Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma
Simple Summary
Abstract
1. Introduction
2. Essential Oils
3. Mechanism of Action of EOs in Melanoma
3.1. Inhibition of Cell Proliferation
3.2. Alteration of Cell Cycle Distribution
3.3. Induction of Apoptosis
3.4. Induction of Necrosis or Modulation of Autophagy
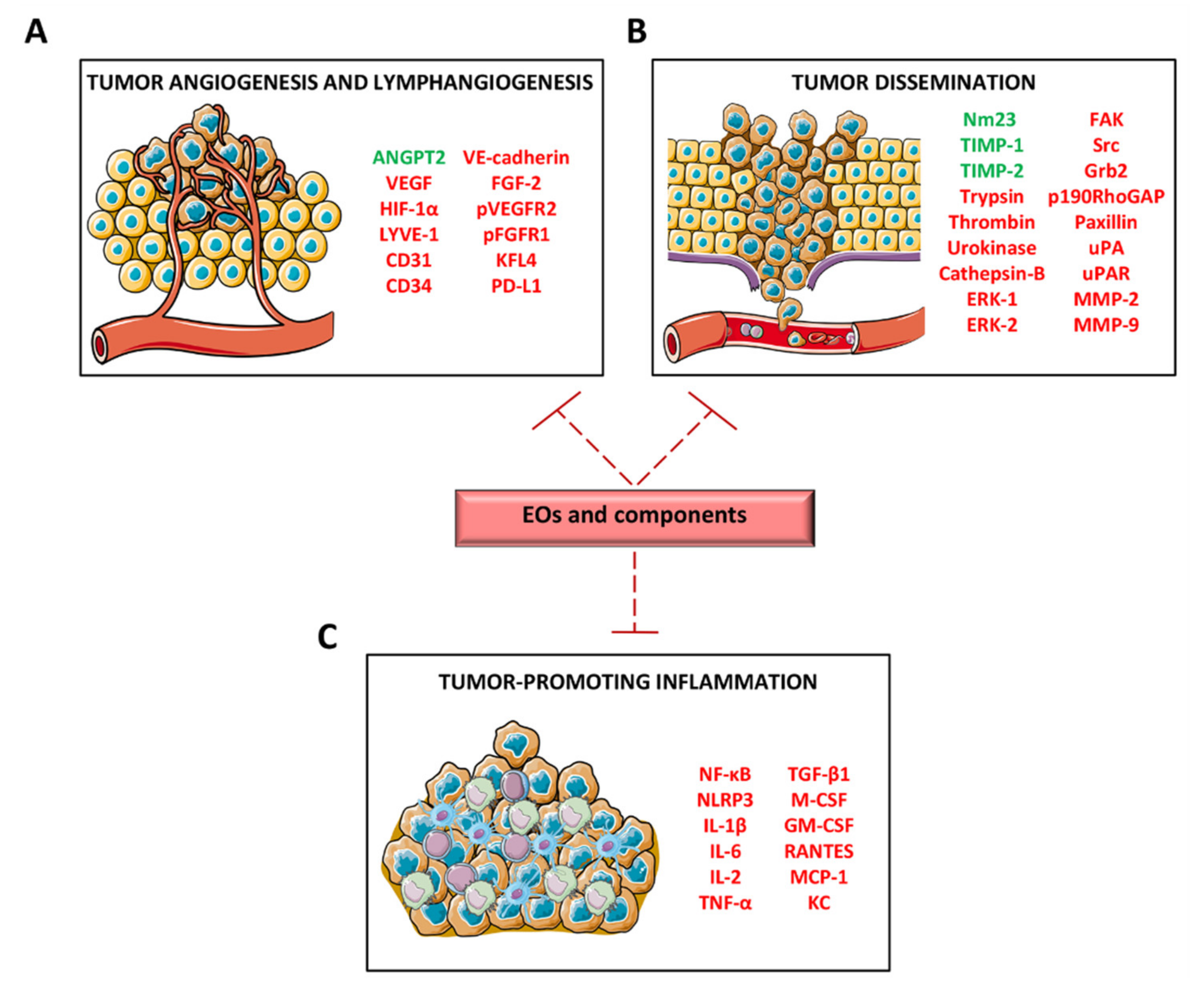
3.5. Inhibition of Angiogenesis and Lymphangiogenesis
3.6. Alteration of In Vitro Tumor Progression-Associated Functions and Inhibition of In Vivo Tumor Growth and Metastasization
3.7. Sensitization of Antitumor Agents
3.8. Chemopreventive Activity
3.9. Antioxidant Effect
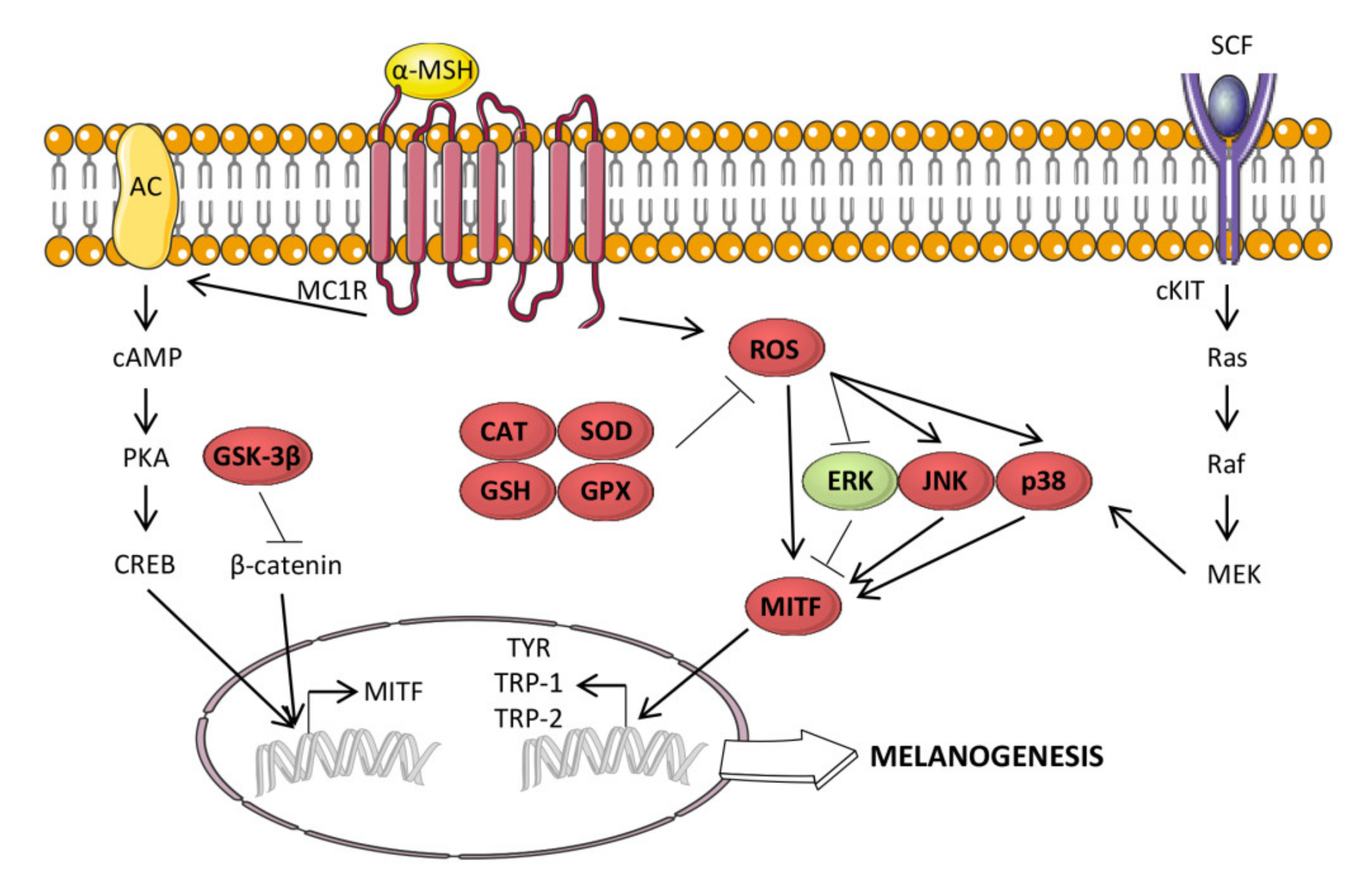
3.10. Antimelanogenic Activity
4. Clinical Use of EOs for Cancer Patients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tímár, J.; Vizkeleti, L.; Doma, V.; Barbai, T.; Rásó, E. Genetic progression of malignant melanoma. Cancer Metastasis Rev. 2016, 35, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, B. Game-changing class of immunotherapy drugs lengthens melanoma survival rates. Nature 2020, 580, S14–S16. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, L.; Khan, S.; Carvajal, R.D.; Yang, J. Novel Targets for the Treatment of Melanoma. Curr. Oncol. Rep. 2019, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Baik, C.; Kirkwood, J.M. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Candido, S.; Falzone, L.; Spandidos, D.A.; Libra, M. Cutaneous melanoma and the immunotherapy revolution (Review). Int. J. Oncol. 2020. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Lee, K.M. Current Insights into Combination Therapies with MAPK Inhibitors and Immune Checkpoint Blockade. Int. J. Mol. Sci. 2020, 21, 2531. [Google Scholar] [CrossRef]
- Becco, P.; Gallo, S.; Poletto, S.; Frascione, M.P.M.; Crotto, L.; Zaccagna, A.; Paruzzo, L.; Caravelli, D.; Carnevale-Schianca, F.; Aglietta, M. Melanoma Brain Metastases in the Era of Target Therapies: An Overview. Cancers 2020, 12, 1640. [Google Scholar] [CrossRef]
- Ishizuka, J.J.; Manguso, R.T.; Cheruiyot, C.K.; Bi, K.; Panda, A.; Iracheta-Vellve, A.; Miller, B.C.; Du, P.P.; Yates, K.B.; Dubrot, J.; et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 2019, 565, 43–48. [Google Scholar] [CrossRef]
- Mangan, B.L.; McAlister, R.K.; Balko, J.M.; Johnson, D.B.; Moslehi, J.J.; Gibson, A.; Phillips, E.J. Evolving Insights into the Mechanisms of Toxicity Associated with Immune Checkpoint Inhibitor Therapy. Br. J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Lu, H.; Liu, S.; Zhang, G.; Bin, W.; Zhu, Y.; Frederick, D.T.; Hu, Y.; Zhong, W.; Randell, S.; Sadek, N.; et al. PAK signalling drives acquired drug resistance to MAPK inhibitors in BRAF-mutant melanomas. Nature 2017, 550, 133–136. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y.; Lau, C.B.S. A Review of the Effects of Natural Compounds, Medicinal Plants, and Mushrooms on the Gut Microbiota in Colitis and Cancer. Front. Pharmacol. 2020, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Biswasroy, P.; Sahu, A.; Sahu, D.K.; Ghosh, G.; Rath, G. Recent advances in herbal nanomedicines for cancer treatment. Curr. Mol. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Burnham, J.F. Scopus database: A review. Biomed. Digit. Libr. 2006, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Salgueiro, L. Essential Oils Chemistry. In Bioactive Essential Oils and Cancer; de Sousa, D.o.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–61. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Mottaghipisheh, J.; Vitalini, S.; Iriti, M. Monoterpenes: Essential Oil Components with Valuable Features. Mini Rev. Med. Chem. 2020. [Google Scholar] [CrossRef]
- De Cássia Da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. Sesquiterpenes from Essential Oils and Anti-Inflammatory Activity. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Zellner, B.D.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review. Flavour Frag. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of Achillea millefolium L. Essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.T.; Tawata, S. Anti-Oxidant, Anti-Aging, and Anti-Melanogenic Properties of the Essential Oils from Two Varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.B.P.; Neto, A.F.S.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.R.C.E.; et al. Antitumour Activity of the Microencapsulation of Annona vepretorum Essential Oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F.; Formisano, C.; Rigano, D.; Senatore, F.; Bruno, M.; Rosselli, S.; Çelik, S. Anthemis wiedemanniana essential oil prevents LPS-induced production of NO in RAW 264.7 macrophages and exerts antiproliferative and antibacterial activities invitro. Nat. Prod. Res. 2012, 26, 1594–1601. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Newman, R.A.; Zhong, Y.; Liu, Z.; Nan, P. Chemical composition and bioactivity of the essential oil of Artemisia anomala from China. J. Essent. Oil Res. 2013, 25, 520–525. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. Dual bioactivities of essential oil extracted from the leaves of Artemisia argyi as an antimelanogenic versus antioxidant agent and chemical composition analysis by GC/MS. Int. J. Mol. Sci. 2012, 13, 14679–14697. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Murray, A.P. Antioxidant activity and chemical composition of essential oil from Atriplex undulata. Nat. Prod. Commun. 2010, 5, 1841–1844. [Google Scholar] [CrossRef]
- Salvador, M.J.; de Carvalho, J.E.; Wisniewski, A., Jr.; Kassuya, C.A.L.; Santos, E.P.; Riva, D.; Stefanello, M.E.A. Chemical composition and cytotoxic activity of the essential oil from the leaves of Casearia lasiophylla. Rev. Bras. Farmacogn. 2011, 21, 864–868. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical Composition, Antioxidant and Anti-melanogenic Activities of Essential Oils from Chrysanthemum boreale Makino at Different Harvesting Stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Chou, S.T.; Chang, W.L.; Chang, C.T.; Hsu, S.L.; Lin, Y.C.; Shih, Y. Cinnamomum cassia essential oil inhibits α-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Arciuli, M.; Arena, M.P.; Benvenuti, S.; Gallone, A. Chemical composition and the anti-melanogenic potential of different essential oils. Flavour Frag. J. 2016, 31, 255–261. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Provenzano, E.; Cindio, B.D.; Menichini, F. In vitro photo-induced cytotoxic activity of Citrus bergamia and C. medica L. cv. Diamante peel essential oils and identified active coumarins. Pharm. Biol. 2010, 48, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Hajlaoui, H.; Mighri, H.; Noumi, E.; Snoussi, M.; Trabelsi, N.; Ksouri, R.; Bakhrouf, A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem. Toxicol. 2010, 48, 2186–2192. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, L.; Yang, Z.; Chen, F.; Zheng, X.; Liu, X. Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crop. Prod. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Huang, Z.; Zhao, M.; Li, P.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; Tang, J.; et al. Variation in Essential Oil and Bioactive Compounds of Curcuma kwangsiensis Collected from Natural Habitats. Chem. Biodivers. 2017, 14, e1700020. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Gao, M.; Wu, J.; Wang, A.; Shi, R. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 220–226. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Lei, X.; Liao, L.; Fu, T.; Yuan, Y.; Huang, X.; Zou, L.; Liu, Y.; Ruan, R.; et al. Chemical composition and evaluation of antioxidant activities, antimicrobial, and anti-melanogenesis effect of the essential oils extracted from Dalbergia pinnata (Lour.) Prain. J. Ethnopharmacol. 2020, 254, 112731. [Google Scholar] [CrossRef]
- Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic Essential Oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) Growing in Central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef]
- Huang, H.C.; Ho, Y.C.; Lim, J.M.; Chang, T.Y.; Ho, C.L.; Chang, T.M. Investigation of the anti-melanogenic and antioxidant characteristics of Eucalyptus camaldulensis flower essential oil and determination of its chemical composition. Int. J. Mol. Sci. 2015, 16, 10470–10490. [Google Scholar] [CrossRef]
- Aranha, E.S.P.; de Azevedo, S.G.; dos Reis, G.G.; Silva Lima, E.; Machado, M.B.; de Vasconcellos, M.C. Essential oils from Eugenia spp.: In vitro antiproliferative potential with inhibitory action of metalloproteinases. Ind. Crop. Prod. 2019, 141, 111736. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Lai, C.C.; Lai, C.P.; Chao, W.W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crop. Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum cambess. ssp. tyrrhenicum bacch., brullo e giusso growing in la maddalena archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef]
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar] [CrossRef]
- Quassinti, L.; Lupidi, G.; Maggi, F.; Sagratini, G.; Papa, F.; Vittori, S.; Bianco, A.; Bramucci, M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27, 862–868. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar]
- Gismondi, A.; Canuti, L.; Grispo, M.; Canini, A. Biochemical composition and antioxidant properties of Lavandula angustifolia Miller essential oil are shielded by propolis against UV radiations. Photochem. Photobiol. 2014, 90, 702–708. [Google Scholar] [CrossRef]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; da Silva, T.B.; Machado, W.J.; Prata, A.P.; Costa, E.V.; Moraes, V.R.; Nogueira, P.C.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef]
- Quassinti, L.; Maggi, F.; Ortolani, F.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Miano, A.; Bramucci, M. Exploring new applications of tulip tree (Liriodendron tulipifera L.): Leaf essential oil as apoptotic agent for human glioblastoma. Environ. Sci. Pollut. Res. 2019, 26, 30485–30497. [Google Scholar] [CrossRef]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; Mondello, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Soo, W.N.; Chen, Y.H.; Shyur, L.F. Essential Oil of Mentha aquatica var. Kenting water mint suppresses two-stage skin carcinogenesis accelerated by BRAF inhibitor vemurafenib. Molecules 2019, 24, 2344. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, M.É.A.; Riva, D.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Salvador, M.J. Chemical Composition and Cytotoxic Activity of Essential Oil from Myrcia laruotteana Fruits. J. Essent. Oil Res. 2011, 23, 7–10. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.; Mahmoud Nassar, Z.D. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012, 5, 294–298. [Google Scholar] [CrossRef]
- Grecco Sdos, S.; Martins, E.G.; Girola, N.; de Figueiredo, C.R.; Matsuo, A.L.; Soares, M.G.; Bertoldo Bde, C.; Sartorelli, P.; Lago, J.H. Chemical composition and in vitro cytotoxic effects of the essential oil from Nectandra leucantha leaves. Pharm. Biol. 2015, 53, 133–137. [Google Scholar] [CrossRef]
- Trevisan, M.T.; Vasconcelos Silva, M.G.; Pfundstein, B.; Spiegelhalder, B.; Owen, R.W. Characterization of the volatile pattern and antioxidant capacity of essential oils from different species of the genus Ocimum. J. Agric. Food Chem. 2006, 54, 4378–4382. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael-Jubeli, R.; Bakar, J.; Dakroub, H.; Rizk, T.; Baillet-Guffroy, A.; Lteif, R.; Tfayli, A. Origanum essential oils reduce the level of melanin in B16-F1 melanocytes. Eur. J. Dermatol. 2019, 29, 596–602. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, J.H.; Moon, K.D.; Jeong, I.Y.; Ahn, D.U.K.; Lee, M.K.; Seo, K.I. Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem. Toxicol. 2014, 65, 97–104. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Guimarães, E.F.; Andrade, E.H.A.; Maia, J.G.S. Essential oils of Amazon Piper species and their cytotoxic, antifungal, antioxidant and anti-cholinesterase activities. Ind. Crop. Prod. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.N.; Ribeiro, A.S.; Santiago, G.M.P.; D’S. Costa, C.O.; Soares, M.B.; Bezerra, D.P.; Shanmugam, S.; Freitas, L.D.S.; Alves, P.B. Antitumor and Aedes aegypti Larvicidal Activities of Essential Oils from Piper klotzschianum, P. hispidum, and P. arboreum. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Loutrari, H.; Magkouta, S.; Pyriochou, A.; Koika, V.; Kolisis, F.N.; Papapetropoulos, A.; Roussos, C. Mastic oil from Pistacia lentiscus var. chia inhibits growth and survival of human K562 leukemia cells and attenuates angiogenesis. Nutr. Cancer 2006, 55, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Krifa, M.; El Mekdad, H.; Bentouati, N.; Pizzi, A.; Ghedira, K.; Hammami, M.; El Meshri, S.E.; Chekir-Ghedira, L. Immunomodulatory and anticancer effects of Pituranthos tortuosus essential oil. Tumor Biol. 2015, 36, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Krifa, M.; El Meshri, S.E.; Bentouati, N.; Pizzi, A.; Sick, E.; Chekir-Ghedira, L.; Rondé, P. In Vitro and in Vivo Anti-Melanoma Effects of Pituranthos tortuosus Essential Oil Via Inhibition of FAK and Src Activities. J. Cell. Biochem. 2016, 117, 1167–1175. [Google Scholar] [CrossRef]
- Manjamalai, A.; Grace, V.M.B. The chemotherapeutic effect of essential oil of Plectranthus amboinicus (Lour) on lung metastasis developed by B16F-10 cell line in C57BL/6 mice. Cancer Investig. 2013, 31, 74–82. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Peng, Y.; He, X.; Pan, S. Anti-oxidant and anti-melanogenic properties of essential oil from peel of Pomelo cv. Guan XI. Molecules 2019, 24, 242. [Google Scholar] [CrossRef]
- Da Silva, E.B.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 277–279. [Google Scholar] [CrossRef]
- Dutra, R.C.; Pittella, F.; Dittz, D.; Marcon, R.; Pimenta, D.S.; Lopes, M.T.P.; Raposo, N.R.B. Chemical composition and cytotoxicity activity of the essential oil of Pterodon emarginatus. Rev. Bras. Farmacogn. 2012, 22, 971–978. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crop. Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Cardile, V.; Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnopharmacol. 2009, 126, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.U.; Affara, N.I. Chemopreventive effects of sage oil on skin papillomas in mice. Phytomedicine 2000, 7, 129–136. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. Essential oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Formisano, C.; Rigano, D.; Canzoneri, M.; Bruno, M.; Senatore, F. Comparison of essential oil components and in vitro anticancer activity in wild and cultivated Salvia verbenaca. Nat. Prod. Res. 2015, 29, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Santha, S.; Dwivedi, C. Anticancer Effects of Sandalwood (Santalum album). Anticancer Res. 2015, 35, 3137–3145. [Google Scholar]
- Popovici, R.A.; Vaduva, D.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.S.; Sartorelli, P.; Guadagnin, R.C.; Matsuo, A.L.; Figueiredo, C.R.; Soares, M.G.; Da Silva, A.M.; Lago, J.H.G. Essential oils from Schinus terebinthifolius leaves chemical composition and in vitro cytotoxicity evaluation. Pharm. Biol. 2012, 50, 1248–1253. [Google Scholar] [CrossRef]
- Conforti, F.; Menichini, F.; Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Comparative chemical composition, free radical-scavenging and cytotoxic properties of essential oils of six Stachys species from different regions of the Mediterranean Area. Food Chem. 2009, 116, 898–905. [Google Scholar] [CrossRef]
- Shakeri, A.; D’Urso, G.; Taghizadeh, S.F.; Piacente, S.; Norouzi, S.; Soheili, V.; Asili, J.; Salarbashi, D. LC-ESI/LTQOrbitrap/MS/MS and GC–MS profiling of Stachys parviflora L. and evaluation of its biological activities. J. Pharm. Biomed. Anal. 2019, 168, 209–216. [Google Scholar] [CrossRef]
- Arung, E.T.; Matsubara, E.; Kusuma, I.W.; Sukaton, E.; Shimizu, K.; Kondo, R. Inhibitory components from the buds of clove (Syzygium aromaticum) on melanin formation in B16 melanoma cells. Fitoterapia 2011, 82, 198–202. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Júnior Dias, H.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.). Ind. Crop. Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Maggi, F. Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharm. Biol. 2017, 55, 1162–1170. [Google Scholar] [CrossRef]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Quassinti, L.; Vitali, L.A.; Maggi, F. Essential Oil of Thymus munbyanus subsp. coloratus from Algeria: Chemotypification and in vitro Biological Activities. Chem. Biodivers. 2017, 14, e1600299. [Google Scholar] [CrossRef]
- Manjamalai, A.; Mahesh Kumar, M.J.; Berlin Grace, V.M. Essential oil of tridax procumbens L induces apoptosis and suppresses angiogenesis and lung metastasis of the B16F-10 cell line in C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012, 13, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Lai, C.C.; Lin, C.C.; Chou, S.T. Effect of Vetiveria zizanioides essential oil on melanogenesis in melanoma cells: Downregulation of tyrosinase expression and suppression of oxidative stress. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, T.Y.; Chang, L.Z.; Wang, H.F.; Yih, K.H.; Hsieh, W.Y.; Chang, T.M. Inhibition of melanogenesis Versus antioxidant properties of essential oil extracted from leaves of vitex negundo linn and chemical composition analysis by GC-MS. Molecules 2012, 17, 3902–3916. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, T.Y.; Jeon, J.H.; Lee, S.H.; Hong, Y.K.; Jin, M.H. Inhibition of melanogenesis by abietatriene from Vitex trifolia leaf oil. Nat. Prod. Sci. 2016, 22, 252–258. [Google Scholar] [CrossRef]
- Manjamalai, A.; Berlin Grace, V.M. Antioxidant activity of essential oils from Wedelia chinensis (Osbeck) in vitro and in vivo lung cancer bearing C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012, 13, 3065–3071. [Google Scholar] [CrossRef]
- Costa, E.V.; Menezes, L.R.A.; Rocha, S.L.A.; Baliza, I.R.S.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Bezerra, D.P. Antitumor properties of the leaf essential oil of Zornia brasiliensis. Planta Med. 2015, 81, 563–567. [Google Scholar] [CrossRef]
- Harris, R. Synergism in the essential oil world. Int. J. Aromather. 2002, 12, 179–186. [Google Scholar] [CrossRef]
- Patsilinakos, A.; Artini, M.; Papa, R.; Sabatino, M.; Bozovic, M.; Garzoli, S.; Vrenna, G.; Buzzi, R.; Manfredini, S.; Selan, L.; et al. Machine Learning Analyses on Data including Essential Oil Chemical Composition and In Vitro Experimental Antibiofilm Activities against Staphylococcus Species. Molecules 2019, 24, 890. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.V.B.; Alves Filho, E.G.; Silva, L.M.A.; Zocolo, G.J.; de Brito, E.S.; Gramosa, N.V. Chemometric analysis of NMR and GC datasets for chemotype characterization of essential oils from different species of Ocimum. Talanta 2018, 180, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S. Chapter 8-Handling, safety and practical applications for use of essential oils. In Essential Chemistry for Aromatherapy, 2nd ed.; Clarke, S., Ed.; Churchill Livingstone: Edinburgh, UK, 2008; pp. 231–264. [Google Scholar] [CrossRef]
- Sabatino, M.; Fabiani, M.; Bozovic, M.; Garzoli, S.; Antonini, L.; Marcocci, M.E.; Palamara, A.T.; De Chiara, G.; Ragno, R. Experimental Data Based Machine Learning Classification Models with Predictive Ability to Select in Vitro Active Antiviral and Non-Toxic Essential Oils. Molecules 2020, 25, 2452. [Google Scholar] [CrossRef]
- Delfine, S.; Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Menichini, F.; Senatore, F. Variation of Malva sylvestris essential oil yield, chemical composition and biological activity in response to different environments across Southern Italy. Ind. Crop. Prod. 2017, 98, 29–37. [Google Scholar] [CrossRef]
- Djarri, L.; Medjroubi, K.; Akkal, S.; Elomri, A.; Seguin, E.; Groult, M.L.; Verite, P. Variability of two essential oils of Kundmannia sicula (L.) DC., a traditional Algerian medicinal plant. Molecules 2008, 13, 812–817. [Google Scholar] [CrossRef]
- Sefidkon, F.; Abbasi, K.; Khaniki, G.B. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem. 2006, 99, 19–23. [Google Scholar] [CrossRef]
- Mohtashami, S.; Rowshan, V.; Tabrizi, L.; Babalar, M.; Ghani, A. Summer savory (Satureja hortensis L.) essential oil constituent oscillation at different storage conditions. Ind. Crop. Prod. 2018, 111, 226–231. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Garzoli, S.; Pirolli, A.; Vavala, E.; Di Sotto, A.; Sartorelli, G.; Bozovic, M.; Angiolella, L.; Mazzanti, G.; Pepi, F.; Ragno, R. Multidisciplinary Approach to Determine the Optimal Time and Period for Extracting the Essential Oil from Mentha suaveolens Ehrh. Molecules 2015, 20, 9640–9655. [Google Scholar] [CrossRef]
- Bozovic, M.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Esential oils extraction: A 24-hour steam distillation systematic methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Padilla-González, G.F.; Frey, M.; Gómez-Zeledón, J.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (Smallanthus sonchifolius, Asteraceae). Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zámboriné Németh, É.; Thi Nguyen, H. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- McAnally, J.A.; Jung, M.; Mo, H. Farnesyl-O-acetylhydroquinone and geranyl-O-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. Cancer Lett. 2003, 202, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Tatman, D.; Jung, M.; Elson, C.E. Farnesyl anthranilate suppresses the growth, in vitro and in vivo, of murine B16 melanomas. Cancer Lett. 2000, 157, 145–153. [Google Scholar] [CrossRef]
- Effenberger, K.; Breyer, S.; Schobert, R. Terpene conjugates of the Nigella sativa seed-oil constituent thymoquinone with enhanced efficacy in cancer cells. Chem. Biodivers. 2010, 7, 129–139. [Google Scholar] [CrossRef]
- Tatman, D.; Mo, H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002, 175, 129–139. [Google Scholar] [CrossRef]
- Fabbri, J.; Maggiore, M.A.; Pensel, P.E.; Denegri, G.M.; Elissondo, M.C. In vitro efficacy study of Cinnamomum zeylanicum essential oil and cinnamaldehyde against the larval stage of Echinococcus granulosus. Exp. Parasitol. 2020, 214, 107904. [Google Scholar] [CrossRef]
- Mediouni, S.; Jablonski, J.A.; Tsuda, S.; Barsamian, A.; Kessing, C.; Richard, A.; Biswas, A.; Toledo, F.; Andrade, V.M.; Even, Y.; et al. Oregano Oil and Its Principal Component, Carvacrol, Inhibit HIV-1 Fusion into Target Cells. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, H.B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-Derived Products as Antibacterial and Antifungal Agents in Human Health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef] [PubMed]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Oprean, C.; Coricovac, D.E.; Andreea, C.; Cimpean, A.; Radeke, H.; Soica, C.; Dehelean, C. Behaviour of four different B16 murine melanoma cell sublines: C57BL/6J skin. Int. J. Exp. Pathol. 2015, 96, 73–80. [Google Scholar] [CrossRef]
- Teicher, B.A. Tumor Models in Cancer Research; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Paschoalin, T.; Carmona, A.K.; Rodrigues, E.G.; Oliveira, V.; Monteiro, H.P.; Juliano, M.A.; Juliano, L.; Travassos, L.R. Characterization of thimet oligopeptidase and neurolysin activities in B16F10-Nex2 tumor cells and their involvement in angiogenesis and tumor growth. Mol. Cancer 2007, 6, 44. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 2002, 70, 791–798. [Google Scholar] [CrossRef]
- Shi, H.; Liu, L.; Liu, L.M.; Geng, J.; Chen, L. Inhibition of tumor growth by beta-elemene through downregulation of the expression of uPA, uPAR, MMP-2, and MMP-9 in a murine intraocular melanoma model. Melanoma Res. 2015, 25, 15–21. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018, 114, e182–e190. [Google Scholar] [CrossRef]
- Xu, M.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. 2015, 71, 1–7. [Google Scholar] [CrossRef]
- Mead, T.J.; Lefebvre, V. Proliferation assays (BrdU and EdU) on skeletal tissue sections. Methods Mol. Biol. 2014, 1130, 233–243. [Google Scholar]
- Griffiths, M.; Sundaram, H. Drug design and testing: Profiling of antiproliferative agents for cancer therapy using a cell-based methyl-[3H]-thymidine incorporation assay. Methods Mol. Biol. 2011, 731, 451–465. [Google Scholar] [PubMed]
- Alsaraf, S.; Hadi, Z.; Al-Lawati, W.M.; Al Lawati, A.A.; Khan, S.A. Chemical composition, in vitro antibacterial and antioxidant potential of Omani Thyme essential oil along with in silico studies of its major constituent. J. King Saud Univ. Sci. 2020, 32, 1021–1028. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT Food Sci. Technol. 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Govindaraju, S.; Arulselvi, P.I. Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. J. Herbs Spices Med. Plants 2018, 24, 37–51. [Google Scholar] [CrossRef]
- Melo, J.O.; Fachin, A.L.; Rizo, W.F.; Jesus, H.C.R.; Arrigoni-Blank, M.F.; Alves, P.B.; Marins, M.A.; França, S.C.; Blank, A.F. Cytotoxic effects of essential oils from three lippia gracilis schauer genotypes on HeLa, B16, and MCF-7 cells and normal human fibroblasts. Genet. Mol. Res. 2014, 13, 2691–2697. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 2019, 71, 461–473. [Google Scholar] [CrossRef]
- Greay, S.J.; Ireland, D.J.; Kissick, H.T.; Levy, A.; Beilharz, M.W.; Riley, T.V.; Carson, C.F. Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen-4-ol. Cancer Chemother. Pharmacol. 2010, 65, 877–888. [Google Scholar] [CrossRef]
- Biswas, R.; Mandal, S.K.; Dutta, S.; Bhattacharyya, S.S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Thujone-Rich Fraction of Thuja occidentalis Demonstrates Major Anti-Cancer Potentials: Evidences from In Vitro Studies on A375 Cells. Evid. Based Complement. Altern. Med. 2011, 2011, 568148. [Google Scholar] [CrossRef]
- Rajput, J.; Bagul, S.; Tadavi, S.; Bendre, R. Comparative Anti-Proliferative Studies of Natural Phenolic Monoterpenoids on Human Malignant Tumour Cells. Med. Aromat. Plants 2016, 5, 1–4. [Google Scholar] [CrossRef]
- Sanches, L.J.; Marinello, P.C.; Panis, C.; Fagundes, T.R.; Morgado-Diaz, J.A.; de-Freitas-Junior, J.C.; Cecchini, R.; Cecchini, A.L.; Luiz, R.C. Cytotoxicity of citral against melanoma cells: The involvement of oxidative stress generation and cell growth protein reduction. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Junior, P.L.; Camara, D.A.; Costa, A.S.; Ruiz, J.L.; Levy, D.; Azevedo, R.A.; Pasqualoto, K.F.; de Oliveira, C.F.; de Melo, T.C.; Pessoa, N.D.; et al. Apoptotic effect of eugenol envolves G2/M phase abrogation accompanied by mitochondrial damage and clastogenic effect on cancer cell in vitro. Phytomedicine 2016, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef]
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol. Cancer 2007, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Kijpornyongpan, T.; Sereemaspun, A.; Chanchao, C. Dose-dependent cytotoxic effects of menthol on human malignant melanoma A-375 cells: Correlation with TRPM8 transcript expression. Asian Pac. J. Cancer Prev. 2014, 15, 1551–1556. [Google Scholar] [CrossRef]
- Yan, H.; Ren, M.Y.; Wang, Z.X.; Feng, S.J.; Li, S.; Cheng, Y.; Hu, C.X.; Gao, S.Q.; Zhang, G.Q. Zerumbone inhibits melanoma cell proliferation and migration by altering mitochondrial functions. Oncol. Lett. 2017, 13, 2397–2402. [Google Scholar] [CrossRef]
- Wang, S.D.; Wang, Z.H.; Yan, H.Q.; Ren, M.Y.; Gao, S.Q.; Zhang, G.Q. Chemotherapeutic effect of Zerumbone on melanoma cells through mitochondria-mediated pathways. Clin. Exp. Dermatol. 2016, 41, 858–863. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.S.; Chun, H.S.; Kwon, S.H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Huang, Y.; Zhao, S.; Xu, Y.; Chen, Y.; Jiang, F.; Tao, L.; Shen, X. EOFAZ inhibits endothelialtomesenchymal transition through downregulation of KLF4. Int. J. Mol. Med. 2020, 46, 300–310. [Google Scholar]
- Bozzuto, G.; Colone, M.; Toccacieli, L.; Stringaro, A.; Molinari, A. Tea tree oil might combat melanoma. Planta Med. 2011, 77, 54–56. [Google Scholar] [CrossRef]
- Martins, B.X.; Arruda, R.F.; Costa, G.A.; Jerdy, H.; de Souza, S.B.; Santos, J.M.; de Freitas, W.R.; Kanashiro, M.M.; de Carvalho, E.C.Q.; Sant’Anna, N.F.; et al. Myrtenal-induced V-ATPase inhibition-A toxicity mechanism behind tumor cell death and suppressed migration and invasion in melanoma. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1–12. [Google Scholar] [CrossRef]
- Siveen, K.S.; Kuttan, G. Thujone inhibits lung metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Can. J. Physiol. Pharmacol. 2011, 89, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Muneer, K.M.; Tamimi, I.A.; Chang, M.E.; Ata, M.O.; Yusuf, N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013, 270, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Abdo Hasson, S.S.A.; Jabri, A.A.; et al. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Oncotarget 2019, 10, 3472–3490. [Google Scholar] [CrossRef]
- Greay, S.J.; Ireland, D.J.; Kissick, H.T.; Heenan, P.J.; Carson, C.F.; Riley, T.V.; Beilharz, M.W. Inhibition of established subcutaneous murine tumour growth with topical Melaleuca alternifolia (tea tree) oil. Cancer Chemother. Pharmacol. 2010, 66, 1095–1102. [Google Scholar] [CrossRef]
- Jedinak, A.; Muckova, M.; Kost’alova, D.; Maliar, T.; Masterova, I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z. Naturforsch. C J. Biosci. 2006, 61, 777–782. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H. Alpha-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Raphael, T.J.; Kuttan, G. Effect of naturally occurring monoterpenes carvone, limonene and perillic acid in the inhibition of experimental lung metastasis induced by B16F-10 melanoma cells. J. Exp. Clin. Cancer Res. 2003, 22, 419–424. [Google Scholar]
- Lee, S.R.; Mun, J.Y.; Jeong, M.S.; Lee, H.H.; Roh, Y.G.; Kim, W.T.; Kim, M.H.; Heo, J.; Choi, Y.H.; Kim, S.J.; et al. Thymoquinone-Induced Tristetraprolin Inhibits Tumor Growth and Metastasis through Destabilization of MUC4 mRNA. Int. J. Mol. Sci. 2019, 20, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079. [Google Scholar] [CrossRef]
- Bommareddy, A.; Brozena, S.; Steigerwalt, J.; Landis, T.; Hughes, S.; Mabry, E.; Knopp, A.; VanWert, A.L.; Dwivedi, C. Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil. Nat. Prod. Res. 2019, 33, 527–543. [Google Scholar] [CrossRef]
- Zhang, X.; Dwivedi, C. Skin cancer chemoprevention by α-santalol. Front. Biosci. Schol. Ed. 2011, 3, S777–S787. [Google Scholar]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mi, J.; Qin, H.; Li, Z.; Chai, J.; Li, M.; Wu, J.; Xu, J. E2F1/IGF-1R Loop Contributes to BRAF Inhibitor Resistance in Melanoma. J. Investig. Dermatol. 2020, 140, 1295–1299. [Google Scholar] [CrossRef]
- Meng, P.; Bedolla, R.G.; Yun, H.; Fitzpatrick, J.E.; Kumar, A.P.; Ghosh, R. Contextual role of E2F1 in suppression of melanoma cell motility and invasiveness. Mol. Carcinog. 2019, 58, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Delmondes, G.A.; Santiago Lemos, I.C.; Dias, D.Q.; Cunha, G.L.D.; Araújo, I.M.; Barbosa, R.; Coutinho, H.D.M. Pharmacological applications of farnesol (C(15)H(26)O): A patent review. Expert Opin. Ther. Pat. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Cerchiara, T.; Straface, S.V.; Brunelli, E.; Tripepi, S.; Gallucci, M.C.; Chidichimo, G. Antiproliferative effect of linalool on RPMI 7932 human melanoma cell line: Ultrastructural studies. Nat. Prod. Commun. 2015, 10, 547–549. [Google Scholar] [CrossRef]
- Slominski, A. Cooling skin cancer: Menthol inhibits melanoma growth. Focus on “TRPM8 activation suppresses cellular viability in human melanoma”. Am. J. Physiol. Cell Physiol. 2008, 295, C293–C295. [Google Scholar] [CrossRef]
- Yamamura, H.; Ugawa, S.; Ueda, T.; Morita, A.; Shimada, S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol. Cell Physiol. 2008, 295, C296–C301. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Krysko, D.V.; Berghe, T.V.; Parthoens, E.; D’Herde, K.; Vandenabeele, P. Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Method. Enzymol. 2008, 442, 307–341. [Google Scholar]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Choucroun, P.; Gillet, D.; Dorange, G.; Sawicki, B.; Dewitte, J.D. Comet assay and early apoptosis. Mutat. Res. 2001, 478, 89–96. [Google Scholar] [CrossRef]
- Giordani, C.; Molinari, A.; Toccacieli, L.; Calcabrini, A.; Stringaro, A.; Chistolini, P.; Arancia, G.; Diociaiuti, M. Interaction of tea tree oil with model and cellular membranes. J. Med. Chem. 2006, 49, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 2012, 17, 6290–6304. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of zerumbone as an anti-cancer agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharm. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox. Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Kuma, A.; Komatsu, M.; Mizushima, N. Autophagy-monitoring and autophagy-deficient mice. Autophagy 2017, 13, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Al Dhaheri, Y.; Iratni, R. Origanum majorana Essential Oil Triggers p38 MAPK-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.C.; Siddiqui, M.S.; Athar, M.; Alam, M.S. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 2012, 31, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Pudełek, M.; Catapano, J.; Kochanowski, P.; Mrowiec, K.; Janik-Olchawa, N.; Czyż, J.; Ryszawy, D. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Russo, R.; Cassiano, M.G.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of D-Limonene in autophagy induced by bergamot essential oil in SH-SY5Y neuroblastoma cells. PLoS ONE 2014, 9, e113682. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Khaw-On, P. Terpinen-4-ol induces autophagic and apoptotic cell death in human leukemic HL-60 cells. Asian Pac. J. Cancer Prev. 2013, 14, 7537–7542. [Google Scholar] [CrossRef]
- Ding, X.F.; Shen, M.; Xu, L.Y.; Dong, J.H.; Chen, G. 13,14-bis(cis-3,5-dimethyl-1-piperazinyl)-beta-elemene, a novel beta-elemene derivative, shows potent antitumor activities via inhibition of mTOR in human breast cancer cells. Oncol. Lett. 2013, 5, 1554–1558. [Google Scholar] [CrossRef]
- Streit, M.; Detmar, M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene 2003, 22, 3172–3179. [Google Scholar] [CrossRef]
- Albini, A.; Tosetti, F.; Li, V.W.; Noonan, D.M.; Li, W.W. Cancer prevention by targeting angiogenesis. Nat. Rev. Clin. Oncol. 2012, 9, 498–509. [Google Scholar] [CrossRef]
- Mabeta, P. Paradigms of vascularization in melanoma: Clinical significance and potential for therapeutic targeting. Biomed. Pharmacother. 2020, 127, 110135. [Google Scholar] [CrossRef]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Jour, G.; Ivan, D.; Aung, P.P. Angiogenesis in melanoma: An update with a focus on current targeted therapies. J. Clin. Pathol. 2016, 69, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Kiyan, H.T.; Demirci, B.; Baser, K.H.; Demirci, F. The in vivo evaluation of anti-angiogenic effects of Hypericum essential oils using the chorioallantoic membrane assay. Pharm. Biol. 2014, 52, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Mishra, P.K.; Iqbal, Z.; Jaggi, M.; Madaan, A.; Bhuyan, K.; Gupta, N.; Gupta, N.; Vats, K.; Verma, R.; et al. Co-Delivery of Eugenol and Dacarbazine by Hyaluronic Acid-Coated Liposomes for Targeted Inhibition of Survivin in Treatment of Resistant Metastatic Melanoma. Pharmaceutics 2019, 11, 163. [Google Scholar] [CrossRef]
- Yousefian Rad, E.; Homayouni Tabrizi, M.; Ardalan, P.; Seyedi, S.M.R.; Yadamani, S.; Zamani-Esmati, P.; Haghani Sereshkeh, N. Citrus lemon essential oil nanoemulsion (CLEO-NE), a safe cell-depended apoptosis inducer in human A549 lung cancer cells with anti-angiogenic activity. J. Microencapsul. 2020, 37, 394–402. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Santos-Júnior, L.; Oliveira, T.V.C.; Cândido, J.F.; Santana, D.S.; Pereira, R.N.F.; Pereyra, B.B.S.; Gomes, M.Z.; Lima, S.O.; Albuquerque-Júnior, R.L.C.; Cândido, E.A.F. Effects of the essential oil of Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm. on healing and tissue repair after partial Achilles tenotomy in rats. Acta Cir. Bras. 2017, 32, 449–458. [Google Scholar] [PubMed]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Hwang, D.I.; Yoon, S.W.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale Makino essential oil induces keratinocyte proliferation and skin regeneration. Nat. Prod. Res. 2015, 29, 562–564. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Eleonora Graziano, A.C.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Labib, R.M.; Ayoub, I.M. Appraisal on the wound healing potential of Melaleuca alternifolia and Rosmarinus officinalis L. essential oil-loaded chitosan topical preparations. PLoS ONE 2019, 14, e0219561. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, G.M.; Kim, J.K. Zerumbone, Sesquiterpene Photochemical from Ginger, Inhibits Angiogenesis. Korean J. Physiol. Pharmacol. 2015, 19, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.A.; Abdul, A.B.; Rahman, H.S.; Rasedee, A.; Tengku Ibrahim, T.A.; Keon, Y.S. Zerumbone Suppresses Angiogenesis in HepG2 Cells through Inhibition of Matrix Metalloproteinase-9, Vascular Endothelial Growth Factor, and Vascular Endothelial Growth Factor Receptor Expressions. Pharmacogn. Mag. 2018, 13, S731–S736. [Google Scholar] [PubMed]
- Shojaei, S.; Kiumarsi, A.; Moghadam, A.R.; Alizadeh, J.; Marzban, H.; Ghavami, S. Perillyl Alcohol (Monoterpene Alcohol), Limonene. Enzymes 2014, 36, 7–32. [Google Scholar] [PubMed]
- Loutrari, H.; Hatziapostolou, M.; Skouridou, V.; Papadimitriou, E.; Roussos, C.; Kolisis, F.N.; Papapetropoulos, A. Perillyl alcohol is an angiogenesis inhibitor. J. Pharmacol. Exp. Ther. 2004, 311, 568–575. [Google Scholar] [CrossRef]
- Zuo, H.X.; Jin, Y.; Wang, Z.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Xing, Y.; Ri, M.H.; Jin, C.H.; Xu, G.H.; et al. Curcumol inhibits the expression of programmed cell death-ligand 1 through crosstalk between hypoxia-inducible factor-1alpha and STAT3 (T705) signaling pathways in hepatic cancer. J. Ethnopharmacol. 2020, 257, 112835. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujisawa, Y.; Sae, I.; Maruyama, H.; Ito, S.; Hasegawa, N.; Sekine, I.; Fujimoto, M. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn. J. Clin. Oncol. 2017, 47, 175–178. [Google Scholar] [CrossRef]
- Scarpati, G.D.; Fusciello, C.; Perri, F.; Sabbatino, F.; Ferrone, S.; Carlomagno, C.; Pepe, S. Ipilimumab in the treatment of metastatic melanoma: Management of adverse events. Oncotargets Ther. 2014, 7, 203–209. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Frag. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, G.; Guan, F. Biological Functions and Analytical Strategies of Sialic Acids in Tumor. Cells 2020, 9, 273. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhu, W.; Chu, H.; Yu, H.; Wei, P.; Wu, X.; Zhu, H.; Gao, H.; Liang, J.; et al. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature 2019, 571, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, M.; Urakami, K.; Masuda, Y.; Zangiacomi, V.; Ishii, H.; Tai, S.; Maruyama, K.; Yamaguchi, K. Fragrant environment with alpha-pinene decreases tumor growth in mice. Biomed. Res. 2012, 33, 57–61. [Google Scholar] [CrossRef]
- Kusuhara, M.; Maruyama, K.; Ishii, H.; Masuda, Y.; Sakurai, K.; Tamai, E.; Urakami, K. A Fragrant Environment Containing α-Pinene Suppresses Tumor Growth in Mice by Modulating the Hypothalamus/Sympathetic Nerve/Leptin Axis and Immune System. Integr. Cancer Ther. 2019, 18, 1534735419845139. [Google Scholar] [CrossRef] [PubMed]
- Lesgards, J.F.; Baldovini, N.; Vidal, N.; Pietri, S. Anticancer activities of essential oils constituents and synergy with conventional therapies: A review. Phytother. Res. 2014, 28, 1423–1446. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The Use of ss-Elemene to Enhance Radio Sensitization of A375 Human Melanoma Cells. Cell J. 2020, 21, 419–425. [Google Scholar] [PubMed]
- Benimetskaya, L.; Lai, J.C.; Khvorova, A.; Wu, S.; Hua, E.; Miller, P.; Zhang, L.M.; Stein, C.A. Relative Bcl-2 independence of drug-induced cytotoxicity and resistance in 518A2 melanoma cells. Clin. Cancer Res. 2004, 10, 8371–8379. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol. Carcinog. 2010, 49, 290–301. [Google Scholar] [CrossRef]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Alam, M.S.; Siddiqui, M.S.; Athar, M. Chemopreventive effect of farnesol on DMBA/TPA-induced skin tumorigenesis: Involvement of inflammation, Ras-ERK pathway and apoptosis. Life Sci. 2009, 85, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Selvan, M.V. Chemopreventive potential of geraniol in 7,12-dimethylbenz(a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. J. Environ. Biol. 2012, 33, 255–260. [Google Scholar] [PubMed]
- Liu, Z.; Shen, C.; Tao, Y.; Wang, S.; Wei, Z.; Cao, Y.; Wu, H.; Fan, F.; Lin, C.; Shan, Y.; et al. Chemopreventive efficacy of menthol on carcinogen-induced cutaneous carcinoma through inhibition of inflammation and oxidative stress in mice. Food Chem. Toxicol. 2015, 82, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lluria-Prevatt, M.; Morreale, J.; Gregus, J.; Alberts, D.S.; Kaper, F.; Giaccia, A.; Powell, M.B. Effects of perillyl alcohol on melanoma in the TPras mouse model. Cancer Epidemiol. Biomark. Prev. 2002, 11, 573–579. [Google Scholar]
- Chaudhary, S.C.; Alam, M.S.; Siddiqui, M.S.; Athar, M. Perillyl alcohol attenuates Ras-ERK signaling to inhibit murine skin inflammation and tumorigenesis. Chem. Biol. Interact. 2009, 179, 145–153. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Blom van Staden, A.; Hosenally, M.; Mahomoodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef]
- Horiba, H.; Nakagawa, T.; Zhu, Q.; Ashour, A.; Watanabe, A.; Shimizu, K. Biological Activities of Extracts from Different Parts of Cryptomeria japonica. Nat. Prod. Commun. 2016, 11, 1337–1342. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, Y.C.; Tsai, M.L.; Cheng, C.Y.; Liu, L.L.; Yen, Y.W.; Chen, W.L. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci. 2015, 37, 550–554. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael Jubeli, R.; El Beyrouthy, M.; Baillet Guffroy, A.; Rizk, T.; Tfayli, A.; Lteif, R. Phytochemical screening and antityrosinase activity of carvacrol, thymoquinone, and four essential oils of Lebanese plants. J. Cosmet. Dermatol. 2019, 18, 944–952. [Google Scholar] [CrossRef]
- Ko, G.A.; Cho, S.K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem. Biol. Interact. 2018, 286, 132–140. [Google Scholar] [CrossRef]
- Jeong, H.; Yu, S.M.; Kim, S.J. Inhibitory effects on melanogenesis by thymoquinone are mediated through the betacatenin pathway in B16F10 mouse melanoma cells. Int. J. Oncol. 2020, 56, 379–389. [Google Scholar] [PubMed]
- Nam, J.H.; Nam, D.Y.; Lee, D.U. Valencene from the Rhizomes of Cyperus rotundus Inhibits Skin Photoaging-Related Ion Channels and UV-Induced Melanogenesis in B16F10 Melanoma Cells. J. Nat. Prod. 2016, 79, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a Tropical Ginger Sesquiterpene of Zingiber officinale Roscoe, Attenuates alpha-MSH-Induced Melanogenesis in B16F10 Cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- McKinney, A.J.; Holmen, S.L. Animal models of melanoma: A somatic cell gene delivery mouse model allows rapid evaluation of genes implicated in human melanoma. Chin. J. Cancer 2011, 30, 153–162. [Google Scholar] [CrossRef]
- Stratton, S.P.; Alberts, D.S.; Einspahr, J.G.; Sagerman, P.M.; Warneke, J.A.; Curiel-Lewandrowski, C.; Myrdal, P.B.; Karlage, K.L.; Nickoloff, B.J.; Brooks, C.; et al. A phase 2a study of topical perillyl alcohol cream for chemoprevention of skin cancer. Cancer Prev. Res. (Phila) 2010, 3, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.P.; Saboda, K.L.; Myrdal, P.B.; Gupta, A.; McKenzie, N.E.; Brooks, C.; Salasche, S.J.; Warneke, J.A.; Ranger-Moore, J.; Bozzo, P.D.; et al. Phase 1 study of topical perillyl alcohol cream for chemoprevention of skin cancer. Nutr. Cancer 2008, 60, 325–330. [Google Scholar] [CrossRef]
- Nishigori, C.; Hattori, Y.; Toyokuni, S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox. Signal. 2004, 6, 561–570. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gong, J.; Han, H.; He, L.; Teng, Y.; Tetley, T.; Sinharay, R.; Chung, K.F.; Islam, T.; Gilliland, F.; et al. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J. Thorac. Dis. 2018, 10, 3088–3097. [Google Scholar] [CrossRef] [PubMed]
- Rahiman, N.; Akaberi, M.; Sahebkar, A.; Emami, S.A.; Tayarani-Najaran, Z. Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc. Res. 2018, 118, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Onyebuchi, C.; Kavaz, D. Chitosan And N, N, N-Trimethyl Chitosan Nanoparticle Encapsulation Of Ocimum Gratissimum Essential Oil: Optimised Synthesis, In Vitro Release And Bioactivity. Int. J. Nanomed. 2019, 14, 7707–7727. [Google Scholar] [CrossRef]
- Do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Gois Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Manjamalai, A.; Grace, B. Chemotherapeutic effect of essential oil of Wedelia chinensis (Osbeck) on inducing apoptosis, suppressing angiogenesis and lung metastasis in C57BL/6 mice model. J. Cancer Sci. Ther. 2013, 5, 271–281. [Google Scholar] [CrossRef]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef]
- Jackett, L.A.; Scolyer, R.A. A Review of Key Biological and Molecular Events Underpinning Transformation of Melanocytes to Primary and Metastatic Melanoma. Cancers 2019, 11, 2041. [Google Scholar] [CrossRef]
- Liu, G.S.; Peshavariya, H.; Higuchi, M.; Brewer, A.C.; Chang, C.W.; Chan, E.C.; Dusting, G.J. Microphthalmia-associated transcription factor modulates expression of NADPH oxidase type 4: A negative regulator of melanogenesis. Free Radic. Biol. Med. 2012, 52, 1835–1843. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents--existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Zhu, Q.; Ishikawa, H.; Ohnuki, K.; Kakino, K.; Horiuchi, N.; Shinotsuka, H.; Naito, T.; Matsumoto, T.; Minamisawa, N. Multiple uses of essential oil and by-products from various parts of the Yakushima native Cedar (Cryptomeria Japonica). J. Wood Chem. Technol. 2016, 36, 42–55. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Khan, F.N.; Ali, S.A.; Khan, K.P. Insight into Mechanistic Action of Thymoquinone Induced Melanogenesis in Cultured Melanocytes. Protein Pept. Lett. 2019, 26, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Cleary, L.; Ragsdale-Lowe, M.; McNeill, S.; Osland, C. The use of aromasticks at a cancer centre: A retrospective audit. Complement. Ther. Clin. Pract. 2014, 20, 203–206. [Google Scholar] [CrossRef]
- Boehm, K.; Büssing, A.; Ostermann, T. Aromatherapy as an adjuvant treatment in cancer care—A descriptive systematic review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Maddocks-Jennings, W.; Wilkinson, J.M.; Cavanagh, H.M.; Shillington, D. Evaluating the effects of the essential oils Leptospermum scoparium (manuka) and Kunzea ericoides (kanuka) on radiotherapy induced mucositis: A randomized, placebo controlled feasibility study. Eur. J. Oncol. Nurs. 2009, 13, 87–93. [Google Scholar] [CrossRef]
- Reis, D.; Jones, T.T. Frankincense Essential Oil as a Supportive Therapy for Cancer-Related Fatigue: A Case Study. Holist. Nurs. Pract. 2018, 32, 140–142. [Google Scholar] [CrossRef]
- Pimenta, F.C.; Alves, M.F.; Pimenta, M.B.; Melo, S.A.; de Almeida, A.A.; Leite, J.R.; Pordeus, L.C.; Diniz Mde, F.; de Almeida, R.N. Anxiolytic Effect of Citrus aurantium L. on Patients with Chronic Myeloid Leukemia. Phytother. Res. 2016, 30, 613–617. [Google Scholar] [CrossRef]
- Mapp, C.P.; Hostetler, D.; Sable, J.F.; Parker, C.; Gouge, E.; Masterson, M.; Willis-Styles, M.; Fortner, C.; Higgins, M. Peppermint Oil: Evaluating Efficacy on Nausea in Patients Receiving Chemotherapy in the Ambulatory Setting. Clin. J. Oncol. Nurs. 2020, 24, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Talasaz-Firoozi, E.; Nasiri, R.; Jalali, N.; Hassanzadeh, M. Antiemetic activity of volatile oil from Mentha spicata and Mentha x piperita in chemotherapy-induced nausea and vomiting. Ecancermedicalscience 2013, 7, 290. [Google Scholar]
- Hamzeh, S.; Safari-Faramani, R.; Khatony, A. Effects of Aromatherapy with Lavender and Peppermint Essential Oils on the Sleep Quality of Cancer Patients: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 7480204. [Google Scholar] [CrossRef] [PubMed]
- Re, L.; Barocci, S.; Sonnino, S.; Mencarelli, A.; Vivani, C.; Paolucci, G.; Scarpantonio, A.; Rinaldi, L.; Mosca, E. Linalool modifies the nicotinic receptor–ion channel kinetics at the mouse neuromuscular junction. Pharmacol. Res. 2000, 42, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Gedney, J.J.; Glover, T.L.; Fillingim, R.B. Sensory and affective pain discrimination after inhalation of essential oils. Psychosom. Med. 2004, 66, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, L.; Achor, S.; Allen, B.; Bauchmire, N.; Dunnington, D.; Klisovic, R.B.; Naber, S.J.; Roblee, K.; Samczak, A.; Tomlinson-Pinkham, K.; et al. The Effect of Aromatherapy on Insomnia and Other Common Symptoms Among Patients With Acute Leukemia. Oncol. Nurs. Forum 2017, 44, E185–E193. [Google Scholar] [CrossRef]
- Evans, A.; Malvar, J.; Garretson, C.; Pedroja Kolovos, E.; Baron Nelson, M. The Use of Aromatherapy to Reduce Chemotherapy-Induced Nausea in Children With Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pediatr. Oncol. Nurs. 2018, 35, 392–398. [Google Scholar] [CrossRef]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement. Ther. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef]
- Tamaki, K.; Fukuyama, A.K.; Terukina, S.; Kamada, Y.; Uehara, K.; Arakaki, M.; Yamashiro, K.; Miyashita, M.; Ishida, T.; McNamara, K.M. Randomized trial of aromatherapy versus conventional care for breast cancer patients during perioperative periods. Breast Cancer Res. Treat. 2017, 162, 523–531. [Google Scholar] [CrossRef]
- Chen, T.H.; Tung, T.H.; Chen, P.S.; Wang, S.H.; Chao, C.M.; Hsiung, N.H.; Chi, C.C. The Clinical Effects of Aromatherapy Massage on Reducing Pain for the Cancer Patients: Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2016, 2016, 9147974. [Google Scholar] [CrossRef]
- Graham, P.H.; Browne, L.; Cox, H.; Graham, J. Inhalation aromatherapy during radiotherapy: Results of a placebo-controlled double-blind randomized trial. J. Clin. Oncol. 2003, 21, 2372–2376. [Google Scholar] [CrossRef]
- Mo, H.; Jeter, R.; Bachmann, A.; Yount, S.T.; Shen, C.L.; Yeganehjoo, H. The Potential of Isoprenoids in Adjuvant Cancer Therapy to Reduce Adverse Effects of Statins. Front. Pharmacol. 2018, 9, 1515. [Google Scholar] [CrossRef]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of monoterpenes and sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Patsilinakos, A.; Papa, R.; Bozovic, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Ragno, R.; et al. Antimicrobial and Antibiofilm Activity and Machine Learning Classification Analysis of Essential Oils from Different Mediterranean Plants against Pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef] [PubMed]
| Plant Name from Which EOs Were Extracted | Plant Common Name | Plant Family Name | Main EO Chemical Components | Reference |
|---|---|---|---|---|
| Achillea millefolium | Yarrow, common yarrow, thousand-leaf | Asteraceae | Artemisia ketone (14.92%), camphor (11.64%), linalyl acetate (11.51%), 1,8-cineole (10.15%) | [23] |
| Alpinia zerumbet | Light Galangal, shell ginger | Zingiberaceae | γ-Terpinene (14.5%), cineole (13.8%), p-cymene (13.5%), sabinene (12.5%), terpinen-4-ol (11.9%), caryophyllene oxide (4.96%), methyl cinnamate (4.24%), caryophyllene (2.4%), γ-terpineol (1.28%) | [24] |
| Annona vepretorum | Araticum, pinha da caatinga, araticum-da-Bahia | Annonaceae | Bicyclogermacrene (35.7%), spathulenol (18.89%), α-phellandrene (8.08%), α-pinene (2.18%), o-cymene (6.24%) | [25] |
| Anthemis wiedemanniana | - | Asteraceae | 9,12-Octadecadienoic acid (12.2%), hexadecanoic acid (10.5%), hexahydrofarnesyl acetone (8.3%), 1,8-cineol (6.2%), carvacrol (5.8%) | [26] |
| Artemisia anomala | - | Asteraceae or Compositae | Camphor (18.3%), 1,8-cineole (17.3%), β-caryophyllene oxide (12.7%), borneol (9.5%) | [27] |
| Artemisia argyi | - | Asteraceae or Compositae | Caryophyllene (10.19%), eucalyptol (23.66%) | [28] |
| Atriplex undulata | - | Chenopodiaceae | p-Acetanisole (28.1%), β-damascenone (9.3%), β-ionone (5.1%), viridiflorene (4.7%), 3-oxo-α-ionol (2.2%) | [29] |
| Casearia lasiophylla | - | Salicaceae | Germacrene D (18.6%), E-caryophyllene (14.7%), δ-cadinene (6.2%), α-cadinol (5.4%) | [30] |
| Chrysanthemum boreale Makino | - | Asteraceae | Germacrene D (10.6–34.9%), β-caryophyllene (10.8%), (–)-camphor (10.8–18.0%), β-thujone (11.7%), α-thujone (9.8%) | [31] |
| Cinnamomum cassia | - | Lauraceae | Cis-2-methoxycinnamic acid (43.06%), cinnamaldehyde (42.37%) | [32] |
| Cinnamomum zeylanicum | - | Lauraceae | Eugenol (70%), β-caryophyllene (2.4%) | [33] |
| Citrus bergamia | Acid lemon | Rutaceae | Limonene (38.1%), linalyl acetate (28.9%), γ-terpinene (7.3%), linalool (6.4%), β-pinene (5.4%), bergapten (1.7%) | [34] |
| Citrus medica | Citron | Rutaceae | Limonene (35.4%), γ-terpinene (24.5%), geranial (5.5%), neral (4.4%), β-pinene (2.6%), α-pinene (2.5%), β-myrcene (2.1%), terpinen-4-ol (1.5%) | [34] |
| Cuminum cyminum | Cumin-jeera | Apiaceae or Umbelliferae | Cuminaldehyde (39.48%), γ-terpinene (15.21%), O-cymene (11.82%), β-pinene (11.13%), 2-caren-10-al (7.93%), trans-carveol (4.49%), and myrtenal (3.5%) | [35] |
| Curcuma aromatica | Wild turmeric | Zingiberaceae | 8,9-Dehydro-9- formyl-cycloisolongifolene (2.66–36.83%), germacrone (4.31–16.53%), ar-turmerone (2.52–17.69%), turmerone (2.62–18.38%), ermanthin (0.75–13.26%), β-sesquiphyllandrene (0.33–11.32%), ar-curcumene (0.29–10.52%) | [36] |
| Curcuma kwangsiensis | Mango-ginger | Zingiberaceae | 8,9-Dehydro-9-formyl-cycloisolongifolene (2.37–42.59%), germacrone (6.53–22.20%), L-camphor (0.19–6.12%) | [37] |
| Curcuma zedoaria | Kua-zedoary | Zingiberaceae | 8,9-Dehydro-9-formyl-cycloisolongifolene (60%), 6-ethenyl-4,5,6,7-tetra-hydro-3,6-dimethyl-5-isopropenyl-trans-benzofuran (12%) | [38] |
| Dalbergia pinnata | Laleng-chali | Fabaceae | Elemicin (91.06%), methyl eugenol (3.69%), 4-allyl-2,6-dimethoxyphenol (1.16%), whiskey lactone (0.55%) | [39] |
| Eryngium amethystinum | - | Apiaceae | Germacrene D (56.7%), β-elemene (4.7%), bicyclogermacrene (3.3%), α-copaene (2.2%), (E)-caryophyllene (1.9%), germacrene B (1.8%), germacra-4(15),5,10(14)-trien-1-α-ol (1.7%), cadin-4-en-10-ol (1.6%) | [40] |
| Eryngium campestre | Eryngo, field eryngo, sea-holly | Apiaceae | Germacrene D (13.8%), allo-aromadendrene (7.7%), spathulenol (7.0%), ledol (5.7%), cadin-4-en-10-ol (3.9%), γ-cadinene (3.6%), epi-α-muurolol (2.1%), germacra-4(15),5,10(14)-trien-1-α-ol (2.0%), δ-cadinene (1.9%), caryophyllene oxide (1.5%) | [40] |
| Eucalyptus camaldulensis | Murray red gum, red gum, red river gum | Myrtaceae | 1,8-Cineole (23.9%), α-eudesmol (11.6%), γ-eudesmol (8.0%), and elemol (5.0%) | [41] |
| Eugenia cuspidifolia | - | Myrtaceae | Caryophyllene oxide (57.46%), α-copaene (3.75%) | [42] |
| Eugenia tapacumensis | - | Myrtaceae | Caryophyllene oxide (55.95%), α-copaene (13.67%) | [42] |
| Eugenia uniflora | Brazil cherry | Myrtaceae | Curzerene (13.4–50.6%), selina-1,3,7(11)-trien-2-one (18.1–43.1%), selina-1,3,7(11)-trien-2-one epoxidem(16.0–30.4%), germacrene B (5.0–18.4%), caryophyllene oxide(1.2–18.1%), (E)-caryophyllene (0.3–9.1%), β-elemene (3.5–8.9%), γ-elemene (2.0–7.8%) | [43] |
| Glechoma hederacea | Ground ivy, field balm, gill over the ground, runaway robin | Lamiaceae or Labiatae | Trans-3-pinanone (41.4%), 4,5,6,7-tetrahydro-5-isopropenyl-3,6-β-dimethyl-6-α-vinylbenzofuran (10.8%), β-caryophyllene (10.2%), and spathulenol (4.3%) | [44] |
| Helichrysum microphyllum | - | Asteraceae or Compositae | Neryl acetate (18.2%), rosifoliol (11.3%), δ-cadinene (8.4%), γ-cadinene (6.7%) | [45] |
| Heracleum sphondylium | Cow parsnip, eltrot | Apiaceae or Umbelliferae | Octyl acetate (54.9–60.2%), octyl butyrate (10.1–13.4%) | [46] |
| Hypericum hircinum | - | Hypericaceae | Cis-β-guaiene (29.3%), δ-selinene (11.3%), isolongifolan-7-α-ol (9.8%), (E)-caryophyllene (7.2%) | [47] |
| Laurus nobilis | Bay Tree, sweet bay, Grecian Laurel, true laurel | Lauraceae | 1,8-cineole (35.15%) | [48] |
| Lavandula augustifolia | English lavender, true lavender | Lamiaceae or Labiatae | α-Pipene, β-pipene, camphene, eucalyptol, D-limonene | [49] |
| Lippia gracilis | - | Verbenaceae | Thymol (55.50%), p-cymene (10.80%), γ-terpinene (5.53%), myrcene (4.03%) | [50] |
| Liriodendron tulipifera | Tulip tree, tulip poplar, yellow poplar, canary whitewood | Magnoliaceae | (Z)-β-Ocimene (12.5–25.2%), (E)-β-ocimene (3.7–6.8%), β-elemene (16.4–17.1%), germacrene D (18.9–27.2%) | [51] |
| Melaleuca alternifolia | Tea Tree | Myrtaceae | Terpinen-4-ol (42.35%), γ-terpinene (20.65%), α-terpinene (9.76%) | [52] |
| Melaleuca quinquenervia | - | Myrtaceae | 1,8-Cineole (21.06%), α-pinene (15.93%), viridiflorol (14.55%), α-terpineol (13.73%) | [53] |
| Mentha aquatica | - | Lamiaceae or Labiatae | β-Ocimene (22.18%), β-pinene (15.41%), 1,8-cineole (12.87%), α-pinene (10.49%) | [54] |
| Myrcia laruotteana | - | Myrtaceae | α-Bisabolol (23.6%), α-bisabolol oxide B (11.5%) | [55] |
| Myristica fragrans | Mace, nutmeg | Myristicaceae | Myristicin, limonene, eugenol and terpinen-4-ol | [56] |
| Nectandra leucantha | - | Lauraceae | Bicyclogermacrene (28.44%), germacrene A (7.34%) | [57] |
| Ocimum basilicum | Sweet basil, common basil, thai basil, tropical basil | Lamiaceae or Labiatae | 1,8 Cineole (11.0%), linalool (42.5%), estragole (33.1%) | [58] |
| Ocimum gratissimum | African basil, east Indian basil, russian basil, shrubby basil | Lamiaceae or Labiatae | Eugenol (54.0%), 1,8 cineole (21.6%), β-selinene (5.5%), β-caryophyllene (5.3%), (Z)-ocimene (4.0%) | [58] |
| Ocimum micranthum | - | Lamiaceae or Labiatae | Eugenol (64.8%), β-caryophyllene (14,3%), bicyclogermacrene (8.1%) | [58] |
| Ocimum tenuiflorum | Sacred basil | Lamiaceae or Labiatae | Eugenol (59.4%), β-caryophyllene (29.4%), germacrene A (8.1%) | [58] |
| Origanum ehrenbergii | - | Lamiaceae or Labiatae | Carvacrol, thymoquinone | [59] |
| Origanum syriacum | Bible hyssop | Lamiaceae or Labiatae | Carvacrol, thymoquinone | [59] |
| Perilla frutescens | Shiso, beefsteakplant, spreading beefsteak plant | Lamiaceae or Labiatae | isoegomaketone | [60] |
| Piper aleyreanum | - | Piperaceae | β-Elemene (16.3%), bicyclogermacrene (9.2%), δ-elemene (8.2%), germacrene D (6.9%), β-caryophyllene (6.2%), spathulenol (5.2%) | [61] |
| Piper cernuum | - | Piperaceae | α-Pinene, camphene, limonene, carvacrol, tymol, myrcene, p-cymene, aterpineol, linalol | [62] |
| Piper klotzschianum | - | Piperaceae | Germacrene D (7.3–22.8%), bicyclogermacrene (13.4–21.6%), E)-caryophyllene (11.9–16.8%), β-pinene (2.3–27.2%), α-pinene (1.4–7.2%) | [63] |
| Pistacia lentiscus | Chios mastictree, aroeira, lentiscus, lentisk, mastic, mastictree | Anacardiaceae | Perillyl alcohol | [64] |
| Pituranthos tortuosus | - | Apiaceae | Sabinene (24.24%), α-pinene (17.98%), limonene (16.12%), and terpinen-4-ol (7.21%) | [65,66] |
| Plectranthus amboinicus | Country borage, Indian borage | Lamiaceae or Labiatae | Carvacrol thymol, cis-caryophyllene, trans-caryophyllene, and p-cymene | [67] |
| Pomelo peel | - | Rutaceae | Limonene (55.92%), β-myrcene (31.17%), β-pinene (3.16%), ocimene (1.42%), β-copaene (1.24%) | [68] |
| Porcelia macrocarpa | - | Annonaceae | Germacrene D (47%), bicyclogermacrene (37%), verbanyl acetate (0.5%), phytol (1.2%) | [69] |
| Pterodon emarginatus | Faveiro, sucupira, sucupira-branca | Fabaceae | β-Elemene (15.3%), trans-caryophyllene (35.9%), α-humulene (6.8%), germacrene-D (9.8%), bicyclogermacrene (5.5%), spathulenol (5.9%) | [70] |
| Salvia aurea | - | Lamiaceae or Labiatae | Caryophyllene oxide (12.5%), α-amorphene (12.0%), aristolone (11.4%), aro-madendrene (10.7%), elemenone (6.0%) | [71] |
| Salvia breacteata | - | Lamiaceae or Labiatae | Caryophyllene oxide (16.6%) | [72] |
| Salvia judaica | - | Lamiaceae or Labiatae | Caryophyllene oxide (12.8%) | [71] |
| Salvia libanotica | - | Lamiaceae or Labiatae | Cineole (57.4%), camphor (8.4%), β-pinene (5.1%), α-pinene (3.9%), camphene (3.0%) | [73] |
| Salvia officinalis | Sage, kitchen sage, small leaf sage, garden sage | Lamiaceae or Labiatae | Caryophyllene (25.634%), camphene (14.139%), eucalyptol (13.902%) | [74] |
| Salvia rubifolia | - | Lamiaceae or Labiatae | γ-Muurolene (11.8%) | [72] |
| Salvia verbenaca | Wild clary | Lamiaceae or Labiatae | Hexadecanoic acid (11–23.1%), Z)-9-octadecenoic acid (5.6–11.1%), benzaldehyde (1.1–7.3%) | [75] |
| Salvia viscosa | - | Lamiaceae or Labiatae | Caryophyllene oxide (12.7%) | [71] |
| Santalum album | White sandal tree, sandalwood, sandal tree, sandal | Santalaceae | α-Santalol (61%), β-santalol (28%) | [76] |
| Satureja hortensis | Summer savory | Lamiaceae or Labiatae | γ-Terpinene (37.862%), o-cymene (15.113%), thymol (13.491%), carvacrol (13.225%) | [77] |
| Schinus terebinthifolius Raddi | Brazilian pepper tree | Anacardiaceae | β-Longipinene (8.1%), germacrene D (23.8%), biclyclogermacrene (15.0%), α-pinene (5.7%), β-pinene (9.1%) | [78] |
| Stachys germanica | Downy woundwort, German hedgenettle | Lamiaceae or Labiatae | (Z,Z,Z)-9,12,15-octa-decatrienoic acid methyl ester (33.3%), exadecanoic acid (22.1%) | [79] |
| Stachys parviflora | - | Lamiaceae or Labiatae | α-Terpenyl acetate (23.6%), β-caryophyllene (16.8%), bicyclogermacrene (9.3%), spathulenol (4.9%), α-pinene (4.2%) | [80] |
| Syzygium aromaticum | Clove, Zanzibar redhead | Myrtaceae | Eugenol (61%), (β-carophillene 5.7%) | [33,81] |
| Tagetes erecta | African marigold, Aztec marigold, big marigold, American marigold | Asteraceae or Compositae | Limonene (10.4%), α-terpinolene (18.1%), (E)-ocimenone (13.0%), dihydrotagetone (11.8%) | [82] |
| Tanacetum macrophyllum | Tansy, rayed tansy, tansy chrysanthemum | Asteraceae or Compositae | Germacrene D (6.9–30.9%), 10-epi-γ-eudesmol (3.9–13.5%), camphor (11.1%), linalool (0.6–10.8%), 1,8-cineole (5.5–8.8%) | [83] |
| Thymus alternans | - | Lamiaceae or Labiatae | (E)-Nerolidol (15.8–31.4%), germacrene D (6.7–7.4%), geranial (6.8–7.7%), (E)-β-ocimene (2.6–7.0%), linalool (1.7–6.4%), geraniol (3.3–6.2%), neral (4.9–5.4%) | [84] |
| Thymus munbyanus | - | Lamiaceae or Labiatae | Borneol (31.2–44.8%), camphor (5.7–13.6%), camphene (3.6–7.5%), 1,8-cineole (4.2–6.0%), germacrene D (3.1–5.0%) | [85] |
| Thymus vulgaris | English thyme, French thyme, garden thyme, thyme | Lamiaceae or Labiatae | γ-Terpinene (68.415%), thymol (24.721%), caryophyllene (5.5%), α-pinene (4.816%) | [74] |
| Tridax procumbens | Coat buttons, coat-button, Mexican daisy | Asteraceae or Compositae | α-Pipene, β-pinene, phellandrene, sabinene | [86] |
| Vetiveria zizanioides | Cuscus grass, khus-khus, khas-khas, vetiver | Poaceae | Cedr-8-en-13-ol (12.4%), α-amorphene (7.80%), β-vatirenene (5.94%), α-gurjunene (5.91%), dehydroaromadendrene (5.45%) | [87] |
| Vitex Negundo | Common chaste tree, negundo, five keaved chaste tree, negundo chastetree, chaste tree | Lamiaceae or Labiatae | Sabinene (19.04%), caryophyllene (18.27%) | [88] |
| Vitex Trifolia | Indian privet, Arabian lilac, Indian three-leaf vitex, hand of mary | Lamiaceae or Labiatae | α-Pinene (11.38%), β-pinene (2.84%), sabinene (10.25%), eucaluptol (8.60%), camphene (12.69%), manoyl oxide (16.11%), abietatriene (9.03%) | [89] |
| Wedelia chinensis | Chinese wedelia | Asteraceae or Compositae | Carvocrol, trans-caryophyllene | [90] |
| Zornia brasiliensis | - | Fabaceae | Trans-nerolidol (48.0%), germacrene D (13.9%), α-humulene (9.3%), trans-caryophyllene (8.4%), and (Z,E)-α-farnesene (7.3%) | [91] |
| Pathway Affected | Plant Name from Which EOs Were Extracted | EO Active Components | In Vitro and In Vivo Models | Reference |
|---|---|---|---|---|
| In vitro cell proliferation | Annona Vepretorum | Spathulenol, o-cymene, α-pinene | B16-F10 | [25] |
| Anthemis wiedemanniana | - | C32 | [26] | |
| Artemisia anomala | - | BRO | [27] | |
| Casearia lasiophylla | - | UACC-62 | [30] | |
| Citrus bergamia | Bergapten | A375 | [34] | |
| Citrus medica | Limonene | A375 | [34,126] | |
| Coleus aromaticus | Carvacrol | A375 | [127] | |
| Curcuma aromatica | - | B16 | [36] | |
| Curcuma kwangsiensis | - | B16 | [37] | |
| Curcuma zedoaria | - | B16-Bl6 | [38] | |
| Eryngium amethystinum | - | A375 | [40] | |
| Eryngium campestre | - | A375 | [40] | |
| Eugenia cuspidifolia | - | SK-MEL-19 | [42] | |
| Eugenia tapacumensis | - | SK-MEL-19 | [42] | |
| Eugenia uniflora | Curzerene | SK-MEL-19 | [43] | |
| Helichrysum microphyllum | - | A375 | [45] | |
| Heracleum sphondylium | Octyl butyrate | A375 | [46] | |
| Hypericum hircinum | - | B16-F1 | [47] | |
| Laurus nobilis | - | C32 | [48] | |
| Lippia gracilis | - | B16-F10 | [50,128] | |
| Liriodendron tulipifera | β-Elemene | A375 | [51] | |
| Melaleuca alternifolia | Terpinen-4-ol | A375, M14, B16-F10 | [52,129,130] | |
| Melaleuca quinquenervia | 1,8-Cineole, α-Pipene, α-Terpineol | B16 | [53] | |
| Myrcia laruotteana | - | UACC-62 | [55] | |
| Nectandra leucantha | Bicyclogermacrene | B16-F10-Nex2 | [57] | |
| Perilla frutescens | Isoegomaketone | B16 | [60] | |
| Piper aleyreanum | - | SK-MEL-19 | [61] | |
| Piper cernuum | Camphene | B16-F10-Nex2 | [62] | |
| Piper klotzschianum | - | B16-F10 | [63] | |
| Porcelia macrocarpa | - | B16-F10-Nex2 | [69] | |
| Pterodon emarginatus | - | MeWo | [70] | |
| Salvia aurea | - | M14, A375, A2058 | [71] | |
| Salvia bracteata | - | M14 | [72] | |
| Salvia judaica | - | M14, A375, A2058 | [71] | |
| Salvia officinalis | - | A375, M14, A2058, B164A5 | [74,101] | |
| Salvia rubifolia | - | M14 | [72] | |
| Salvia verbenaca | - | M14 | [75] | |
| Salvia viscosa | - | M14, A375, A2058 | [71] | |
| Satureja hortensis | - | B164A5, A375 | [77] | |
| Schinus terebinthifolius Raddi | α-Pipene, β-pipene, pipane | B16-F10-Nex2, A2058 | [78] | |
| Stachys germanica | - | C32 | [79] | |
| Stachys parviflora | - | B16-F10 | [80] | |
| Syzygium aromaticum | Eugenol | B16 | [81] | |
| Tagetes erecta | - | B16-F10 | [82] | |
| Tanacetum macrophyllum | - | A375 | [83] | |
| Thuja occidentalis | Thujone | A375 | [131] | |
| Thymus alternans | - | A375 | [84] | |
| Thymus munbyanus | - | A375 | [85] | |
| Thymus vulgaris | - | B164A5, A375 | [74] | |
| Vitex Trifolia | Abietatriene | B16-F10 | [89] | |
| Carvacrol | SK-MEL-2 | [132] | ||
| Citral | B16-F10, SK-MEL-147, UACC-257 | [133] | ||
| Eugenol | SK-MEL-2, A2058, SK-MEL-28, Sbcl2, WM3211, WM98-1, WM1205Lu, LCM-MEL GR-MEL, 13443 | [132,134,135,136] | ||
| Farnesol | B16, B16-F10 | [106,109] | ||
| Farnesyl anthranilate | B16 | [106,107] | ||
| Farnesyl-O-acetylhydroquinone | B16 | [106] | ||
| Menthol | A375 | [137] | ||
| Neridol | B16 | [109] | ||
| Thymol | SK-MEL-2 | [132] | ||
| Zerumbone | CHL-1, A375 | [138,139] | ||
| β-Caryophyllene | B16-F10 | [140] | ||
| In vitro tumor progression-associated functions | Alpinia zerumbet | - | HUVEC | [141] |
| Eugenia uniflora | Curzerene | SK-MEL-19 | [43] | |
| Melaleuca alternifolia | Terpinen-4-Ol | M14 | [142] | |
| Pituranthos tortuosus | - | B16-F10 | [66] | |
| Satureja hortensis | - | B164A5, A375 | [77] | |
| Myrtenal | B16-F0, B16-F10, SK-MEL-5 | [143] | ||
| Thujone | B16-F10 | [144] | ||
| Thymoquinone | B16-F10, A375 | [145] | ||
| Zerumbone | CHL-1 | [138] | ||
| In vivo tumor growth and metastasization | Annona Vepretorum | Spathulenol, o-cymene, α-pinene | B16-F10 (C57BL/6J) | [25] |
| Boswellia carterii | - | B16-F10 (C57BL/6) | [146] | |
| Curcuma zedoaria | - | B16-Bl6 (C57BL/6) | [38] | |
| Melaleuca alternifolia | - | B16-F10 (C57BL/6J) | [147] | |
| Perilla frutescens | Isoegomaketone | B16 (C57BL/6N) | [60] | |
| Piper cernuum | Camphene | B16-F10-Nex2 (C57BL/6) | [62] | |
| Pituranthos tortuosus | - | B16-F10 (BALB/c) | [65,66] | |
| Plectranthus amboinicus | - | B16-F10 (C57BL/6) | [67] | |
| Salvia officinalis | β-Ursolic acid | B16 (C57BL/6) | [148] | |
| Schinus terebinthifolius Raddi | α-Pipene | B16-F10-Nex2 (C57BL/6) | [149] | |
| Tridax procumbens | - | B16-F10 (C57BL/6) | [86] | |
| Zornia brasiliensis | - | B16-F10 (C57BL/6) | [91] | |
| Eugenol | B16 (B6D2F1) | [135] | ||
| Limonene | B16-F10 (C57BL/6) | [150] | ||
| Myrtenal | B16-F10 (C57BL/6) | [143] | ||
| Perillic Acid | B16-F10 (C57BL/6) | [150] | ||
| Thujone | B16-F10 (C57BL/6) | [144] | ||
| Thymoquinone | B16-F10 (C57BL/6) | [121,151] | ||
| α-Pinene | B16-F10-Nex2 (C57BL/6) | [149] | ||
| β-Caryophyllene | B16-F10 (C57BL/6N) | [140] | ||
| β-Elemene | B16-F10 (C57BL/6) | [120,152] |
| Pathway Affected | Plant Name from Which EOs Were Extracted | EO Active Components | In Vitro and In Vivo Melanoma Models | Reference |
|---|---|---|---|---|
| Cell cycle | Melaleuca alternifolia | Terpinen-4-ol | A375, B16 | [129,130] |
| Santalum album | α-Santol | UACC-62 | [155] | |
| Eugenol | A2058, WM1205Lu, Sbcl2, WM3211 | [134,135] | ||
| Farnesol | B16 | [109] | ||
| Neridol | B16 | [109] | ||
| Apoptosis | Annona Vepretorum | Spathulenol, o-cymene, α-pinene | B16-F10 | [25] |
| Boswellia carterii | B16-F10, FM94 | [146] | ||
| Coleus aromaticus | Carvacrol | A375 | [127] | |
| Eugenia uniflora | Curzerene | SK-MEL-19 | [43] | |
| Melaleuca alternifolia | Terpinen-4-Ol | A375, M14 | [52,129] | |
| Perilla frutescens | Isoegomaketone | B16 | [60] | |
| Piper cernuum | Camphene | B16-F10-Nex2 | [62] | |
| Pituranthos tortuosus | - | B16-F10 | [66] | |
| Salvia aurea | - | M14 | [71] | |
| Salvia bracteata | - | M14 | [72] | |
| Salvia judaica | - | M14 | [71] | |
| Salvia officinalis | - | A375, M14, A2058 | [101] | |
| Salvia verbenaca | - | M14 | [75] | |
| Salvia viscosa | - | M14 | [71] | |
| Salvia rubifolia | - | M14 | [72] | |
| Schinus terebinthifolius Raddi | α-Pipene | B16-F10-Nex2, A2058 | [78,149] | |
| Thuja occidentalis | Thujone | A375 | [131] | |
| Tridax procumbens | - | B16-F10 (C57BL/6) | [86] | |
| Citral | B16-F10, SK-MEL-147, UACC-257 | [133] | ||
| Eugenol | A2058, SK-MEL-28 | [134,135,136] | ||
| Linalool | RPMI-7932 | [160] | ||
| Menthol | A375, G-361 | [161,162] | ||
| Thymoquinone | B16-F10 | [121] | ||
| Zerumbone | CHL-1, A375 | [138,139] | ||
| β-Carophyllene | B16-F10 (C57BL/6N) | [140] | ||
| Necrosis and autophagy | Melaleuca alternifolia | Terpinen-4-Ol | B16 | [130] |
| Salvia aurea | - | M14 | [71] | |
| Salvia bracteata | - | M14 | [72] | |
| Salvia judaica | - | M14 | [71] | |
| Salvia viscosa | - | M14 | [71] | |
| Salvia rubifolia | - | M14 | [72] | |
| Citral | B16-F10 | [133] |
| Plant Name from Which EOs Were Extracted | EO Active Components | In Vitro and In Vivo Models | Reference |
|---|---|---|---|
| Citrus lemon | - | CAM | [189] |
| Curcuma zedoaria | - | HUVEC, CAM, rat aortic ring assay, B16-Bl6 (C57BL/6) | [38] |
| Hypericum perforatum | - | EAhy.926 | [187] |
| Myristica fragrans | - | EAhy.926 | [56] |
| Pistacia lentiscus | - | B16 | [64] |
| Plectranthus amboinicus | - | B16-F10 (C57BL/6) | [67] |
| Salvia officinalis | - | Infected wound model (BALB/c) | [190] |
| Tridax procumbens | - | B16-F10 (C57BL/6) | [86] |
| Curcumol | HUVEC | [199] | |
| Eugenol | EAhy.926 | [188] | |
| Perillyl Alcohol | BLMVEC, HUVEC, B16-F10 | [198] | |
| Zerumbone | HUVEC, CAM, rat aortic ring assay | [195,196] | |
| β-Caryophyllene | B16-F10 (C57BL/6N) | [140] | |
| β-Elemene | CAM, rat aortic ring assay, B16-F10 (C57BL/6) | [120,152] |
| Pathway Affected | Plant Name from Which EOs Were Extracted | EO Active Components | In Vitro and In Vivo Models | Reference |
|---|---|---|---|---|
| Sensitization of antitumor agents | Eugenol | SK-MEL-28, B16-F10 | [188] | |
| Thymoquinone | B16-F10 | [121] | ||
| β-Elemene | A375 | [209] | ||
| Chemoprevention | Mentha aquatica | - | DMBA/TPA (FVB/NJ) | [54] |
| Salvia libanotica | - | DMBA/TPA (BALB/c) | [73] | |
| Santalum album | α-Santol | DMBA/TPA (CD1, SENCAR) | [76] | |
| Eugenol | DMBA/TPA, DMBA/croton oil (Swiss albino) | [212,213] | ||
| Farnesol | DMBA/TPA (Swiss albino) | [214] | ||
| Geraniol | DMBA/TPA (Swiss albino) | [215] | ||
| Limonene | DMBA/TPA (Swiss albino) | [177] | ||
| Menthol | DMBA/TPA (ICR) | [216] | ||
| Perillyl Alcohol | DMBA/TPras mut, DMBA/TPA (Swiss albino) | [217,218] | ||
| Melanogenesis | Achillea millefolium | Linalyl Acetate | B16 | [23] |
| Alpinia zerumbet | - | B16-F10 | [24] | |
| Artemisia argyi | - | B16-F10 | [28] | |
| Cinnamomum cassia | Cinnamaldehyde | B16 | [32] | |
| Cinnamomum zeylanicum | - | B16 | [33] | |
| Citrus grandis | - | B16-F10 | [219] | |
| Citrus hystrix | - | B16-F10 | [219] | |
| Citrus reticulata | - | B16-F10 | [219] | |
| Cryptomeria japonica | - | B16 | [220] | |
| Chrysanthemum boreale Makino | Cuminaldehyde | B16-Bl6 | [31] | |
| Dalbergia pinnata | - | Zebrafish embryos | [39] | |
| Eucalyptus camaldulensis | - | B16-F10 | [41] | |
| Glechoma hederacea | - | B16 | [44] | |
| Melaleuca quinquenervia | 1,8-Cineole, α-pipene, α-terpineol | B16 | [53] | |
| Mentha aquatica | β-Caryophyllene | B16-F10 | [221] | |
| Origanum syriacum | Carvacrol | B16-F1 | [59,222] | |
| Origanum ehrenbergii | Carvacrol | B16-F1 | [59] | |
| Pomelo peel | - | B16 | [68] | |
| Psiadia terebinthina | - | B16-F10 | [219] | |
| Syzygium aromaticum | Eugenol | B16 | [81] | |
| Vetiveria zizanioides | Cedr-8-En-13-Ol | B16 | [87] | |
| Vitex Negundo | - | B16-F10 | [88] | |
| Vitex Trifolia | Abietatriene | B16-F10 | [89] | |
| Phytol | B16-F10 | [223] | ||
| Thymoquinone | B16-F10 | [224] | ||
| Valencene | B16-F10 | [225] | ||
| Zerumbone | B16-F10 | [226] |
| Plant Name from Which EOs Were Extracted | EO Active Components | Cell Free Assay and Melanoma Models | Reference |
|---|---|---|---|
| Achillea millefolium | Linalyl Acetate | B16 | [23] |
| Alpinia zerumbet | - | DPPH, ABTS, nitric oxide, hydroxyl radical scavenging activity, xanthine oxidase | [24] |
| Artemisia argyi | - | DPPH, ABTS, metal-ion chelation | [28] |
| Atriplex undulata | - | Crocin bleaching inhibition, DPPH | [29] |
| Cinnamomum cassia | Cinnamaldehyde | B16 | [32] |
| Chrysanthemum boreale Makino | - | DPPH, ABTS | [31] |
| Cryptomeria japonica | - | B16 | [220] |
| Cuminum Cyminum | - | DPPH, superoxide anion radical-scavenging activity, β-carotene/linoleic acid | [35] |
| Curcuma aromatica | - | DPPH | [36] |
| Curcuma kwangsiensis | - | DPPH | [37] |
| Dalbergia pinnata | - | DPPH, ABTS | [39] |
| Eucalyptus camaldulensis | - | DPPH, ABTS, B16-F10 | [41] |
| Eugenia uniflora | - | DPPH, β-carotene/linoleic acid | [43] |
| Glechoma hederacea | - | β-carotene/linoleic acid, B16 | [44] |
| Helichrysum microphyllum | - | DPPH, ABTS | [45] |
| Hypericum hircinum | - | DPPH, ABTS | [47] |
| Lavandula augustifolia | - | B16-F10 | [49] |
| Melaleuca quinquenervia | 1,8-Cineole, α-pipene, α-terpineol | B16 | [53] |
| Ocimum basilicum | - | DPPH, hypoxanthine/xanthine oxidase | [58] |
| Ocimum gratissimum | - | DPPH, hypoxanthine/xanthine oxidase | [58,237] |
| Ocimum micranthum | - | DPPH, hypoxanthine/xanthine oxidase | [58] |
| Ocimum tenuiflorum | - | DPPH, hypoxanthine/xanthine oxidase | [58] |
| Piper aleyreanum | - | DPPH | [61] |
| Psidium guineense | - | DPPH, ABTS | [238] |
| Pomelo peel | - | DPPH, ABTS | [68] |
| Satureja hortensis | - | DPPH | [77] |
| Stachys cretica | - | DPPH | [79] |
| Stachys hydrophila | - | DPPH | [79] |
| Stachys palustri | - | DPPH | [79] |
| Stachys parviflora | - | DPPH, β-carotene/linoleic acid | [80] |
| Tanacetum macrophyllum | - | DPPH, ABTS, FRAP | [83] |
| Thymus munbyanus | - | DPPH, ABTS, FRAP | [85] |
| Thymus vulgaris | - | DPPH | [125] |
| Vetiveria zizanioides | - | β-carotene/linoleic acid, B16 | [87] |
| Vitex Negundo | - | DPPH, ABTS, metal-ion chelation | [88] |
| Wedelia chinensis | - | B16-F10 (C57BL/6) | [90,239] |
| Eugenol | A2058, SK-MEL 28 | [134] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Martile, M.; Garzoli, S.; Ragno, R.; Del Bufalo, D. Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma. Cancers 2020, 12, 2650. https://doi.org/10.3390/cancers12092650
Di Martile M, Garzoli S, Ragno R, Del Bufalo D. Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma. Cancers. 2020; 12(9):2650. https://doi.org/10.3390/cancers12092650
Chicago/Turabian StyleDi Martile, Marta, Stefania Garzoli, Rino Ragno, and Donatella Del Bufalo. 2020. "Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma" Cancers 12, no. 9: 2650. https://doi.org/10.3390/cancers12092650
APA StyleDi Martile, M., Garzoli, S., Ragno, R., & Del Bufalo, D. (2020). Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma. Cancers, 12(9), 2650. https://doi.org/10.3390/cancers12092650








