Mediated Antioxidant Activation in Solid Tumor Radioresistance. J. Oncol. 2019, 2019, 5956867. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Clemencon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Cormerais, Y.V.; Vučetić, M.; Parks, S.K.; Pouyssegur, J. Amino Acid Transporters Are a Vital Focal Point in the Control of mTORC1 Signaling and Cancer. Int. J. Mol. Sci. 2021, 22, 23. [Google Scholar] [CrossRef]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.; Shuai, Y.; Huang, Y.; Jin, R.; Wang, X.; Luo, J. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 122, 82–93. [Google Scholar] [CrossRef]
- Malik, A.R.; Szydlowska, K.; Nizinska, K.; Asaro, A.; van Vliet, E.A.; Popp, O.; Dittmar, G.; Fritsche-Guenther, R.; Kirwan, J.A.; Nykjaer, A.; et al. SorCS2 Controls Functional Expression of Amino Acid Transporter EAAT3 and Protects Neurons from Oxidative Stress and Epilepsy-Induced Pathology. Cell Rep. 2019, 26, 2792–2804.e6. [Google Scholar] [CrossRef]
- Aoyama, K.; Suh, S.W.; Hamby, A.M.; Liu, J.; Chan, W.Y.; Chen, Y.; Swanson, R.A. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat. Neurosci. 2006, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, K.; Sun, B.; Xu, D.; Tong, L.; Yin, H.; Liao, Y.; Song, H.; Wang, T.; Jing, B.; et al. Dysregulated Glutamate Transporter SLC1A1 Propels Cystine Uptake via Xc- for Glutathione Synthesis in Lung Cancer. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Almilaji, A.; Pakladok, T.; Guo, A.; Munoz, C.; Foller, M.; Lang, F. Regulation of the glutamate transporter EAAT3 by mammalian target of rapamycin mTOR. Biochem. Biophys. Res. Commun. 2012, 421, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Fairweather, S.; Broer, S. Disruption of Amino Acid Homeostasis by Novel ASCT2 Inhibitors Involves Multiple Targets. Front. Pharmacol. 2018, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hardie, R.A.; Hoy, A.J.; van Geldermalsen, M.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Ren, P.; Yue, M.; Xiao, D.; Xiu, R.; Gan, L.; Liu, H.; Qing, G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J. Pathol. 2015, 235, 90–100. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Lane, A.N.; Robertson, B.; Kemp, S.; Liu, Y.; Hill, B.G.; Dean, D.C.; Clem, B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2014, 33, 556–566. [Google Scholar] [CrossRef]
- Wang, Q.; Tiffen, J.; Bailey, C.G.; Lehman, M.L.; Ritchie, W.; Fazli, L.; Metierre, C.; Feng, Y.J.; Li, E.; Gleave, M.; et al. Targeting amino acid transport in metastatic castration-resistant prostate cancer: Effects on cell cycle, cell growth, and tumor development. J. Natl. Cancer Inst. 2013, 105, 1463–1473. [Google Scholar] [CrossRef]
- Qi, J.; Tripathi, M.; Mishra, R.; Sahgal, N.; Fazli, L.; Ettinger, S.; Placzek, W.J.; Claps, G.; Chung, L.W.; Bowtell, D.; et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 2013, 23, 332–346. [Google Scholar] [CrossRef]
- Broer, A.; Rahimi, F.; Broer, S. Deletion of Amino Acid Transporter ASCT2 (SLC1A5) Reveals an Essential Role for Transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to Sustain Glutaminolysis in Cancer Cells. J. Biol. Chem. 2016, 291, 13194–13205. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Gauthier-Coles, G.; Rahimi, F.; van Geldermalsen, M.; Dorsch, D.; Wegener, A.; Holst, J.; Broer, S. Ablation of the ASCT2 (SLC1A5) gene encoding a neutral amino acid transporter reveals transporter plasticity and redundancy in cancer cells. J. Biol. Chem. 2019, 294, 4012–4026. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Park, S.J.; Nam, M.; Kang, J.; Kim, K.; Yeo, J.H.; Kim, J.K.; Heo, Y.; Lee, H.S.; Lee, M.Y.; et al. A Variant of SLC1A5 Is a Mitochondrial Glutamine Transporter for Metabolic Reprogramming in Cancer Cells. Cell Metab. 2020, 31, 267–283.e212. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lv, B.; Zhang, X. Knock-down of LncRNA-XIST induced glioma cell death and inhibited tumorigenesis by regulating miR-137/SLC1A5 axis-mediated ROS production. Naunyn Schmiedebergs Arch. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Wu, L.; Zhang, K.; Wang, H.; Zhang, T.; Gutierrez, L.; O’Connell, D.; Zhang, P.; Li, Y.; Gao, T.; et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018, 25, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Kanai, Y.; Palacin, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, Y.; Wang, Y.; Li, W.; Liu, X.; Lv, Y.; Li, X.; Mi, J. Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics 2017, 7, 1036–1046. [Google Scholar] [CrossRef]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Lock, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.-I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 Heavy Chain Is a Marker and Regulator of Head and Neck Squamous Cell Carcinoma Radiosensitivity. Clin. Cancer Res. 2019, 25, 3152–3163. [Google Scholar] [CrossRef]
- Chiduza, G.N.; Johnson, R.M.; Wright, G.S.A.; Antonyuk, S.V.; Muench, S.P.; Hasnain, S.S. LAT1 (SLC7A5) and CD98hc (SLC3A2) complex dynamics revealed by single-particle cryo-EM. Acta Crystallogr. D Struct. Biol. 2019, 75, 660–669. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 1675613. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Albuquerque, C.P.; Braas, D.; Zhang, W.; Villa, G.R.; Bi, J.; Ikegami, S.; Masui, K.; Gini, B.; Yang, H.; et al. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Mol. Cell 2017, 67, 128–138.e127. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Engevik, A.C.; Willet, S.G.; Williams, J.A.; Zou, Y.; Massion, P.P.; Mills, J.C.; Choi, E.; Goldenring, J.R. Cystine/Glutamate Antiporter (xCT) Is Required for Chief Cell Plasticity After Gastric Injury. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Goji, T.; Takahara, K.; Negishi, M.; Katoh, H. Cystine uptake through the cystine/glutamate antiporter xCT triggers glioblastoma cell death under glucose deprivation. J. Biol. Chem. 2017, 292, 19721–19732. [Google Scholar] [CrossRef]
- Cano-Crespo, S.; Chillaron, J.; Junza, A.; Fernandez-Miranda, G.; Garcia, J.; Polte, C.; Laura, R.; Ignatova, Z.; Yanes, Ó.; Zorzano, A.; et al. CD98hc (SLC3A2) sustains amino acid and nucleotide availability for cell cycle progression. Sci. Rep. 2019, 9, 14065. [Google Scholar] [CrossRef]
- De la Ballina, L.R.; Cano-Crespo, S.; Gonzalez-Munoz, E.; Bial, S.; Estrach, S.; Cailleteau, L.; Tissot, F.; Daniel, H.; Zorzano, A.; Ginsberg, M.H.; et al. Amino Acid Transport Associated to Cluster of Differentiation 98 Heavy Chain (CD98hc) Is at the Cross-road of Oxidative Stress and Amino Acid Availability. J. Biol. Chem. 2016, 291, 9700–9711. [Google Scholar] [CrossRef]
- Digomann, D.; Linge, A.; Dubrovska, A. SLC3A2/CD98hc, autophagy and tumor radioresistance: A link confirmed. Autophagy 2019, 15, 1850–1851. [Google Scholar] [CrossRef]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Cormerais, Y.; Giuliano, S.; LeFloch, R.; Front, B.; Durivault, J.; Tambutte, E.; Massard, P.A.; de la Ballina, L.R.; Endou, H.; Wempe, M.F.; et al. Genetic Disruption of the Multifunctional CD98/LAT1 Complex Demonstrates the Key Role of Essential Amino Acid Transport in the Control of mTORC1 and Tumor Growth. Cancer Res. 2016, 76, 4481–4492. [Google Scholar] [CrossRef]
- Torrents, D.; Estevez, R.; Pineda, M.; Fernandez, E.; Lloberas, J.; Shi, Y.B.; Zorzano, A.; Palacin, M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J. Biol. Chem. 1998, 273, 32437–32445. [Google Scholar] [CrossRef]
- Averous, J.; Lambert-Langlais, S.; Mesclon, F.; Carraro, V.; Parry, L.; Jousse, C.; Bruhat, A.; Maurin, A.C.; Pierre, P.; Proud, C.G.; et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci. Rep. 2016, 6, 27698. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Ye, P.; Mimura, J.; Okada, T.; Sato, H.; Liu, T.; Maruyama, A.; Ohyama, C.; Itoh, K. Nrf2- and ATF4-dependent upregulation of xCT modulates the sensitivity of T24 bladder carcinoma cells to proteasome inhibition. Mol. Cell. Biol. 2014, 34, 3421–3434. [Google Scholar] [CrossRef]
- Lim, J.K.M.; Delaidelli, A.; Minaker, S.W.; Zhang, H.F.; Colovic, M.; Yang, H.; Negri, G.L.; von Karstedt, S.; Lockwood, W.W.; Schaffer, P.; et al. Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. Proc. Natl. Acad. Sci. USA 2019, 116, 9433–9442. [Google Scholar] [CrossRef]
- Park, Y.; Reyna-Neyra, A.; Philippe, L.; Thoreen, C.C. mTORC1 Balances Cellular Amino Acid Supply with Demand for Protein Synthesis through Post-transcriptional Control of ATF4. Cell Rep. 2017, 19, 1083–1090. [Google Scholar] [CrossRef]
- Enyedi, B.; Varnai, P.; Geiszt, M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid. Redox Signal. 2010, 13, 721–729. [Google Scholar] [CrossRef]
- Cuozzo, J.W.; Kaiser, C.A. Competition between glutathione and protein thiols for disulphide-bond formation. Nat. Cell Biol. 1999, 1, 130–135. [Google Scholar] [CrossRef]
- Haynes, C.M.; Titus, E.A.; Cooper, A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell 2004, 15, 767–776. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, C.; Zhang, Z.; Gao, X.; Jia, Z.; Chen, H.; Jia, Q.; Zhao, X.; Liu, J.; et al. SLC3A2 is a novel endoplasmic reticulum stress-related signaling protein that regulates the unfolded protein response and apoptosis. PLoS ONE 2018, 13, e0208993. [Google Scholar] [CrossRef]
- Li, W.; Zhu, J.; Dou, J.; She, H.; Tao, K.; Xu, H.; Yang, Q.; Mao, Z. Phosphorylation of LAMP2A by p38 MAPK couples ER stress to chaperone-mediated autophagy. Nat. Commun. 2017, 8, 1763. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, L.; Gong, J.; Shi, C.; Wang, Z.; Ye, B.; Xuan, A.; He, X.; Long, D.; Zhu, X.; et al. NF-kappaB pathway link with ER stress-induced autophagy and apoptosis in cervical tumor cells. Cell Death Discov. 2017, 3, 17059. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Zhao, R.; Yan, F.; Ma, Y.; Zhao, L.; Qiao, H. xCT deficiency induces autophagy via endoplasmic reticulum stress activated p38-mitogen-activated protein kinase and mTOR in sut melanocytes. Eur. J. Cell Biol. 2016, 95, 175–181. [Google Scholar] [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Sato, H.; Shiiya, A.; Kimata, M.; Maebara, K.; Tamba, M.; Sakakura, Y.; Makino, N.; Sugiyama, F.; Yagami, K.; Moriguchi, T.; et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 2005, 280, 37423–37429. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.X.; Singh, G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef]
- Daher, B.; Parks, S.K.; Durivault, J.; Cormerais, Y.; Baidarjad, H.; Tambutte, E.; Pouyssegur, J.; Vucetic, M. Genetic Ablation of the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. Cancer Res. 2019, 79, 3877–3890. [Google Scholar] [CrossRef]
- Arensman, M.D.; Yang, X.S.; Leahy, D.M.; Toral-Barza, L.; Mileski, M.; Rosfjord, E.C.; Wang, F.; Deng, S.; Myers, J.S.; Abraham, R.T.; et al. Cystine-glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9533–9542. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, Y.; Zhang, Z.; Tan, N.; Zhao, F.; Ge, C.; Liang, L.; Jia, D.; Chen, T.; Yao, M.; et al. Disruption of xCT inhibits cell growth via the ROS/autophagy pathway in hepatocellular carcinoma. Cancer Lett. 2011, 312, 55–61. [Google Scholar] [CrossRef]
- Sugiyama, A.; Ohta, T.; Obata, M.; Takahashi, K.; Seino, M.; Nagase, S. xCT inhibitor sulfasalazine depletes paclitaxel-resistant tumor cells through ferroptosis in uterine serous carcinoma. Oncol. Lett. 2020, 20, 2689–2700. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Torrence, M.E.; MacArthur, M.R.; Hosios, A.M.; Valvezan, A.; Asara, J.M.; Mitchell, J.R.; Manning, B.D. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. bioRxiv 2020. [Google Scholar] [CrossRef]
- Quiros, P.M.; Mottis, A.; Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Biol. 2016, 17, 213–226. [Google Scholar] [CrossRef]
- Wang, S.F.; Chen, M.S.; Chou, Y.C.; Ueng, Y.F.; Yin, P.H.; Yeh, T.S.; Lee, H.C. Mitochondrial dysfunction enhances cisplatin resistance in human gastric cancer cells via the ROS-activated GCN2-eIF2alpha-ATF4-xCT pathway. Oncotarget 2016, 7, 74132–74151. [Google Scholar] [CrossRef]
- Sehm, T.; Rauh, M.; Wiendieck, K.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N.E. Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget 2016, 7, 74630–74647. [Google Scholar] [CrossRef]
- Cobler, L.; Zhang, H.; Suri, P.; Park, C.; Timmerman, L.A. xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget 2018, 9, 32280–32297. [Google Scholar] [CrossRef]
- Cojoc, M.; Mabert, K.; Muders, M.H.; Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin. Cancer Biol. 2015, 31, 16–27. [Google Scholar] [CrossRef]
- Peitzsch, C.; Nathansen, J.; Schniewind, S.I.; Schwarz, F.; Dubrovska, A. Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers 2019, 11, 616. [Google Scholar] [CrossRef]
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862. [Google Scholar] [CrossRef]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Ju, H.Q.; Lu, Y.X.; Chen, D.L.; Tian, T.; Mo, H.Y.; Wei, X.L.; Liao, J.W.; Wang, F.; Zeng, Z.L.; Pelicano, H.; et al. Redox Regulation of Stem-like Cells Though the CD44v-xCT Axis in Colorectal Cancer: Mechanisms and Therapeutic Implications. Theranostics 2016, 6, 1160–1175. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Nagano, O.; Okazaki, S.; Saya, H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene 2013, 32, 5191–5198. [Google Scholar] [CrossRef]
- Wu, H.M.; Chi, K.H.; Lin, W.W. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett. 2002, 526, 101–105. [Google Scholar] [CrossRef]
- Paniagua Soriano, G.; De Bruin, G.; Overkleeft, H.S.; Florea, B.I. Toward understanding induction of oxidative stress and apoptosis by proteasome inhibitors. Antioxid. Redox Signal. 2014, 21, 2419–2443. [Google Scholar] [CrossRef]
- Yoo, Y.D.; Lee, D.H.; Cha-Molstad, H.; Kim, H.; Mun, S.R.; Ji, C.; Park, S.H.; Sung, K.S.; Choi, S.A.; Hwang, J.; et al. Glioma-derived cancer stem cells are hypersensitive to proteasomal inhibition. EMBO Rep. 2017, 18, 150–168. [Google Scholar] [CrossRef]
- Monticone, M.; Biollo, E.; Fabiano, A.; Fabbi, M.; Daga, A.; Romeo, F.; Maffei, M.; Melotti, A.; Giaretti, W.; Corte, G.; et al. z-Leucinyl-leucinyl-norleucinal induces apoptosis of human glioblastoma tumor-initiating cells by proteasome inhibition and mitotic arrest response. Mol. Cancer Res. 2009, 7, 1822–1834. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Ge, C.; Cao, B.; Feng, D.; Zhou, F.; Zhang, J.; Yang, N.; Feng, S.; Wang, G.; Aa, J. The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci. Rep. 2017, 7, 3791. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflug. Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Stretton, C.; Lipina, C.; Hyde, R.; Cwiklinski, E.; Hoffmann, T.M.; Taylor, P.M.; Hundal, H.S. CDK7 is a component of the integrated stress response regulating SNAT2 (SLC38A2)/System A adaptation in response to cellular amino acid deprivation. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Bridges, E.; Valli, A.; Choudhry, H.; Sheldon, H.; Wigfield, S.; Gray, N.; Zois, C.E.; Grimm, F.; Jones, D.; et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 12452–12461. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van ‘t Veer, L.J.; et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Roderick, H.L.; Camacho, P.; Jiang, J.X. Identification and characterization of an amino acid transporter expressed differentially in liver. Proc. Natl. Acad. Sci. USA 2000, 97, 3230–3235. [Google Scholar] [CrossRef]
- Lister, A.; Bourgeois, S.; Imenez Silva, P.H.; Rubio-Aliaga, I.; Marbet, P.; Walsh, J.; Shelton, L.M.; Keller, B.; Verrey, F.; Devuyst, O.; et al. NRF2 regulates the glutamine transporter Slc38a3 (SNAT3) in kidney in response to metabolic acidosis. Sci. Rep. 2018, 8, 5629. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; King, M.S.; Ruprecht, J.J.; Thangaratnarajah, C. The SLC25 Carrier Family: Important Transport Proteins in Mitochondrial Physiology and Pathology. Physiology 2020, 35, 302–327. [Google Scholar] [CrossRef]
- Zhou, X.; Paredes, J.A.; Krishnan, S.; Curbo, S.; Karlsson, A. The mitochondrial carrier SLC25A10 regulates cancer cell growth. Oncotarget 2015, 6, 9271–9283. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Bijian, K.; Qiu, D.; da Silva, S.D.; Marques, M.; Chang, C.H.; Nassour, H.; Ramotar, D.; Damaraju, S.; Mackey, J.; et al. Insights into a novel nuclear function for Fascin in the regulation of the amino-acid transporter SLC3A2. Sci. Rep. 2016, 6, 36699. [Google Scholar] [CrossRef]
- Menga, A.; Palmieri, E.M.; Cianciulli, A.; Infantino, V.; Mazzone, M.; Scilimati, A.; Palmieri, F.; Castegna, A.; Iacobazzi, V. SLC25A26 overexpression impairs cell function via mtDNA hypermethylation and rewiring of methyl metabolism. FEBS J. 2017, 284, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Dituri, F.; Convertini, P.; Santarsiero, A.; Palmieri, F.; Todisco, S.; Mancarella, S.; Giannelli, G.; Iacobazzi, V. Epigenetic upregulation and functional role of the mitochondrial aspartate/glutamate carrier isoform 1 in hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Carrer, A.; Shah, S.; Snyder, N.W.; Wei, S.; Venneti, S.; Worth, A.J.; Yuan, Z.F.; Lim, H.W.; Liu, S.; et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014, 20, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.M.; Griffiths, A.D.; Tawfik, D.S.; Loakes, D. Determinants of cofactor binding to DNA methyltransferases: Insights from a systematic series of structural variants of S-adenosylhomocysteine. Org. Biomol. Chem. 2005, 3, 152–161. [Google Scholar] [CrossRef]
- Pritchard, J.B. Intracellular alpha-ketoglutarate controls the efficacy of renal organic anion transport. J. Pharmacol. Exp. Ther. 1995, 274, 1278–1284. [Google Scholar]
- Mentch, S.J.; Mehrmohamadi, M.; Huang, L.; Liu, X.; Gupta, D.; Mattocks, D.; Gomez Padilla, P.; Ables, G.; Bamman, M.M.; Thalacker-Mercer, A.E.; et al. Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab. 2015, 22, 861–873. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef]
- Mastroberardino, L.; Spindler, B.; Pfeiffer, R.; Skelly, P.J.; Loffing, J.; Shoemaker, C.B.; Verrey, F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998, 395, 288–291. [Google Scholar] [CrossRef]
- Kaira, K.; Sunose, Y.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; Itoh, H.; Nagamori, S.; et al. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 2012, 107, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Furuya, M.; Horiguchi, J.; Nakajima, H.; Kanai, Y.; Oyama, T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012, 103, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, T.; Shimizu, K.; Kaira, K.; Nagashima, T.; Ohtaki, Y.; Atsumi, J.; Obayashi, K.; Nagamori, S.; Kanai, Y.; Oyama, T.; et al. Clinical significance of coexpression of L-type amino acid transporter 1 (LAT1) and ASC amino acid transporter 2 (ASCT2) in lung adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1126–1139. [Google Scholar] [PubMed]
- Xu, M.; Sakamoto, S.; Matsushima, J.; Kimura, T.; Ueda, T.; Mizokami, A.; Kanai, Y.; Ichikawa, T. Up-Regulation of LAT1 during Antiandrogen Therapy Contributes to Progression in Prostate Cancer Cells. J. Urol. 2016, 195, 1588–1597. [Google Scholar] [CrossRef]
- Honjo, H.; Kaira, K.; Miyazaki, T.; Yokobori, T.; Kanai, Y.; Nagamori, S.; Oyama, T.; Asao, T.; Kuwano, H. Clinicopathological significance of LAT1 and ASCT2 in patients with surgically resected esophageal squamous cell carcinoma. J. Surg. Oncol. 2016, 113, 381–389. [Google Scholar] [CrossRef]
- Dann, S.G.; Ryskin, M.; Barsotti, A.M.; Golas, J.; Shi, C.; Miranda, M.; Hosselet, C.; Lemon, L.; Lucas, J.; Karnoub, M.; et al. Reciprocal regulation of amino acid import and epigenetic state through Lat1 and EZH2. EMBO J. 2015, 34, 1773–1785. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Howden, A.J.; Brenes, A.; Spinelli, L.; Hukelmann, J.L.; Macintyre, A.N.; Liu, X.; Thomson, S.; Taylor, P.M.; Rathmell, J.C.; et al. Antigen receptor control of methionine metabolism in T cells. Elife 2019, 8. [Google Scholar] [CrossRef]
- Hodgson, N.; Trivedi, M.; Muratore, C.; Li, S.; Deth, R. Soluble oligomers of amyloid-beta cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 2013, 36, 197–209. [Google Scholar] [CrossRef]
- Campbell, B.; Roberts, M.; Kerksick, C.; Wilborn, C.; Marcello, B.; Taylor, L.; Nassar, E.; Leutholtz, B.; Bowden, R.; Rasmussen, C.; et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine alpha-ketoglutarate in trained adult men. Nutrition 2006, 22, 872–881. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Milandu, Y.; Auger, C.; Bignucolo, A.; Appanna, V.P.; Appanna, V.D. Histidine is a source of the antioxidant, alpha-ketoglutarate, in Pseudomonas fluorescens challenged by oxidative stress. FEMS Microbiol. Lett. 2010, 309, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.J.; Robinson, I.M. Biosynthesis of alpha-ketoglutarate by the reductive carboxylation of succinate in Bacteroides ruminicola. J. Bacteriol. 1970, 104, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, M.E.; Abdel-Wahab, O.; Lu, C.; Ward, P.S.; Patel, J.; Shih, A.; Li, Y.; Bhagwat, N.; Vasanthakumar, A.; Fernandez, H.F.; et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010, 18, 553–567. [Google Scholar] [CrossRef]
- Borger, D.R.; Goyal, L.; Yau, T.; Poon, R.T.; Ancukiewicz, M.; Deshpande, V.; Christiani, D.C.; Liebman, H.M.; Yang, H.; Kim, H.; et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2014, 20, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Yang, X.J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Violante, S.; Ijlst, L.; Ruiter, J.; Koster, J.; van Lenthe, H.; Duran, M.; de Almeida, I.T.; Wanders, R.J.; Houten, S.M.; Ventura, F.V. Substrate specificity of human carnitine acetyltransferase: Implications for fatty acid and branched-chain amino acid metabolism. Biochim. Biophys. Acta 2013, 1832, 773–779. [Google Scholar] [CrossRef]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Liu, H.; Xing, R.; Cheng, X.; Li, Q.; Liu, F.; Ye, H.; Zhao, M.; Wang, H.; Wang, G.; Hao, H. De-novo NAD+ synthesis regulates SIRT1-FOXO1 apoptotic pathway in response to NQO1 substrates in lung cancer cells. Oncotarget 2016, 7, 62503–62519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, Y.; Dalmasso, G.; Sitaraman, S.; Merlin, D. Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G535–G545. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Dalmasso, G.; Yan, Y.; Laroui, H.; Dahan, S.; Mayer, L.; Sitaraman, S.V.; Merlin, D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J. Biol. Chem. 2010, 285, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Charania, M.A.; Ayyadurai, S.; Ingersoll, S.A.; Xiao, B.; Viennois, E.; Yan, Y.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Intestinal epithelial CD98 synthesis specifically modulates expression of colonic microRNAs during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1282–G1291. [Google Scholar] [CrossRef][Green Version]
- Amasheh, S.; Fromm, M.; Gunzel, D. Claudins of intestine and nephron—A correlation of molecular tight junction structure and barrier function. Acta Physiol. 2011, 201, 133–140. [Google Scholar] [CrossRef]
- Han, M.K.; Baker, M.; Zhang, Y.; Yang, C.; Zhang, M.; Garg, P.; Viennois, E.; Merlin, D. Overexpression of CD98 in intestinal epithelium dysregulates miRNAs and their targeted proteins along the ileal villus-crypt axis. Sci. Rep. 2018, 8, 16220. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Jin, M.; Hou, L.; Chen, X.; Zhang, R.; Zhang, J.; Zhu, J. CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Mol. Cancer 2018, 17, 165. [Google Scholar] [CrossRef]
- Garcia-Bermudez, J.; Williams, R.T.; Guarecuco, R.; Birsoy, K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020, 33, 67–82. [Google Scholar] [CrossRef]
- Tanabe, A.; Sahara, H. The Metabolic Heterogeneity and Flexibility of Cancer Stem Cells. Cancers 2020, 12, 2780. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, B.; Krakoff, I.; Burchenal, J.; Karnofsky, D.; Golbey, R.; Dowling, M.; Oettgen, H.; Lipton, A. Clinical results of treatment with E. coli L-asparaginase in adults with leukemia, lymphoma, and solid tumors. Cancer 1970, 25, 279–305. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar] [PubMed]
- Bhutia, Y.D.; Babu, E.; Ramachandran, S.; Ganapathy, V. Amino Acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015, 75, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, E.; Funicello, M.; Rauen, T.; Gobbi, M.; Mennini, T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008, 578, 171–176. [Google Scholar] [CrossRef]
- Carbone, M.; Duty, S.; Rattray, M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int. 2012, 60, 31–38. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Riluzole increases glutamate uptake by cultured C6 astroglial cells. Int. J. Dev. Neurosci. 2013, 31, 482–486. [Google Scholar] [CrossRef]
- El-Gebali, S.; Bentz, S.; Hediger, M.A.; Anderle, P. Solute carriers (SLCs) in cancer. Mol. Asp. Med. 2013, 34, 719–734. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Masuram, S.; Fredriksson, R.; Schioth, H.B. Solute carriers as drug targets: Current use, clinical trials and prospective. Mol. Asp. Med. 2013, 34, 702–710. [Google Scholar] [CrossRef]
- Johnston, R.A.; Rawling, T.; Chan, T.; Zhou, F.; Murray, M. Selective inhibition of human solute carrier transporters by multikinase inhibitors. Drug Metab. Dispos. 2014, 42, 1851–1857. [Google Scholar] [CrossRef]
- Wu, P.; Bjorn-Yoshimoto, W.E.; Staudt, M.; Jensen, A.A.; Bunch, L. Identification and Structure-Activity Relationship Study of Imidazo[1,2-a]pyridine-3-amines as First Selective Inhibitors of Excitatory Amino Acid Transporter Subtype 3 (EAAT3). ACS Chem. Neurosci. 2019, 10, 4414–4429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Shchepakin, D.; Kavanaugh, M.P.; Trauner, D. Photoswitchable Inhibitor of a Glutamate Transporter. ACS Chem. Neurosci. 2017, 8, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, Z.; Peng, J.; Li, Y.; Huang, H.; Liao, Y.; Zhou, M.; Sun, L.; Huang, N.; Shi, M.; et al. Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. Int. J. Cancer 2018, 142, 2578–2588. [Google Scholar] [CrossRef]
- Marshall, A.D.; van Geldermalsen, M.; Otte, N.J.; Lum, T.; Vellozzi, M.; Thoeng, A.; Pang, A.; Nagarajah, R.; Zhang, B.; Wang, Q.; et al. ASCT2 regulates glutamine uptake and cell growth in endometrial carcinoma. Oncogenesis 2017, 6, e367. [Google Scholar] [CrossRef]
- Wang, Q.; Beaumont, K.A.; Otte, N.J.; Font, J.; Bailey, C.G.; van Geldermalsen, M.; Sharp, D.M.; Tiffen, J.C.; Ryan, R.M.; Jormakka, M.; et al. Targeting glutamine transport to suppress melanoma cell growth. Int. J. Cancer 2014, 135, 1060–1071. [Google Scholar] [CrossRef]
- Schulte, M.L.; Khodadadi, A.B.; Cuthbertson, M.L.; Smith, J.A.; Manning, H.C. 2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids: A novel scaffold for inhibition of ASCT2-mediated glutamine transport. Bioorg. Med. Chem. Lett. 2016, 26, 1044–1047. [Google Scholar] [CrossRef]
- Kasai, N.; Sasakawa, A.; Hosomi, K.; Poh, T.W.; Chua, B.L.; Yong, W.P.; So, J.; Chan, S.L.; Soong, R.; Kono, K.; et al. Anti-tumor efficacy evaluation of a novel monoclonal antibody targeting neutral amino acid transporter ASCT2 using patient-derived xenograft mouse models of gastric cancer. Am. J. Transl. Res. 2017, 9, 3399–3410. [Google Scholar]
- Suzuki, M.; Toki, H.; Furuya, A.; Ando, H. Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem. Biophys. Res. Commun. 2017, 482, 651–657. [Google Scholar] [CrossRef]
- Hara, Y.; Minami, Y.; Yoshimoto, S.; Hayashi, N.; Yamasaki, A.; Ueda, S.; Masuko, K.; Masuko, T. Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Med. 2020, 9, 302–312. [Google Scholar] [CrossRef]
- Rajasinghe, L.D.; Hutchings, M.; Gupta, S.V. Delta-Tocotrienol Modulates Glutamine Dependence by Inhibiting ASCT2 and LAT1 Transporters in Non-Small Cell Lung Cancer (NSCLC) Cells: A Metabolomic Approach. Metabolites 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Quandt, G.; Hofner, G.; Pabel, J.; Dine, J.; Eder, M.; Wanner, K.T. First photoswitchable neurotransmitter transporter inhibitor: Light-induced control of gamma-aminobutyric acid transporter 1 (GAT1) activity in mouse brain. J. Med. Chem. 2014, 57, 6809–6821. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.O.F.; Sivaprakasam, S.; Brown, T.P.; Thangaraju, M.; Bhutia, Y.D.; Ganapathy, V. SLC6A14, a Na+/Cl—Coupled amino acid transporter, functions as a tumor promoter in colon and is a target for Wnt signaling. Biochem. J. 2020, 477, 1409–1425. [Google Scholar] [CrossRef] [PubMed]
- Coothankandaswamy, V.; Cao, S.; Xu, Y.; Prasad, P.D.; Singh, P.K.; Reynolds, C.P.; Yang, S.; Ogura, J.; Ganapathy, V.; Bhutia, Y.D. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br. J. Pharmacol. 2016, 173, 3292–3306. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Zheng, H.; Chen, Z.; Lin, X.; Li, C.; Yao, Q.; Bhutia, Y.D.; Ganapathy, V.; Chen, R.; Kou, L. Synergism between SLC6A14 blockade and gemcitabine in pancreactic cancer: A 1H-NMR-based metabolomic study in pancreatic cancer cells. Biochem. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kongpracha, P.; Nagamori, S.; Wiriyasermkul, P.; Tanaka, Y.; Kaneda, K.; Okuda, S.; Ohgaki, R.; Kanai, Y. Structure-activity relationship of a novel series of inhibitors for cancer type transporter L-type amino acid transporter 1 (LAT1). J. Pharmacol. Sci. 2017, 133, 96–102. [Google Scholar] [CrossRef]
- Enomoto, K.; Sato, F.; Tamagawa, S.; Gunduz, M.; Onoda, N.; Uchino, S.; Muragaki, Y.; Hotomi, M. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci. Rep. 2019, 9, 14616. [Google Scholar] [CrossRef]
- Cormerais, Y.; Pagnuzzi-Boncompagni, M.; Schrotter, S.; Giuliano, S.; Tambutte, E.; Endou, H.; Wempe, M.F.; Pages, G.; Pouyssegur, J.; Picco, V. Inhibition of the amino-acid transporter LAT1 demonstrates anti-neoplastic activity in medulloblastoma. J. Cell. Mol. Med. 2019, 23, 2711–2718. [Google Scholar] [CrossRef]
- Ueno, S.; Kimura, T.; Yamaga, T.; Kawada, A.; Ochiai, T.; Endou, H.; Sakurai, H. Metformin enhances anti-tumor effect of L-type amino acid transporter 1 (LAT1) inhibitor. J. Pharmacol. Sci. 2016, 131, 110–117. [Google Scholar] [CrossRef]
- Rosilio, C.; Nebout, M.; Imbert, V.; Griessinger, E.; Neffati, Z.; Benadiba, J.; Hagenbeek, T.; Spits, H.; Reverso, J.; Ambrosetti, D.; et al. L-type amino-acid transporter 1 (LAT1): A therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia 2015, 29, 1253–1266. [Google Scholar] [CrossRef]
- Ji, X.; Qian, J.; Rahman, S.M.J.; Siska, P.J.; Zou, Y.; Harris, B.K.; Hoeksema, M.D.; Trenary, I.A.; Heidi, C.; Eisenberg, R.; et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 2018, 37, 5007–5019. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, Y.; Yamasaki, J.; Suina, K.; Okazaki, S.; Koike, N.; Saya, H.; Nagano, O. Vasodilator oxyfedrine inhibits aldehyde metabolism and thereby sensitizes cancer cells to xCT-targeted therapy. Cancer Sci. 2020, 111, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Investig. 2020, 130, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Umene, K.; Yamasaki, J.; Suina, K.; Otsuki, Y.; Yoshikawa, M.; Minami, Y.; Masuko, T.; Kawaguchi, S.; Nakayama, H.; et al. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019, 110, 3453–3463. [Google Scholar] [CrossRef]
- Ogihara, K.; Kikuchi, E.; Okazaki, S.; Hagiwara, M.; Takeda, T.; Matsumoto, K.; Kosaka, T.; Mikami, S.; Saya, H.; Oya, M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019, 110, 1431–1441. [Google Scholar] [CrossRef]
- Dai, L.; Cao, Y.; Chen, Y.; Kaleeba, J.A.; Zabaleta, J.; Qin, Z. Genomic analysis of xCT-mediated regulatory network: Identification of novel targets against AIDS-associated lymphoma. Oncotarget 2015, 6, 12710–12722. [Google Scholar] [CrossRef]
- Dai, L.; Cao, Y.; Chen, Y.; Parsons, C.; Qin, Z. Targeting xCT, a cystine-glutamate transporter induces apoptosis and tumor regression for KSHV/HIV-associated lymphoma. J. Hematol. Oncol. 2014, 7, 30. [Google Scholar] [CrossRef]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Valle, A.; Oliver, J.; Roca, P. UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free Radic. Biol. Med. 2015, 86, 67–77. [Google Scholar] [CrossRef]
- Kawanishi, M.; Fukuda, T.; Shimomura, M.; Inoue, Y.; Wada, T.; Tasaka, R.; Yasui, T.; Sumi, T. Expression of UCP2 is associated with sensitivity to platinum-based chemotherapy for ovarian serous carcinoma. Oncol. Lett. 2018, 15, 9923–9928. [Google Scholar] [CrossRef]
- Wang, Q.; Grkovic, T.; Font, J.; Bonham, S.; Pouwer, R.H.; Bailey, C.G.; Moran, A.M.; Ryan, R.M.; Rasko, J.E.; Jormakka, M.; et al. Monoterpene glycoside ESK246 from Pittosporum targets LAT3 amino acid transport and prostate cancer cell growth. ACS Chem. Biol. 2014, 9, 1369–1376. [Google Scholar] [CrossRef]
- Zhou, P.; Liang, X.; Zhou, C.; Qin, J.; Hou, C.; Zhu, Z.; Zhang, W.; Wang, S.; Zhong, D. Glutamine-beta-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2. J. Mater. Chem. B 2019, 7, 5363–5375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, J.; Wang, Z.; Yang, Z.; Li, Z.; Deng, H.; Li, L.; Peng, X.; Feng, M. Glutamine addiction activates polyglutamine-based nanocarriers delivering therapeutic siRNAs to orthotopic lung tumor mediated by glutamine transporter SLC1A5. Biomaterials 2018, 183, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, T.; Li, Z.; Wang, L.; Yuan, S.; Sun, L. The role of ASCT2 in cancer: A review. Eur. J. Pharmacol. 2018, 837, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Qian, J.; Hoeksema, M.D.; Wang, J.; Jacobovitz, M.; Ji, X.; Harris, F.T.; Harris, B.K.; Boyd, K.L.; Chen, H.; et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int. J. Cancer 2015, 137, 1587–1597. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Lu, Y.; Qiu, S.; Fan, Z. ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis. Cancer Lett. 2016, 381, 23–30. [Google Scholar] [CrossRef]
- Tao, X.; Lu, Y.; Qiu, S.; Wang, Y.; Qin, J.; Fan, Z. AP1G1 is involved in cetuximab-mediated downregulation of ASCT2-EGFR complex and sensitization of human head and neck squamous cell carcinoma cells to ROS-induced apoptosis. Cancer Lett. 2017, 408, 33–42. [Google Scholar] [CrossRef]
- Osanai-Sasakawa, A.; Hosomi, K.; Sumitomo, Y.; Takizawa, T.; Tomura-Suruki, S.; Imaizumi, M.; Kasai, N.; Poh, T.W.; Yamano, K.; Yong, W.P.; et al. An anti-ASCT2 monoclonal antibody suppresses gastric cancer growth by inducing oxidative stress and antibody dependent cellular toxicity in preclinical models. Am. J. Cancer Res. 2018, 8, 1499–1513. [Google Scholar]
- Zhuang, X.; Tong, H.; Ding, Y.; Wu, L.; Cai, J.; Si, Y.; Zhang, H.; Shen, M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019, 10, 620. [Google Scholar] [CrossRef]
- Ding, J.; Gou, Q.; Jin, J.; Shi, J.; Liu, Q.; Hou, Y. Metformin inhibits PPARdelta agonist-mediated tumor growth by reducing Glut1 and SLC1A5 expressions of cancer cells. Eur. J. Pharmacol. 2019, 857, 172425. [Google Scholar] [CrossRef]
- Sun, J.; Nagel, R.; Zaal, E.A.; Ugalde, A.P.; Han, R.; Proost, N.; Song, J.Y.; Pataskar, A.; Burylo, A.; Fu, H.; et al. SLC1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019, 38, e102147. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Jia, L.; Chen, X.; Awaleh Moumin, F.; Cai, J. SLC1A3 promotes gastric cancer progression via the PI3K/AKT signalling pathway. J. Cell. Mol. Med. 2020, 24, 14392–14404. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Gynther, M.; Huttunen, J.; Puris, E.; Spicer, J.A.; Denny, W.A. A Selective and Slowly Reversible Inhibitor of l-Type Amino Acid Transporter 1 (LAT1) Potentiates Antiproliferative Drug Efficacy in Cancer Cells. J. Med. Chem. 2016, 59, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Jutabha, P.; Maeda, S.; Supak, Y.; Ouchi, M.; Endou, H.; Fujita, T.; Chida, M.; Anzai, N. LAT1 acts as a crucial transporter of amino acids in human thymic carcinoma cells. J. Pharmacol. Sci. 2016, 132, 201–204. [Google Scholar] [CrossRef]
- Higuchi, K.; Sakamoto, S.; Ando, K.; Maimaiti, M.; Takeshita, N.; Okunushi, K.; Reien, Y.; Imamura, Y.; Sazuka, T.; Nakamura, K.; et al. Characterization of the expression of LAT1 as a prognostic indicator and a therapeutic target in renal cell carcinoma. Sci. Rep. 2019, 9, 16776. [Google Scholar] [CrossRef]
- Yun, D.W.; Lee, S.A.; Park, M.G.; Kim, J.S.; Yu, S.K.; Park, M.R.; Kim, S.G.; Oh, J.S.; Kim, C.S.; Kim, H.J.; et al. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J. Pharmacol. Sci. 2014, 124, 208–217. [Google Scholar] [CrossRef]
- Skvortsov, S.; Skvortsova, I.-I.; Tang, D.G.; Dubrovska, A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef]
- Strauss, L.G. Positron Emission Tomography: Current Role for Diagnosis and Therapy Monitoring in Oncology. Oncologist 1997, 2, 381–388. [Google Scholar] [CrossRef]
- Liu, Y. The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Front. Oncol. 2016, 6, 201. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Q.; Ma, Y.; Wang, Y.; Feng, Y.; Zhao, B.; Yang, Y. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of (1)(8)F-FDG PET/CT. PLoS ONE 2013, 8, e78552. [Google Scholar] [CrossRef]
- Kumar, R.; Rani, N.; Patel, C.; Basu, S.; Alavi, A. False-Negative and False-Positive Results in FDG-PET and PET/CT in Breast Cancer. PET Clin. 2009, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Lee, H.J.; Goo, J.M.; Lee, H.Y.; Lee, J.J.; Chung, J.K.; Im, J.G. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J. Radiol. 2006, 7, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Pan, Q.; Gao, S.; Sun, A.; Wen, F.; Tang, G. Excitatory glutamate transporter EAAC1 as an important transporter of N-(2-[(18)F]fluoropropionyl)-L-glutamate in oncology PET imaging. Nucl. Med. Biol. 2020, 84–85, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, Y.; Li, J.; Qin, M.; Fu, Q.; Wang, C.; Liu, Z. (18)F-Alanine Derivative Serves as an ASCT2 Marker for Cancer Imaging. Mol. Pharm. 2018, 15, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, M.; Hight, M.R.; Buck, J.R.; Tantawy, M.N.; Nickels, M.L.; Hoeksema, M.D.; Harris, B.K.; Boyd, K.; Massion, P.P.; Manning, H.C. Preclinical Evaluation of 4-[18F]Fluoroglutamine PET to Assess ASCT2 Expression in Lung Cancer. Mol. Imaging Biol. 2016, 18, 18–23. [Google Scholar] [CrossRef]
- Ono, M.; Oka, S.; Okudaira, H.; Nakanishi, T.; Mizokami, A.; Kobayashi, M.; Schuster, D.M.; Goodman, M.M.; Shirakami, Y.; Kawai, K. [(14)C]Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl. Med. Biol. 2015, 42, 887–892. [Google Scholar] [CrossRef]
- Saarinen, I.; Jambor, I.; Kim, M.; Kuisma, A.; Kemppainen, J.; Merisaari, H.; Eskola, O.; Koskenniemi, A.R.; Perez, I.M.; Bostrom, P.; et al. Correlation between (18)F-1-amino-3-fluorocyclobutane-1-carboxylic acid ((18)F-fluciclovine) uptake and expression of alanine-serine-cysteine-transporter 2 (ASCT2) and L-type amino acid transporter 1 (LAT1) in primary prostate cancer. EJNMMI Res. 2019, 9, 50. [Google Scholar] [CrossRef]
- Deuschle, F.C.; Schiefner, A.; Skerra, A. Structural differences between the ectodomains of murine and human CD98hc. Proteins 2019, 87, 693–698. [Google Scholar] [CrossRef]
- Verhoeven, J.; Baguet, T.; Piron, S.; Pauwelyn, G.; Bouckaert, C.; Descamps, B.; Raedt, R.; Vanhove, C.; De Vos, F.; Goethals, I. 2-[(18)F]FELP, a novel LAT1-specific PET tracer, for the discrimination between glioblastoma, radiation necrosis and inflammation. Nucl. Med. Biol. 2020, 82–83, 9–16. [Google Scholar] [CrossRef]
- Verhoeven, J.; Hulpia, F.; Kersemans, K.; Bolcaen, J.; De Lombaerde, S.; Goeman, J.; Descamps, B.; Hallaert, G.; Van den Broecke, C.; Deblaere, K.; et al. New fluoroethyl phenylalanine analogues as potential LAT1-targeting PET tracers for glioblastoma. Sci. Rep. 2019, 9, 2878. [Google Scholar] [CrossRef]
- Habermeier, A.; Graf, J.; Sandhofer, B.F.; Boissel, J.P.; Roesch, F.; Closs, E.I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 2015, 47, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Nakatani, Y.; Mawatari, A.; Shibata, N.; Hume, W.E.; Hayashinaka, E.; Wada, Y.; Doi, H.; Watanabe, Y. (18)F-FIMP: A LAT1-specific PET probe for discrimination between tumor tissue and inflammation. Sci. Rep. 2019, 9, 15718. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Watabe, T.; Nagamori, S.; Naka, S.; Ikeda, H.; Kongpracha, P.; Horitsugi, G.; Kanai, Y.; Shimosegawa, E.; Kanai, Y.; et al. Distribution of LAT1-targeting PET tracer was independent of the tumor blood flow in rat xenograft models of C6 glioma and MIA PaCa-2. Ann. Nucl. Med. 2019, 33, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tominaga, H.; Ohgaki, R.; Wiriyasermkul, P.; Hagiwara, K.; Okuda, S.; Kaira, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y. Specific transport of 3-fluoro-l-alpha-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 2016, 107, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Ohshima, M.; Hiruta, Y.; Nishimura, T.; Nagase, K.; Kanazawa, H. LAT1-Targeting Thermoresponsive Fluorescent Polymer Probes for Cancer Cell Imaging. Int. J. Mol. Sci. 2018, 19, 1646. [Google Scholar] [CrossRef] [PubMed]
- Papin-Michault, C.; Bonnetaud, C.; Dufour, M.; Almairac, F.; Coutts, M.; Patouraux, S.; Virolle, T.; Darcourt, J.; Burel-Vandenbos, F. Study of LAT1 Expression in Brain Metastases: Towards a Better Understanding of the Results of Positron Emission Tomography Using Amino Acid Tracers. PLoS ONE 2016, 11, e0157139. [Google Scholar] [CrossRef]
- Dadone-Montaudie, B.; Ambrosetti, D.; Dufour, M.; Darcourt, J.; Almairac, F.; Coyne, J.; Virolle, T.; Humbert, O.; Burel-Vandenbos, F. [18F] FDOPA standardized uptake values of brain tumors are not exclusively dependent on LAT1 expression. PLoS ONE 2017, 12, e0184625. [Google Scholar] [CrossRef]
- Li, C.; Huang, S.; Guo, J.; Wang, C.; Huang, Z.; Huang, R.; Liu, L.; Liang, S.; Wang, H. Metabolic Evaluation of MYCN-Amplified Neuroblastoma by 4-[(18)F]FGln PET Imaging. Mol. Imaging Biol. 2019, 21, 1117–1126. [Google Scholar] [CrossRef]
- Cantor, J.M.; Ginsberg, M.H. CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 2012, 125, 1373–1382. [Google Scholar] [CrossRef]
- Langen, K.J.; Hamacher, K.; Weckesser, M.; Floeth, F.; Stoffels, G.; Bauer, D.; Coenen, H.H.; Pauleit, D. O-(2-[18F]fluoroethyl)-L-tyrosine: Uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006, 33, 287–294. [Google Scholar] [CrossRef]
- Bashir, A.; Mathilde Jacobsen, S.; Molby Henriksen, O.; Broholm, H.; Urup, T.; Grunnet, K.; Andree Larsen, V.; Moller, S.; Skjoth-Rasmussen, J.; Skovgaard Poulsen, H.; et al. Recurrent glioblastoma versus late posttreatment changes: Diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 2019, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Ikeda, H.; Nagamori, S.; Wiriyasermkul, P.; Tanaka, Y.; Naka, S.; Kanai, Y.; Hagiwara, K.; Aoki, M.; Shimosegawa, E.; et al. (18)F-FBPA as a tumor-specific probe of L-type amino acid transporter 1 (LAT1): A comparison study with (18)F-FDG and (11)C-Methionine PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; De Melo, J.; Hickman, K.A.; Lourenco, C.; Redel, C.; Resetca, D.; Tamachi, A.; Tu, W.B.; Penn, L.Z. MYC Deregulation in Primary Human Cancers. Genes 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Jiang, J.; Gao, P.; Liu, H.; Qing, G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017, 21, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Lafita-Navarro, M.C.; Hao, Y.H.; Kilgore, J.A.; Perez-Castro, L.; Braverman, J.; Borenstein-Auerbach, N.; Kim, M.; Lesner, N.P.; Mishra, P.; et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019, 33, 1236–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, X.; Yuan, F.; Zhang, L.; Wang, Y.; Wang, L.; Mao, Z.; Luo, J.; Zhang, H.; Zhu, W.G.; et al. Increased Amino Acid Uptake Supports Autophagy-Deficient Cell Survival upon Glutamine Deprivation. Cell Rep. 2018, 23, 3006–3020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, X.; Yuan, F.; Zhu, W.G.; Zhao, Y. Autophagy-deficient tumor cells rely on extracellular amino acids to survive upon glutamine deprivation. Autophagy 2018, 14, 1652–1653. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Lippincott-Schwartz, J.; Yin, X.M.; Weiss, W.A.; Takebe, N.; Timmer, W.; DiPaola, R.S.; Lotze, M.T.; White, E. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 2011, 17, 654–666. [Google Scholar] [CrossRef]
- Broer, S.; Fairweather, S.J. Amino Acid Transport Across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef]
- Bouthelier, A.; Aragones, J. Role of the HIF oxygen sensing pathway in cell defense and proliferation through the control of amino acid metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118733. [Google Scholar] [CrossRef]
- Mishra, R.; Haldar, S.; Placencio, V.; Madhav, A.; Rohena-Rivera, K.; Agarwal, P.; Duong, F.; Angara, B.; Tripathi, M.; Liu, Z.; et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J. Clin. Investig. 2018, 128, 4472–4484. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.P.; Ward, N.P.; DeNicola, G.M. Recent advances in cancer metabolism: A technological perspective. Exp. Mol. Med. 2018, 50, 31. [Google Scholar] [CrossRef] [PubMed]
- Silva-Almeida, C.; Ewart, M.A.; Wilde, C. 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin. Cell Dev. Biol. 2020, 98, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Kremer, D.M.; Huang, H.; Breitkopf, S.B.; Ben-Sahra, I.; Manning, B.D.; Lyssiotis, C.A.; Asara, J.M. Ex vivo and in vivo stable isotope labelling of central carbon metabolism and related pathways with analysis by LC-MS/MS. Nat. Protoc. 2019, 14, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Scalise, M.; Galluccio, M.; Wieder, M.; Seidel, T.; Langer, T.; Indiveri, C.; Ecker, G.F. Discovery of Potent Inhibitors for the Large Neutral Amino Acid Transporter 1 (LAT1) by Structure-Based Methods. Int. J. Mol. Sci. 2018, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using Genome-scale Models to Predict Biological Capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef]


| Chemical Inhibition | |||||
|---|---|---|---|---|---|
| Transporter | Inhibitor | Experimental Model | Tissue Type | Refs | |
| SLC1A1 | 2-(furan-2-yl)-8-methyl-N-(o-tolyl)imidazo[1,2-a]pyridin-3-amine | In vitro (Cell line) | Human Embryonic Kidney cells | [161] | |
| SLC1A2 | Azo-TFB-TBOA | In vitro (Xenopus laevis oocytes) | Xenopus laevis oocytes | [162] | |
| SLC1A5, SLC38A2, SLC7A5 | V-9302 | In vitro (Cell line) | A panel of human cancer cell lines [163] Head and Neck Squamous Cell Carcinoma [29] | [29,163] | |
| In vivo (Cell line derived-xenograft) | patient-derived xenografts (PDX) model [163], Head and Neck Squamous Cell Carcinoma cell line xenograft [29] | ||||
| SLC1A5 | L-g-glutamyl-p-nitroanilide (GPNA) | In vitro (Cell line) | Colorectal Carcinoma [164], Endometrial Carcinoma [165] | [164,165] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC1A5 | Benzylserine (BenSer) | In vitro (Cell line) | Endometrial Carcinoma [165], Melanoma [166] | [165,166] | |
| SLC1A5 | Benzyloxybenzyl analogues | In vitro (Cell line) | Human Embryonic Kidney cells, C6 Rat cells | [167] | |
| SLC1A5 | KM8094 mAB | In vivo (Patient derived-xenograft) | Patient-derived Gastric cancer tissue | [168] | |
| SLC1A5 | KM4008 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | KM4012 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | KM4018 mAB | In vitro (Cell line) | A panel of cell lines | [169] | |
| SLC1A5 | Ab3-8 mAb | In vitro (Cell line) | Colorectal Carcinoma | [170] | |
| SLC1A5, SLC7A5 | Delta-tocotrienol (δT) | In vitro (Cell line) | Non-Small Cell Lung Cancer | [171] | |
| SLC6A1 | Photoswitchable inhibitor | In vitro (Cell line) | Human Embryonic Kidney cells and Dentate gyrus granule cells | [172] | |
| SLC6A14 | α-methyltryptophan | In vitro (Cell line) | Colon cancer [173], Pancreatic cancer [174,175] | [173,174,175] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC7A5 | SKN103 | In vitro (Cell line) | A panel of human cancer cell lines | [176] | |
| SLC7A5 | BCH | In vitro (Cell line) | Melanoma | [166] | |
| SLC7A5 | JPH203 | In vitro (Cell line) | Anaplastic thyroid cancer [177], Medulloblastoma [178], Head and Neck Squamous Cell Carcinoma [179], T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia [180] | [177,178,179,180] | |
| In vivo (Cell line derived-xenograft) | |||||
| SLC7A11 | Sulfasalazine (SSZ) | In vitro (Cell line) | Non-Small Cell Lung Cancer [181], A panel of human cancer cell lines [182], Lung Adenocarcinoma [183], Head and Neck Squamous Cell Carcinoma [184], Bladder Cancer [185], Lymphoma [186,187] | [181,182,183,184,185,186,187] | |
| In vivo (Cell line derived-xenograft) | |||||
| In vivo (KRAS Mouse model) | |||||
| In vivo (Murine metastasis model) | |||||
| SLC25A8 | Genipin | In vitro (Cell line) | Breast Cancer [188], Ovarian Cancer [189] | [189] | |
| SLC43A1 | ESK246 | In vitro (Cell line) | Prostate Cancer | [190] | |
| Drug Delivery | |||||
| Through which Transporter | Molecule | Delivery | Experimental Model | Tissue Type | Refs |
| SLC1A5 | GLN-CD (conjugation of Gln with β-cyclodextrin) | Doxorubicin | In vitro (Cell line) | Triple-negative Breast Cancer | [191] |
| SLC1A5 | Polyglutamine (PGS) | various agents including siRNAs | In vitro (Cell line) | Lung Cancer | [192] |
| Transporter | Tracer | Experimental Model | Tissue Type | References |
|---|---|---|---|---|
| SLC1A1 | N-(2-(18F)fluoropropionyl)-L-glutamate ((18F)FPGLU) | In vitro (Cell line) | Rat C6 glioma cell line, SPC-A-1 lung adenocarcinoma cell line | [213] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC1A5 | 18F-Ala-BF3 | In vivo (Cell line derived-xenograft) | Gastric cancer cell line xenograft | [214] |
| SLC1A5 | 4-(18F)Fluoro-Gln | In vivo (Cell line derived-xenograft) | Non-Small Cell Lung Cancer and Colorectal Carcinoma xenografts | [215] |
| In vivo (EGFR-mutant Mouse model) | ||||
| SLC1A5, SLC7A5 | (18F)fluciclovine | In vitro (Cell line) | Castration-resistant prostate cancer | [216,217] |
| SLC3A2 | Anticalin | Primary prostate cancer tissue | Primary prostate cancer tissue | [218] |
| SLC7A5 | 2-(18F)FELP | In vitro (Cell line) | Glioblastoma [219,220] | [219,220] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC7A5 | [18F]FET | In vitro (Cell line) | Glioblastoma [219,220,221] | [219,220,221] |
| In vivo (Cell line derived-xenograft) | ||||
| SLC7A5 | 18F-FIMP | In vivo (Cell line derived-xenograft) | Glioblastoma | [222] |
| SLC7A5 | 18F-FBPA | In vivo (Cell line derived-xenograft) | C6 Glioma and MIA PaCa-2 xenografts | [223] |
| SLC7A5 | 18F-FAMT | In vitro (Cell line) | C6 Glioma and MIA PaCa-2 xenografts [223], Xenopus oocytes [224] | [223,224] |
| In vivo (Cell line derived-xenograft) | ||||
| In vitro (Xenopus laevis oocytes) | ||||
| SLC7A5 | P(NIPAAm-co-DMAAm) | In vitro (Cell line) | Cervical carcinoma (HeLa cells) | [225] |
| SLC7A5 | (18F)-FDOPA | In vitro (Cell line) | Non tumoral brain tissues and brain metastases [226], Glioblastoma [227] | [226,227] |
| Brain tumor tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahya, U.; Köseer, A.S.; Dubrovska, A. Amino Acid Transporters on the Guard of Cell Genome and Epigenome. Cancers 2021, 13, 125. https://doi.org/10.3390/cancers13010125
Kahya U, Köseer AS, Dubrovska A. Amino Acid Transporters on the Guard of Cell Genome and Epigenome. Cancers. 2021; 13(1):125. https://doi.org/10.3390/cancers13010125
Chicago/Turabian StyleKahya, Uğur, Ayşe Sedef Köseer, and Anna Dubrovska. 2021. "Amino Acid Transporters on the Guard of Cell Genome and Epigenome" Cancers 13, no. 1: 125. https://doi.org/10.3390/cancers13010125
APA StyleKahya, U., Köseer, A. S., & Dubrovska, A. (2021). Amino Acid Transporters on the Guard of Cell Genome and Epigenome. Cancers, 13(1), 125. https://doi.org/10.3390/cancers13010125