Establishment of Prostate Tumor Growth and Metastasis Is Supported by Bone Marrow Cells and Is Mediated by PIP5K1α Lipid Kinase
Simple Summary
Abstract
1. Introduction
2. Results
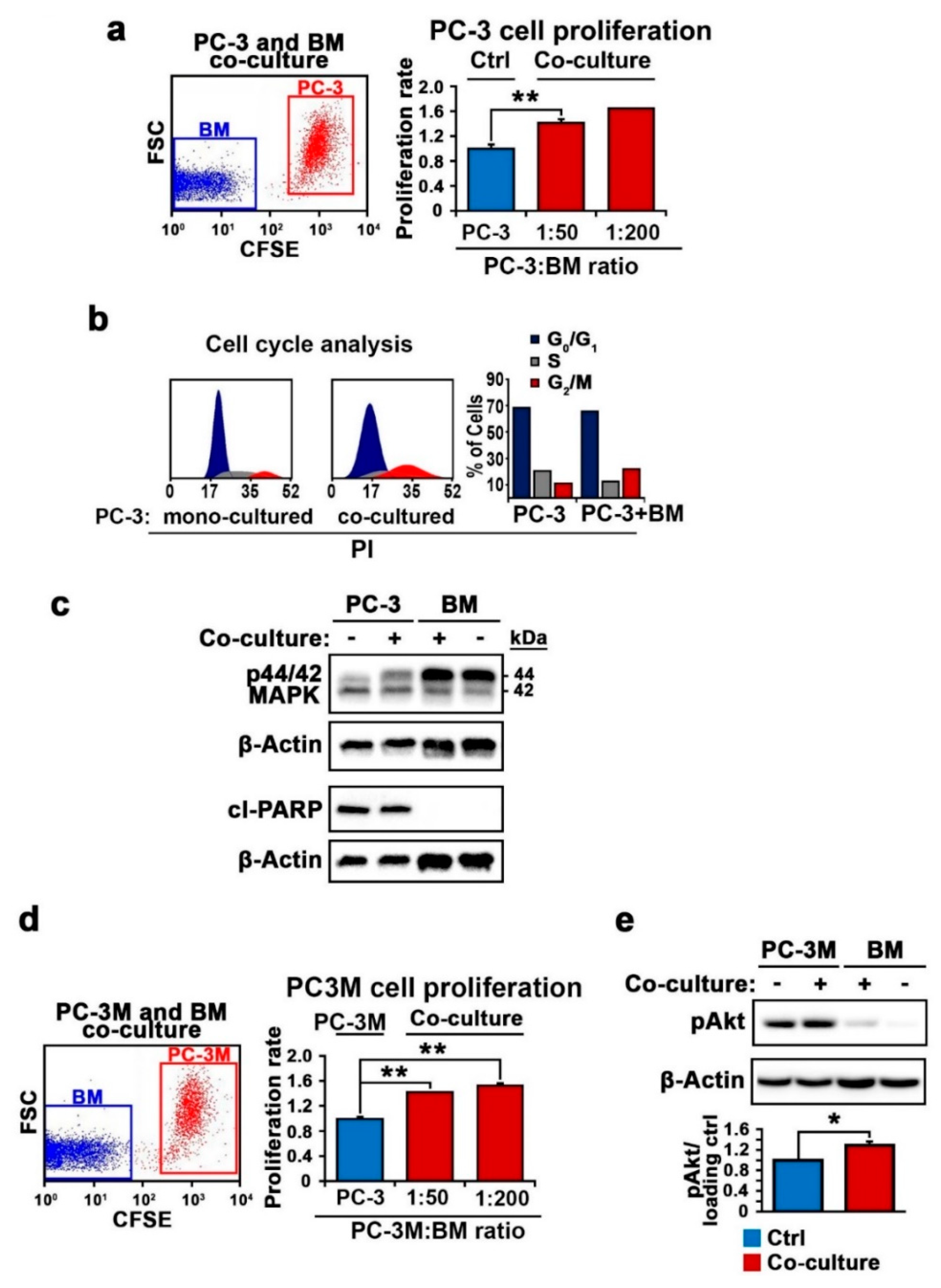
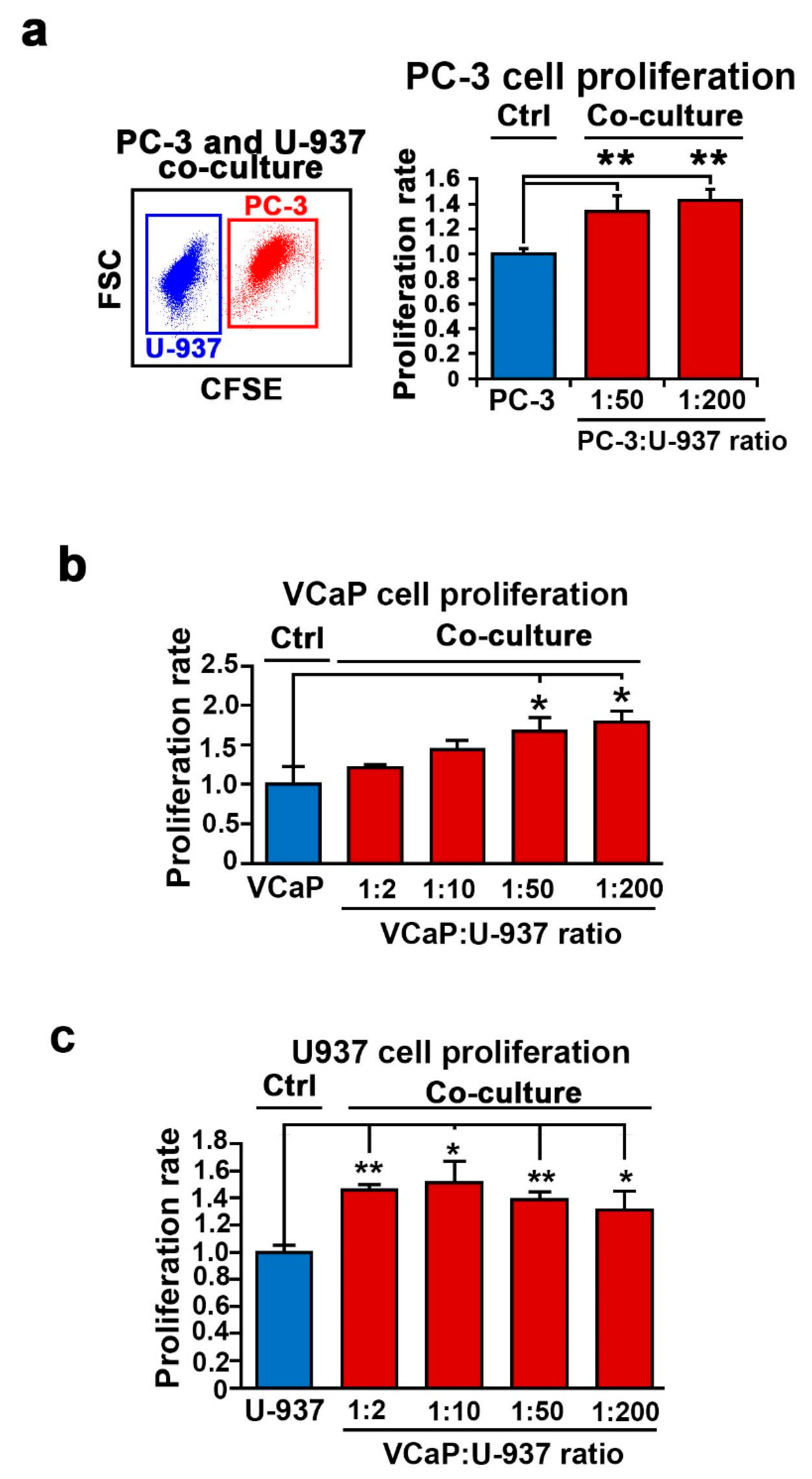
2.1. The Functional Consequences of the Interaction between PCa Cells and a Bone-Marrow Cells
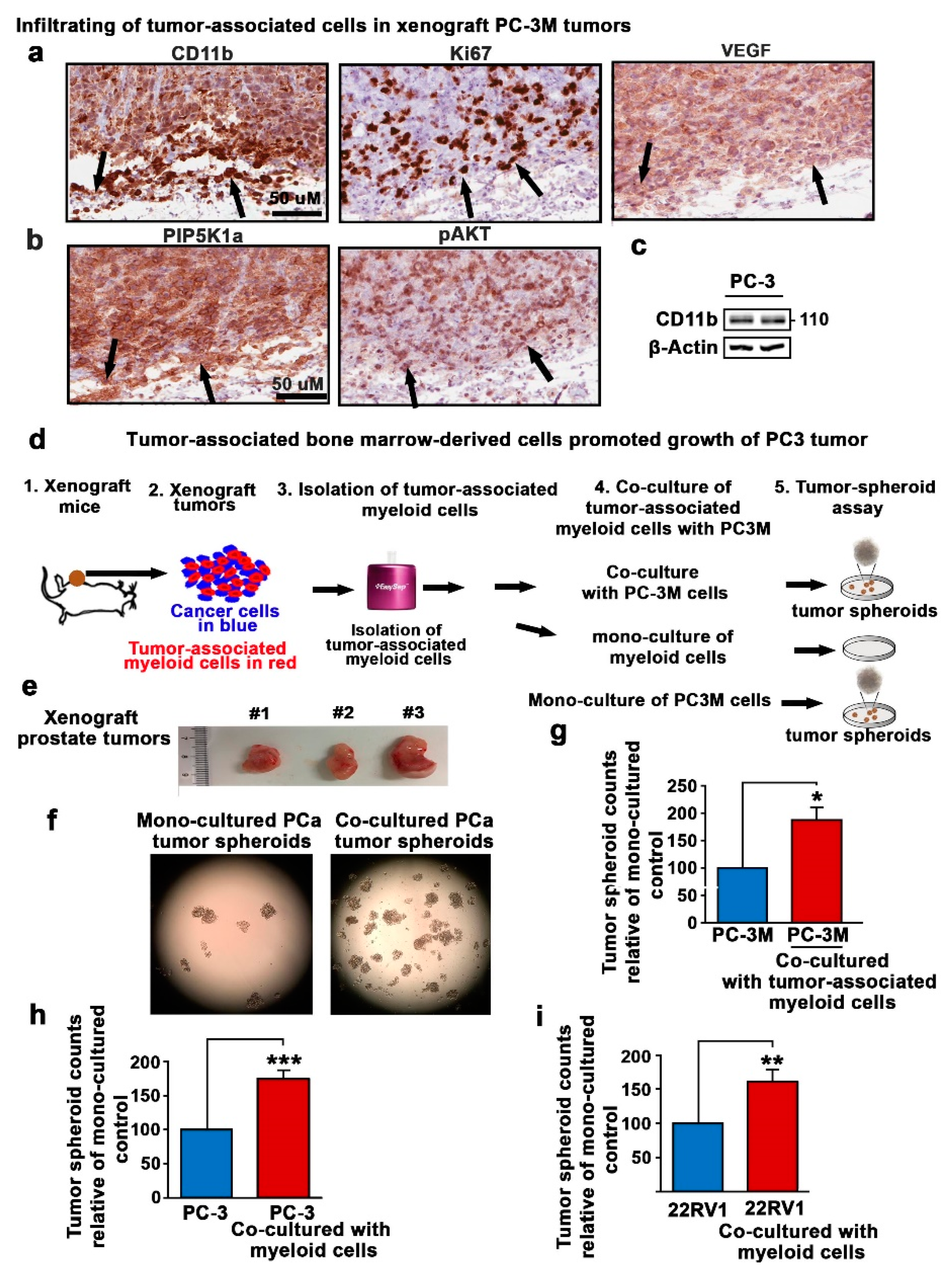
2.2. Tumor-Associated Myeloid Cells Promote Tumor Growth and Expansion in Xenograft Mouse Model
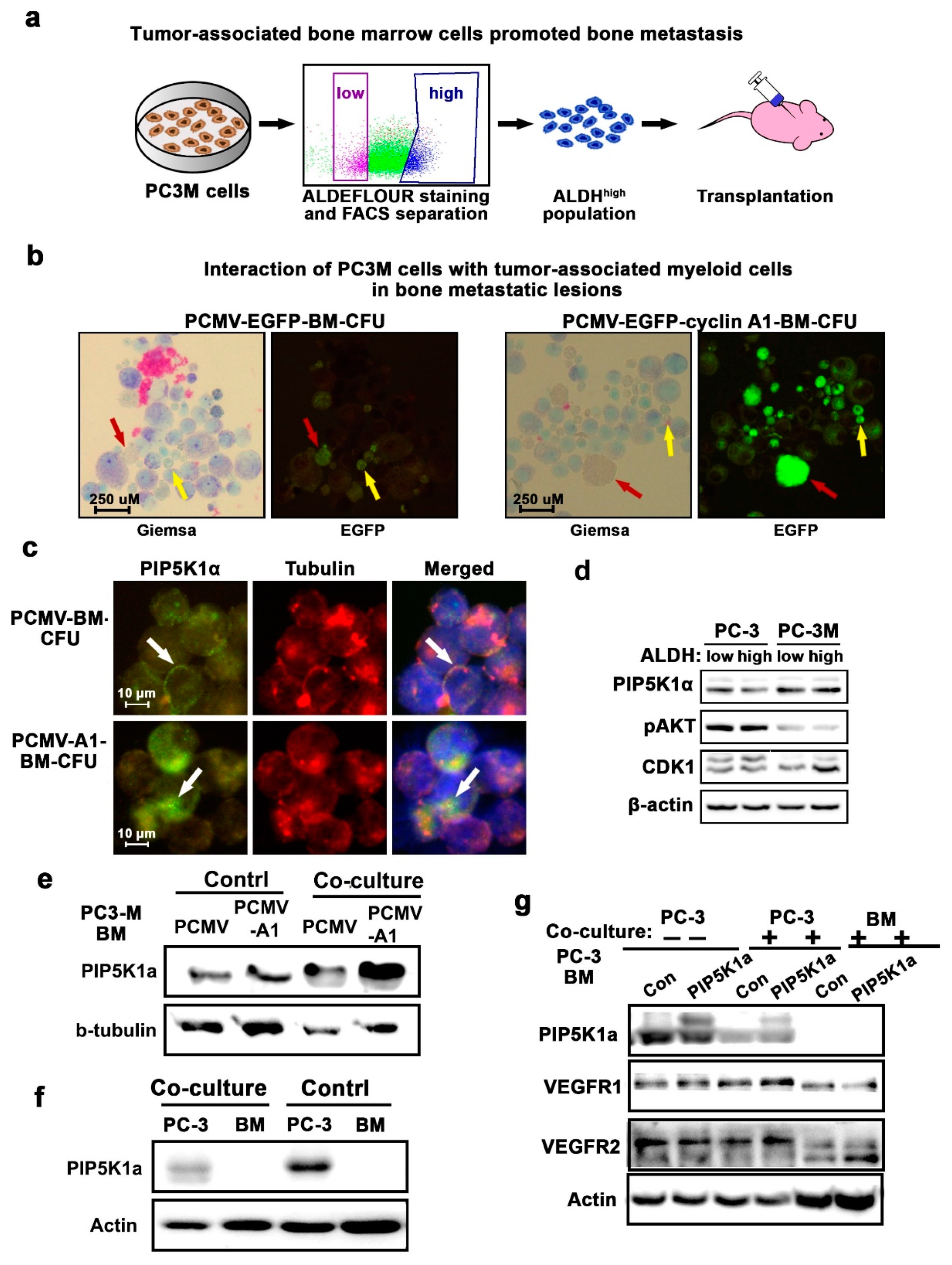
2.3. Interaction between PCa Cells and Bone Marrow Cells Correlates with Increased BM Metastasis in a Xenograft Mouse Model
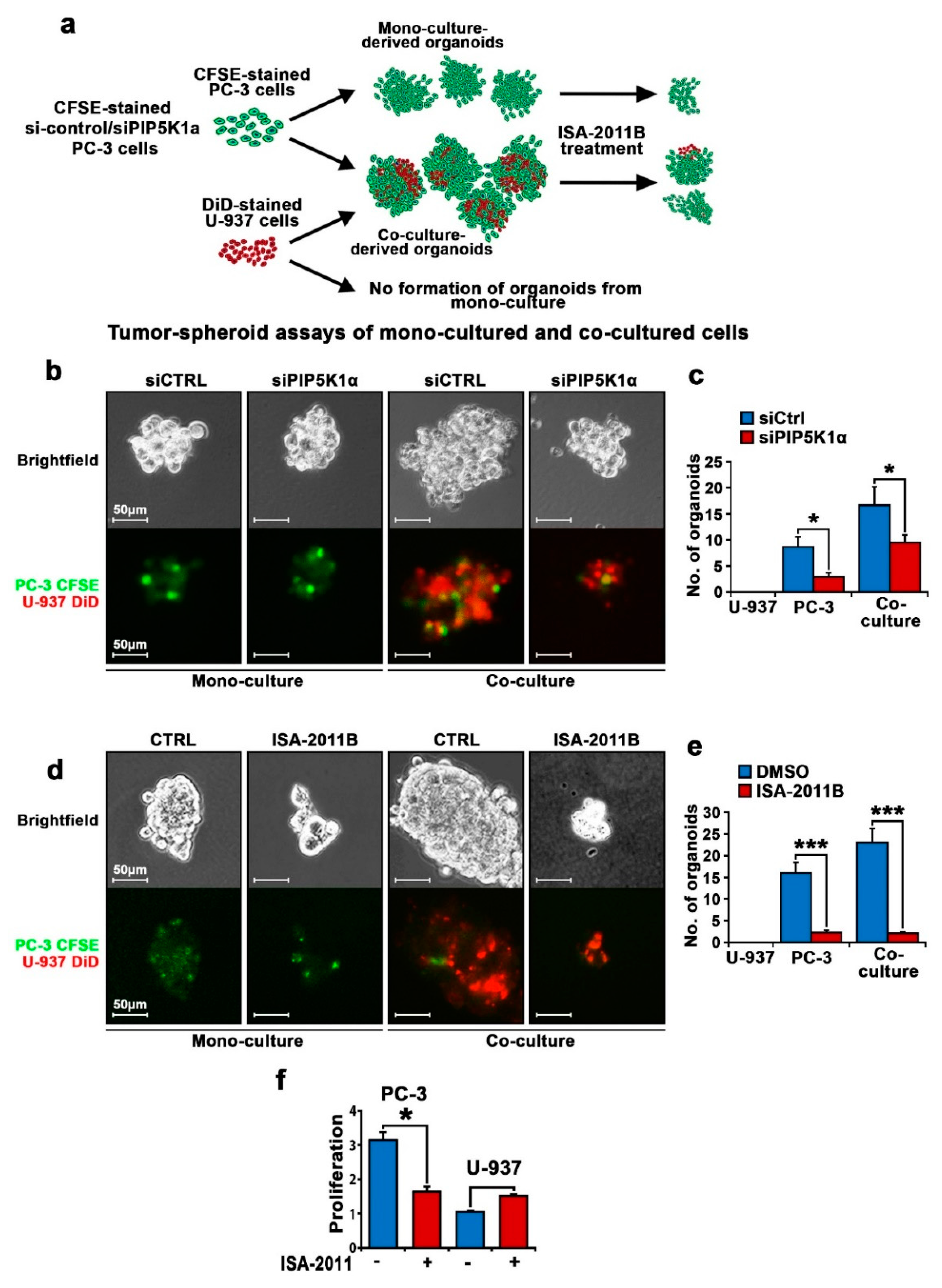
2.4. PIP5K1α Mediates Interaction between PCa Cells and Tumor-Associated Myeloid Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Co-Culture of PCa Cells with U-937 Cells or Bone Marrow Cells
4.3. Fluorescence-Activated Cell Sorting (FACS) and Analysis of Co-Cultured Cells
4.4. FACS-Based Cell Cycle Analysis
4.5. Plasmids, Transfection and siRNA Knockdowns
4.6. Colony-Forming Unit (CFU) Assays Using Methylcellulose-Based Medium
4.7. Prostate Cancer Stem-Like Cell-Derived Tumor-Spheroid Formation Assay
4.8. Mouse Model of PCa Xenograft Tumors and Distant Metastases
4.9. Isolation of Bone Marrow-Derived Cells from the Xenograft Tumors
4.10. Immunoblot Analysis
4.11. Immunohistochemical Analysis
4.12. Immunofluorescence Analysis
4.13. Field-Emission Scanning Electron Microscopic Imaging of PC-3 and U-937 Cells
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef]
- Strasser, K.; Birnleitner, H.; Beer, A.; Pils, D.; Gerner, M.C.; Schmetterer, K.G.; Bachleitner-Hofmann, T.; Stift, A.; Bergmann, M.; Oehler, R. Immunological differences between colorectal cancer and normal mucosa uncover a prognostically relevant immune cell profile. Oncoimmunology 2019, 8, e1537693. [Google Scholar] [CrossRef]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.; van Vugt, M.; de Vries, E.G.E.; Schroder, C.P.; Fehrmann, R.S.N. Relevance of Tumor-Infiltrating Immune Cell Composition and Functionality for Disease Outcome in Breast Cancer. J. Natl. Cancer Inst. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- de Brot, S.; Ntekim, A.; Cardenas, R.; James, V.; Allegrucci, C.; Heery, D.M.; Bates, D.O.; Odum, N.; Persson, J.L.; Mongan, N.P. Regulation of vascular endothelial growth factor in prostate cancer. Endocr. Relat. Cancer 2015, 22, R107–R123. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.S.; Weinberg, R.A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 2014, 16, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Bekes, E.M.; Schweighofer, B.; Kupriyanova, T.A.; Zajac, E.; Ardi, V.C.; Quigley, J.P.; Deryugina, E.I. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am. J. Pathol. 2011, 179, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Dai, Z.; Zhou, Z.J.; Chen, Q.; Wang, Z.; Xiao, Y.S.; Hu, Z.Q.; Huang, X.Y.; Yang, G.H.; Shi, Y.H.; et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis 2014, 35, 597–605. [Google Scholar] [CrossRef]
- Melani, C.; Chiodoni, C.; Forni, G.; Colombo, M.P. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 2003, 102, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.; Borrello, I.; Bronte, V. Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 2006, 16, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Almand, B.; Clark, J.I.; Nikitina, E.; van Beynen, J.; English, N.R.; Knight, S.C.; Carbone, D.P.; Gabrilovich, D.I. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J. Immunol. 2001, 166, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, J.; Ren, X.; Gorska, A.E.; Chytil, A.; Aakre, M.; Carbone, D.P.; Matrisian, L.M.; Richmond, A.; Lin, P.C.; et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 2008, 13, 23–35. [Google Scholar] [CrossRef] [PubMed]
- van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Semenas, J.; Hedblom, A.; Miftakhova, R.R.; Sarwar, M.; Larsson, R.; Shcherbina, L.; Johansson, M.E.; Harkonen, P.; Sterner, O.; Persson, J.L. The role of PI3K/AKT-related PIP5K1alpha and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3689–E3698. [Google Scholar] [CrossRef]
- Drake, J.M.; Huang, J. PIP5K1alpha inhibition as a therapeutic strategy for prostate cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 12578–12579. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Semenas, J.; Miftakhova, R.; Simoulis, A.; Robinson, B.; Gjorloff Wingren, A.; Mongan, N.P.; Heery, D.M.; Johnsson, H.; Abrahamsson, P.A.; et al. Targeted suppression of AR-V7 using PIP5K1alpha inhibitor overcomes enzalutamide resistance in prostate cancer cells. Oncotarget 2016, 7, 63065–63081. [Google Scholar] [CrossRef]
- Choi, S.; Hedman, A.C.; Sayedyahossein, S.; Thapa, N.; Sacks, D.B.; Anderson, R.A. Agonist-stimulated phosphatidylinositol-3,4,5-trisphosphate generation by scaffolded phosphoinositide kinases. Nat. Cell Biol. 2016, 18, 1324–1335. [Google Scholar] [CrossRef]
- Lee, S.H.; Johnson, D.; Luong, R.; Sun, Z. Crosstalking between androgen and PI3K/AKT signaling pathways in prostate cancer cells. J. Biol. Chem. 2015, 290, 2759–2768. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Miftakhova, R.; Hedblom, A.; Semenas, J.; Robinson, B.; Simoulis, A.; Malm, J.; Rizvanov, A.; Heery, D.M.; Mongan, N.P.; Maitland, N.J.; et al. Cyclin A1 and P450 Aromatase Promote Metastatic Homing and Growth of Stem-like Prostate Cancer Cells in the Bone Marrow. Cancer Res. 2016, 76, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Hedblom, A.; Laursen, K.B.; Miftakhova, R.; Sarwar, M.; Anagnostaki, L.; Bredberg, A.; Mongan, N.P.; Gudas, L.J.; Persson, J.L. CDK1 interacts with RARgamma and plays an important role in treatment response of acute myeloid leukemia. Cell Cycle 2013, 12, 1251–1266. [Google Scholar] [CrossRef] [PubMed]
- Miftakhova, R.; Hedblom, A.; Batkiewicz, L.; Anagnosaki, L.; Zhang, Y.; Sjolander, A.; Wingren, A.G.; Wolgemuth, D.J.; Persson, J.L. Cyclin A1 regulates the interactions between mouse haematopoietic stem and progenitor cells and their niches. Cell Cycle 2015, 14, 1948–1960. [Google Scholar] [CrossRef]
- Larsson, P.; Syed Khaja, A.S.; Semenas, J.; Wang, T.; Sarwar, M.; Dizeyi, N.; Simoulis, A.; Hedblom, A.; Wai, S.N.; Odum, N.; et al. The functional interlink between AR and MMP9/VEGF signaling axis is mediated through PIP5K1alpha/pAKT in prostate cancer. Int. J. Cancer 2019, 146, 1686–1699. [Google Scholar] [CrossRef]
- Sarwar, M.; Syed Khaja, A.S.; Aleskandarany, M.; Karlsson, R.; Althobiti, M.; Odum, N.; Mongan, N.P.; Dizeyi, N.; Johnson, H.; Green, A.R.; et al. The role of PIP5K1alpha/pAKT and targeted inhibition of growth of subtypes of breast cancer using PIP5K1alpha inhibitor. Oncogene 2019, 38, 375–389. [Google Scholar] [CrossRef]
- Pettaway, C.A.; Pathak, S.; Greene, G.; Ramirez, E.; Wilson, M.R.; Killion, J.J.; Fidler, I.J. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin. Cancer Res. 1996, 2, 1627–1636. [Google Scholar]
- Wegiel, B.; Bjartell, A.; Tuomela, J.; Dizeyi, N.; Tinzl, M.; Helczynski, L.; Nilsson, E.; Otterbein, L.E.; Harkonen, P.; Persson, J.L. Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J. Natl. Cancer Inst. 2008, 100, 1022–1036. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsson, R.; Larsson, P.; Miftakhova, R.; Syed Khaja, A.S.; Sarwar, M.; Semenas, J.; Chen, S.; Hedblom, A.; Wang, T.; Ekström-Holka, K.; et al. Establishment of Prostate Tumor Growth and Metastasis Is Supported by Bone Marrow Cells and Is Mediated by PIP5K1α Lipid Kinase. Cancers 2020, 12, 2719. https://doi.org/10.3390/cancers12092719
Karlsson R, Larsson P, Miftakhova R, Syed Khaja AS, Sarwar M, Semenas J, Chen S, Hedblom A, Wang T, Ekström-Holka K, et al. Establishment of Prostate Tumor Growth and Metastasis Is Supported by Bone Marrow Cells and Is Mediated by PIP5K1α Lipid Kinase. Cancers. 2020; 12(9):2719. https://doi.org/10.3390/cancers12092719
Chicago/Turabian StyleKarlsson, Richard, Per Larsson, Regina Miftakhova, Azharuddin Sajid Syed Khaja, Martuza Sarwar, Julius Semenas, Sa Chen, Andreas Hedblom, Tianyan Wang, Kristina Ekström-Holka, and et al. 2020. "Establishment of Prostate Tumor Growth and Metastasis Is Supported by Bone Marrow Cells and Is Mediated by PIP5K1α Lipid Kinase" Cancers 12, no. 9: 2719. https://doi.org/10.3390/cancers12092719
APA StyleKarlsson, R., Larsson, P., Miftakhova, R., Syed Khaja, A. S., Sarwar, M., Semenas, J., Chen, S., Hedblom, A., Wang, T., Ekström-Holka, K., Simoulis, A., Kumar, A., Ødum, N., Grundström, T., & Persson, J. L. (2020). Establishment of Prostate Tumor Growth and Metastasis Is Supported by Bone Marrow Cells and Is Mediated by PIP5K1α Lipid Kinase. Cancers, 12(9), 2719. https://doi.org/10.3390/cancers12092719





