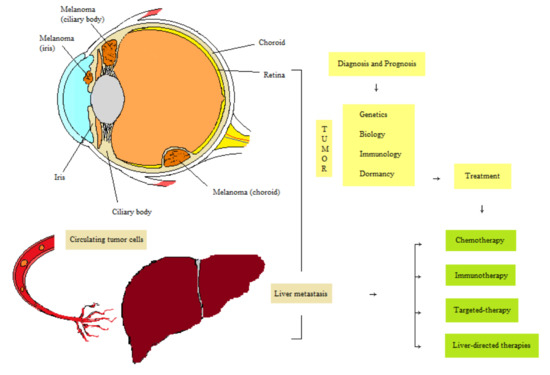
Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma
Simple Summary
Abstract
1. Introduction
2. Epidemiology and Uveal Melanoma Characteristics
3. Prognosis of UM
Cytogenetic Alterations in UM
4. Adjuvant Therapies and Surveillance of UM
5. Metastatic Dormancy and Therapeutic Opportunities
6. Treatment of Metastatic Disease
6.1. Chemotherapy
6.2. Liver-Directed Therapies
6.2.1. Surgery
6.2.2. Regional Perfusion Therapies
6.2.3. Radioembolization
6.2.4. Immunoembolization (IE)
6.3. Immunotherapy
6.4. Targeted Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C.; Wu, X.C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E. Incidence of Uveal Melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef]
- Mallone, S.; De Vries, E.; Guzzo, M.; Midena, E.; Verne, J.; Coebergh, J.W.; Marcos-Gragera, R.; Ardanaz, E.; Martinez, R.; Chirlaque, M.D.; et al. Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur. J. Cancer 2012, 48, 1167–1175. [Google Scholar] [CrossRef]
- Damato, E.M.; Damato, B.E. Detection and time to treatment of uveal melanoma in the United Kingdom: An evaluation of 2384 patients. Ophthalmology 2012, 119, 1582–1589. [Google Scholar] [CrossRef]
- Mahendraraj, K.; Lau, C.S.M.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7,516 patients from the surveillance, epidemiology, and end results database (1973–2012). Clin. Ophthalmol. 2016, 10, 2113–2119. [Google Scholar] [CrossRef]
- Aronow, M.E.; Topham, A.K.; Singh, A.D. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013). Ocul. Oncol. Pathol. 2018, 4, 145–151. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, L.; Wang, Y.; Miao, Q.; Jin, K.; Chen, M.; Ye, J. Epidemiological Study of Uveal Melanoma from US Surveillance, Epidemiology, and End Results Program (2010–2015). J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Hu, D.N.; Yu, G.P.; McCormick, S.A.; Schneider, S.; Finger, P.T. Population-based incidence of uveal melanoma in various races and ethnic groups. Am. J. Ophthalmol. 2005, 140, 612.e1–612.e8. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-P.; Hu, D.-N.; McCormick, S.A. Latitude and Incidence of Ocular Melanoma. Photochem. Photobiol. 2006, 82, 1621. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Cohen, M.N.; Shields, P.W.; Furuta, M.; Shields, J.A. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 Doyne Lecture. Eye 2015, 29, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Livesey, M.; Walker, B.; Garoon, R.; Bucci, M.; Feinstein, E.; Pesch, A.; Gonzalez, C.; Lally, S.E.; et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013, 131, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; De Potter, P.; Fijal, B.A.; Shields, C.L.; Shields, J.A.; Elston, R.C. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology 1998, 105, 195–198. [Google Scholar] [CrossRef]
- Hammer, H.; Oláh, J.; Tóth-Molnár, E. Dysplastic nevi are a risk factor for uveal melanoma. Eur. J. Ophthalmol. 1996, 6, 472–474. [Google Scholar] [CrossRef]
- McDonald, K.A.; Krema, H.; Chan, A.-W. Cutaneous Signs and Risk Factors for Ocular Melanoma. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Barker, C.A.; Salama, A.K. New NCCN guidelines for uveal melanoma and treatment of recurrent or progressive distant metastatic melanoma. J. Natl. Compr. Cancer Netw. 2018, 16, 646–650. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Koning, L.; Coupland, S.E.; Jochemsen, A.G.; Marais, R.; Stern, M.H.; Valente, A.; Barnhill, R.; Cassoux, N.; Evans, A.; et al. So close, yet so far: Discrepancies between uveal and other melanomas. a position paper from UM cure 2020. Cancers 2019, 11, 1032. [Google Scholar] [CrossRef]
- Shields, C.L.; Cater, J.; Shields, J.A.; Singh, A.D.; Santos, M.C.M.; Carvalho, C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch. Ophthalmol. 2000, 118, 360–364. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: Analysis of 2514 consecutive cases. Arch. Ophthalmol. 2009, 127, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Melia, B.M.; Diener-West, M.; Bennett, S.R.; Folk, J.C.; Montague, P.R.; Weingeist, T.A.; Hawkins, B.S. Factors predictive of growth and treatment of small choroidal melanoma: COMS report no. 5. Arch. Ophthalmol. 1997, 115, 1537–1544. [Google Scholar] [CrossRef]
- Accuracy of Diagnosis of Choroidal Melanomas in the Collaborative Ocular Melanoma Study: COMS Report No. 1. Arch. Ophthalmol. 1990, 108, 1268–1273. [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Ehya, H.; Eagle, R.C.; De Potter, P. Fine-needle aspiration biopsy of suspected intraocular tumors. Int. Ophthalmol. Clin. 1993, 33, 77–82. [Google Scholar] [CrossRef]
- Hawkins, B.S. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report no. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef]
- Hawkins, B.S. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am. J. Ophthalmol. 2004, 138, 936–951. [Google Scholar] [CrossRef]
- Bishop, K.D.; Olszewski, A.J. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int. J. Cancer 2014, 134, 2961–2971. [Google Scholar] [CrossRef]
- Burr, J.M.; Mitry, E.; Rachet, B.; Coleman, M.P. Survival from uveal melanoma in England and Wales 1986 to 2001. Ophthalmic Epidemiol. 2007, 14, 3–8. [Google Scholar] [CrossRef]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma From the Time of First Metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef]
- Seibel, I.; Cordini, D.; Rehak, M.; Hager, A.; Riechardt, A.I.; Böker, A.; Heufelder, J.; Weber, A.; Gollrad, J.; Besserer, A.; et al. Local Recurrence after Primary Proton Beam Therapy in Uveal Melanoma: Risk Factors, Retreatment Approaches, and Outcome. Am. J. Ophthalmol. 2015, 160, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Sidiki, S.; Clark, B.J.; McClaren, K.; Kyle, P.; Lee, W.R. Metastatic choroidal melanoma to the contralateral orbit 40 years after enucleation. Arch. Ophthalmol. 1996, 114, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Augsburger, J.J.; Donoso, L.A.; Bernardino, V.B.; Portenar, M. Hepatic metastasis and orbital recurrence of uveal melanoma after 42 years. Am. J. Ophthalmol. 1985, 100, 666–668. [Google Scholar] [CrossRef]
- Dithmar, S.; Diaz, C.E.; Grossniklaus, H.E. Intraocular melanoma spread to regional lymph nodes. Retina 2000, 20, 76–79. [Google Scholar] [CrossRef]
- Badve, S.S.; Fisher, C. AJCC 8th edition—A step forward. Breast J. 2020, 26, 1263–1264. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer classification of posterior uveal melanoma (tumor size category) predicts prognosis in 7731 patients. Ophthalmology 2013, 120, 2066–2071. [Google Scholar] [CrossRef]
- Gill, H.S.; Char, D.H. Uveal melanoma prognostication: From lesion size and cell type to molecular class. Can. J. Ophthalmol. 2012, 47, 246–253. [Google Scholar] [CrossRef]
- Bagger, M.; Andersen, M.T.; Andersen, K.K.; Heegaard, S.; Kiilgaard, J.F. The prognostic effect of American joint committee on cancer staging and genetic status in patients with choroidal and ciliary body melanoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 438–444. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Cassoux, N.; Rodrigues, M.J.; Plancher, C.; Asselain, B.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Piperno-Neumann, S.; Dendale, R.; Sastre, X.; Desjardins, L.; et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 2014, 98, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Duke, C.; Coupland, S.E.; Hiscott, P.; Smith, P.A.; Campbell, I.; Douglas, A.; Howard, P. Cytogenetics of Uveal Melanoma. A 7-Year Clinical Experience. Ophthalmology 2007, 114, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Say, E.A.T.; Hasanreisoglu, M.; Saktanasate, J.; Lawson, B.M.; Landy, J.E.; Badami, A.U.; Sivalingam, M.D.; Hauschild, A.J.; House, R.J.; et al. Personalized Prognosis of Uveal Melanoma Based on Cytogenetic Profile in 1059 Patients over an 8-Year Period: The 2017 Harry S. Gradle Lecture. Ophthalmology 2017, 124, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative ocular oncology group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef]
- Damato, B.; Coupland, S.E. A reappraisal of the significance of largest basal diameter of posterior uveal melanoma. Eye 2009, 23, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Dopierala, J.; Klaasen, A.; van Dijk, M.; Sibbring, J.; Coupland, S.E. Multiplex ligation-dependent probe amplification of uveal melanoma: Correlation with metastatic death. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3048–3055. [Google Scholar] [CrossRef]
- Vaquero-Garcia, J.; Lalonde, E.; Ewens, K.G.; Ebrahimzadeh, J.; Richard-Yutz, J.; Shields, C.L.; Barrera, A.; Green, C.J.; Barash, Y.; Ganguly, A. PRiMeUM: A model for predicting risk of metastasis in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4096–4105. [Google Scholar] [CrossRef]
- Walter, S.D.; Chao, D.L.; Feuer, W.; Schiffman, J.; Char, D.H.; Harbour, J.W. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 2016, 134, 734–740. [Google Scholar] [CrossRef]
- Eleuteri, A.; Damato, B.; Coupland, S.E.; Taktak, A.F.G. Enhancing survival prognostication in patients with choroidal melanoma by integrating pathologic clinical and genetic predictors of metastasis. Int. J. Biomed. Eng. Technol. 2012, 8, 18–35. [Google Scholar] [CrossRef]
- Jager, M.J.; Brouwer, N.J.; Esmaeli, B. The Cancer Genome Atlas Project: An Integrated Molecular View of Uveal Melanoma. Ophthalmology 2018, 125, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Mazloumi, M.; Vichitvejpaisal, P.; Dalvin, L.A.; Yaghy, A.; Ewens, K.G.; Ganguly, A.; Shields, C.L. Accuracy of the Cancer Genome Atlas Classification vs American Joint Committee on Cancer Classification for Prediction of Metastasis in Patients with Uveal Melanoma. JAMA Ophthalmol. 2020, 138, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed]
- Vichitvejpaisal, P.; Dalvin, L.A.; Mazloumi, M.; Ewens, K.G.; Ganguly, A.; Shields, C.L. Genetic Analysis of Uveal Melanoma in 658 Patients Using the Cancer Genome Atlas Classification of Uveal Melanoma as A, B, C, and D. Ophthalmology 2019, 126, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W. The genetics of uveal melanoma: An emerging framework for targeted therapy. Pigment Cell Melanoma Res. 2012, 25, 171–181. [Google Scholar] [CrossRef]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jöckel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef]
- Tschentscher, F.; Prescher, G.; Zeschnigk, M.; Horsthemke, B.; Lohmann, D.R. Identification of chromosomes 3, 6, and 8 aberrations in uveal melanoma by microsatellite analysis in comparison to comparative genomic hybridization. Cancer Genet. Cytogenet. 2000, 122, 13–17. [Google Scholar] [CrossRef]
- Versluis, M.; De Lange, M.J.; Van Pelt, S.I.; Ruivenkamp, C.A.L.; Kroes, W.G.M.; Cao, J.; Jager, M.J.; Luyten, G.P.M.; Van Der Velden, P.A. Digital PCR validates 8q dosage as prognostic tool in uveal melanoma. PLoS ONE 2015, 10, e0116371. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.O.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Carbone, M.; Ferris, L.K.; Baumann, F.; Napolitano, A.; Lum, C.A.; Flores, E.G.; Gaudino, G.; Powers, A.; Bryant-Greenwood, P.; Krausz, T.; et al. BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J. Transl. Med. 2012, 10, 179. [Google Scholar] [CrossRef]
- Laíns, I.; Bartosch, C.; Mondim, V.; Healy, B.; Kim, I.K.; Husain, D.; Miller, J.W. Second Primary Neoplasms in Patients With Uveal Melanoma: A SEER Database Analysis. Am. J. Ophthalmol. 2016, 165, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.R.; Kalirai, H.; Sacco, J.J.; Azevedo, R.A.; Duckworth, A.; Slupsky, J.R.; Coulson, J.M.; Coupland, S.E. Loss of BAP1 expression is associated with an immunosuppressive microenvironment in uveal melanoma, with implications for immunotherapy development. J. Pathol. 2020, 250, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Nilsson, L.M.; Mitra, S.; Alsén, S.; Shelke, G.V.; Sah, V.R.; Forsberg, E.M.V.; Stierner, U.; All-Eriksson, C.; Einarsdottir, B.; et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Louie, B.H.; Kurzrock, R. BAP1: Not Just a BRCA1-Associated Protein. Cancer Treat. Rev. 2020, 90, 102091. [Google Scholar] [CrossRef]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; Van Bodegom, A.; Paridaens, D.; Kiliç, E.; De Klein, A. Uveal Melanomas with SF3B1 Mutations: A Distinct Subclass Associated with Late-Onset Metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef]
- Harbour, J.W.; Chen, R. The DecisionDx-UM Gene Expression Profile Test Provides Risk Stratification and Individualized Patient Care in Uveal Melanoma. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Luscan, A.; Just, P.A.; Briand, A.; Burin Des Roziers, C.; Goussard, P.; Nitschké, P.; Vidaud, M.; Avril, M.F.; Terris, B.; Pasmant, E. Uveal melanoma hepatic metastases mutation spectrum analysis using targeted next-generation sequencing of 400 cancer genes. Br. J. Ophthalmol. 2015, 99, 437–439. [Google Scholar] [CrossRef]
- Martin, M.; Maßhöfer, L.; Temming, P.; Rahmann, S.; Metz, C.; Bornfeld, N.; Van De Nes, J.; Hitpass, L.K.; Hinnebusch, A.G.; Horsthemke, B.; et al. Exome sequencing identifies recurrent somatic mutations in EIF1AX and SF3B1 in uveal melanoma with disomy 3. Nat. Genet. 2013, 45, 933–936. [Google Scholar] [CrossRef]
- Höglund, M.; Gisselsson, D.; Hansen, G.B.; White, V.A.; Säll, T.; Mitelman, F.; Horsman, D. Dissecting karyotypic patterns in malignant melanomas: Temporal clustering of losses and gains in melanoma karyotypic evolution. Int. J. Cancer 2004, 108, 57–65. [Google Scholar] [CrossRef]
- Damato, B.E.; Heimann, H.; Kalirai, H.; Coupland, S.E. Age, survival predictors, and metastatic death in patients with choroidal melanoma tentative evidence of a therapeutic effect on survival. JAMA Ophthalmol. 2014, 132, 605–613. [Google Scholar] [CrossRef]
- Aalto, Y.; Eriksson, L.; Seregard, S.; Larsson, O.; Knuutila, S. Concomitant loss of chromosome 3 and whole arm losses and gains of chromosome 1, 6, or 8 in metastasizing primary uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 313–317. [Google Scholar]
- Ewens, K.G.; Kanetsky, P.A.; Richards-Yutz, J.; Al-Dahmash, S.; de Luca, M.C.; Bianciotto, C.G.; Shields, C.L.; Ganguly, A. Genomic profile of 320 uveal melanoma cases: Chromosome 8p-loss and metastatic outcome. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Naus, N.C.; Verhoeven, A.C.A.; van Drunen, E.; Slater, R.; Mooy, C.M.; Paridaens, D.A.; Luyten, G.P.M.; de Klein, A. Detection of Genetic Prognostic Markers in Uveal Melanoma Biopsies Using Fluorescence in Situ Hybridization. Clin. Cancer Res. 2002, 8, 534–539. [Google Scholar]
- Sisley, K.; Nichols, C.; Parsons, M.A.; Farr, R.; Rees, R.C.; Rennie, I.G. Clinical applications of chromosome analysis, from fine needle aspiration biopsies, of posterior uveal melanomas. Eye 1998, 12, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Ganguly, A.; Materin, M.A.; Teixeira, L.; Mashayekhi, A.; Swanson, L.A.; Marr, B.P.; Shields, J.A. Chromosome 3 analysis of uveal melanoma using fine-needle aspiration biopsy at the time of plaque radiotherapy in 140 consecutive cases: The Deborah Iverson, MD, Lectureship. Arch. Ophthalmol. 2007, 125, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Klofas, L.K.; Bogan, C.M.; Coogan, A.; Schultenover, S.J.; Weiss, V.L.; Daniels, A.B. Instrument gauge and type in uveal melanoma fine needle biopsy: Implications for diagnostic yield and molecular prognostication. Am. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Young, T.A.; Rao, N.P.; Glasgow, B.J.; Moral, J.N.; Straatsma, B.R. Fluorescent In Situ Hybridization for Monosomy 3 via 30-Gauge Fine-Needle Aspiration Biopsy of Choroidal Melanoma In Vivo. Ophthalmology 2007, 114, 142–146. [Google Scholar] [CrossRef]
- Cross, N.A.; Ganesh, A.; Parpia, M.; Murray, A.K.; Rennie, I.G.; Sisley, K. Multiple locations on chromosome 3 are the targets of specific deletions in uveal melanoma. Eye 2006, 20, 476–481. [Google Scholar] [CrossRef]
- Lake, S.L.; Kalirai, H.; Dopierała, J.; Damato, B.E.; Coupland, S.E. Comparison of Formalin-Fixed and Snap-Frozen Samples Analyzed by Multiplex Ligation-Dependent Probe Amplification for Prognostic Testing in Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2647–2652. [Google Scholar] [CrossRef]
- Worley, L.A.; Onken, M.D.; Person, E.; Robirds, D.; Branson, J.; Char, D.H.; Perry, A.; Harbour, J.W. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin. Cancer Res. 2007, 13, 1466–1471. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Tuscan, M.D.; Harbour, J.W. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J. Mol. Diagn. 2010, 12, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Field, M.G.; Harbour, J.W. Recent developments in prognostic and predictive testing in uveal melanoma. Curr. Opin. Ophthalmol. 2014, 25, 234–239. [Google Scholar] [CrossRef]
- Field, M.G.; Decatur, C.L.; Kurtenbach, S.; Gezgin, G.; Van Der Velden, P.A.; Jager, M.J.; Kozak, K.N.; Harbour, J.W. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin. Cancer Res. 2016, 22, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Singh, A.D. Adjuvant Therapy of Uveal Melanoma: Current Status. Ocul. Oncol. Pathol. 2014, 1, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Messersmith, H.; Kaur, V.; Kirkwood, J.M.; Kudchadkar, R.; McQuade, J.L.; Provenzano, A.; Swami, U.; Weber, J.; Alluri, K.C.; et al. Systemic Therapy for Melanoma: ASCO Guideline. J. Clin. Oncol. 2020, JCO.20.00198. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.; Romaniuk, C.; Ghaneh, P.; Wong, H.; McKay, M.; Chopra, M.; Coupland, S.E.; Damato, B.E. MRI in the detection of hepatic metastases from high-risk uveal melanoma: A prospective study in 188 patients. Br. J. Ophthalmol. 2013, 97, 159–163. [Google Scholar] [CrossRef]
- Nathan, P.; Cohen, V.; Coupland, S.; Curtis, K.; Damato, B.; Evans, J.; Fenwick, S.; Kirkpatrick, L.; Li, O.; Marshall, E.; et al. Uveal Melanoma UK National Guidelines. Eur. J. Cancer 2015, 51, 2404–2412. [Google Scholar] [CrossRef]
- Desjardins, L.; Dorval, T.; Levy, C.; Cojean, I.; Schlienger, P.; Salmon, R.J.; Validire, P.; Asselain, B. Etude randomisée de chimiothérapie adjuvante par le Déticène dans le mélanome choroïdien. Ophtalmologie 1998, 12, 168–173. [Google Scholar]
- McLean, I.W.; Berd, D.; Mastrangelo, M.J.; Shields, J.A.; Davidorf, F.H.; Grever, M.; Makley, T.A.; Gamel, J.W. A randomized study of methanol-extraction residue of bacille Calmette-Guerin as postsurgical adjuvant therapy of uveal melanoma. Am. J. Ophthalmol. 1990, 110, 522–526. [Google Scholar] [CrossRef]
- Richtig, E.; Langmann, G.; Schlemmer, G.; Müllner, K.; Papaefthymiou, G.; Bergthaler, P.; Smolle, J. Verträglichkeit und wirksamkeit einer adjuvanten interferon-alfa-2b-behandlung beim aderhautmelanom. Ophthalmologe 2006, 103, 506–511. [Google Scholar] [CrossRef]
- Lane, A.M.; Egan, K.M.; Harmon, D.; Holbrook, A.; Munzenrider, J.E.; Gragoudas, E.S. Adjuvant Interferon Therapy for Patients with Uveal Melanoma at High Risk of Metastasis. Ophthalmology 2009, 116, 2206–2212. [Google Scholar] [CrossRef]
- Voelter, V.; Schalenbourg, A.; Pampallona, S.; Peters, S.; Halkic, N.; Denys, A.; Goitein, G.; Zografos, L.; Leyvraz, S. Adjuvant intra-arterial hepatic fotemustine for high-risk uveal melanoma patients. Melanoma Res. 2008, 18, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Piperno-Neumann, S.; Rodrigues, M.J.; Servois, V.; Pierron, G.; Gastaud, L.; Negrier, S.; Levy-Gabriel, C.; Lumbroso, L.; Cassoux, N.; Bidard, F.-C.; et al. A randomized multicenter phase 3 trial of adjuvant fotemustine versus surveillance in high risk uveal melanoma (UM) patients (FOTEADJ). J. Clin. Oncol. 2017, 35, 9502. [Google Scholar] [CrossRef]
- Binkley, E.; Triozzi, P.L.; Rybicki, L.; Achberger, S.; Aldrich, W.; Singh, A. A prospective trial of adjuvant therapy for high-risk uveal melanoma: Assessing 5-year survival outcomes. Br. J. Ophthalmol. 2020, 104, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Han, F.; Yamamoto, A. The biology and management of uveal melanoma. Curr. Oncol. Rep. 2008, 10, 431–438. [Google Scholar] [CrossRef]
- Surriga, O.; Rajasekhar, V.K.; Ambrosini, G.; Dogan, Y.; Huang, R.; Schwartz, G.K. Crizotinib, a c-Met Inhibitor, Prevents Metastasis in a Metastatic Uveal Melanoma Model. Mol. Cancer Ther. 2013, 12, 2817–2826. [Google Scholar] [CrossRef]
- Search of: NCT02223819—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02223819&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Valsecchi, M.E.; Orloff, M.; Sato, R.; Chervoneva, I.; Shields, C.L.; Shields, J.A.; Mastrangelo, M.J.; Sato, T. Adjuvant Sunitinib in High-Risk Patients with Uveal Melanoma: Comparison with Institutional Controls. Ophthalmology 2018, 125, 210–217. [Google Scholar] [CrossRef]
- Search of: NCT00489944—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT00489944&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT02068586—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02068586&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Landreville, S.; Agapova, O.A.; Matatall, K.A.; Kneass, Z.T.; Onken, M.D.; Lee, R.S.; Bowcock, A.M.; Harbour, J.W. Histone Deacetylase Inhibitors Induce Growth Arrest and Differentiation in Uveal Melanoma. AACR 2011, 18, 408–416. [Google Scholar] [CrossRef]
- Fagone, P.; Caltabiano, R.; Russo, A.; Lupo, G.; Anfuso, C.D.; Basile, M.S.; Longo, A.; Nicoletti, F.; De Pasquale, R.; Libra, M.; et al. Identification of novel chemotherapeutic strategies for metastatic uveal melanoma. Sci. Rep. 2017, 7, 44564. [Google Scholar] [CrossRef]
- Bol, K.; van den Bosch, T.; Schreibelt, G.; Punt, C.; Figdor, C.; Paridaens, D.; de Vries, J. Adjuvant dendritic cell vaccination in high-risk uveal melanoma patients. J. Immunother. Cancer 2015, 3, 127. [Google Scholar] [CrossRef]
- Search of: NCT01983748—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01983748&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT00929019—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT00929019&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Schank, T.E.; Hassel, J.C. cancers Immunotherapies for the Treatment of Uveal Melanoma-History and Future. Cancers 2019, 11, 1048. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.A.; Rodriguez, D.A.; Kurtenbach, S.; Kuznetsov, J.N.; Sanchez, M.I.; Decatur, C.L.; Snyder, H.; Feun, L.G.; Livingstone, A.S.; Harbour, J.W. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fountain, E.; Bassett, R.L.; Cain, S.; Posada, L.; Gombos, D.S.; Hwu, P.; Bedikian, A.; Patel, S.P. Adjuvant ipilimumab in high-risk Uveal melanoma. Cancers 2019, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Search of: NCT02519322—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02519322&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT03528408—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03528408&cntry=&state=&city=&dist= (accessed on 11 September 2020).
- Search of: NCT02336763—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02336763&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Bustamante, P.; Miyamoto, D.; Goyeneche, A.; de Alba Graue, P.G.; Jin, E.; Tsering, T.; Dias, A.B.; Burnier, M.N.; Burnier, J.V. Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med. 2019, 8, 7265–7277. [Google Scholar] [CrossRef]
- Croock, D.L. Metastatic uveal melanoma: Diagnosis and treatment. A literature review. Bull. Société Belg. Ophtalmol. 2002, 286, 59. [Google Scholar]
- Freton, A.; Chin, K.J.; Raut, R.; Tena, L.B.; Kivelä, T.; Finger, P.T. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur. J. Ophthalmol. 2011, 22, 236–243. [Google Scholar] [CrossRef]
- Grossniklaus, H.E. Understanding Uveal Melanoma Metastasis to the Liver: The Zimmerman Effect and the Zimmerman Hypothesis. Ophthalmology 2019, 126, 483–487. [Google Scholar] [CrossRef]
- Singh, A.D. Uveal melanoma: Implications of tumor doubling time. Ophthalmology 2001, 108, 829–830. [Google Scholar] [CrossRef]
- Torres, V.; Triozzi, P.; Eng, C.; Tubbs, R.; Schoenfiled, L.; Crabb, J.W.; Saunthararajah, Y.; Singh, A.D. Circulating tumor cells in uveal melanoma. Futur. Oncol. 2011, 7, 101–109. [Google Scholar] [CrossRef]
- Ossowski, L.; Aguirre-Ghiso, J.A. Dormancy of metastatic melanoma. Pigment Cell Melanoma Res. 2010, 23, 41–56. [Google Scholar] [CrossRef]
- Blanco, P.L.; Lim, L.A.; Miyamoto, C.; Burnier, M.N. Uveal melanoma dormancy: An acceptable clinical endpoint? Melanoma Res. 2012, 22, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Ah-Fat, F.G.; Damato, B.E. Delays in the diagnosis of uveal melanoma and effect on treatment. Eye 1998, 12, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, P.; Panageas, K.S.; Hanlon, C.; Patel, A.; Abramson, D.H.; Chapman, P.B. Variates of survival in metastatic uveal melanoma. J. Clin. Oncol. 2005, 23, 8076–8080. [Google Scholar] [CrossRef] [PubMed]
- Mouriaux, F.; Zaniolo, K.; Bergeron, M.A.; Weidmann, C.; De La Fouchardière, A.; Fournier, F.; Droit, A.; Morcos, M.W.; Landreville, S.; Guérin, S.L. Effects of long-term serial passaging on the characteristics and properties of cell lines derived from uveal melanoma primary tumors. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5288–5301. [Google Scholar] [CrossRef]
- Almog, N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010, 294, 139–146. [Google Scholar] [CrossRef]
- Hedley, B.D.; Allan, A.L.; Chambers, A.F. Tumor dormancy and the role of metastasis suppressor genes in regulating ectopic growth. Futur. Oncol. 2006, 2, 627–641. [Google Scholar] [CrossRef]
- Horak, C.E.; Lee, J.H.; Marshall, J.C.; Shreeve, S.M.; Steeg, P.S. The role of metastasis suppressor genes in metastatic dormancy. Apmis 2008, 116, 586–601. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F. The Prometastatic Microenvironment of the Liver. Cancer Microenviron. 2008, 1, 113–129. [Google Scholar] [CrossRef]
- Krishna, Y.; Mccarthy, C.; Kalirai, H.; Coupland, S.E.; Yamini, K.; Conni, M.; Helen, K. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Hum. Pathol. 2017, 66, 159–166. [Google Scholar] [CrossRef]
- Eyles, J.; Puaux, A.; Wang, X. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig. 2010, 120, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Mouriaux, F.; Casagrande, F.; Pillaire, M.-J.; Manenti, S.; Malecaze, F.; Darbon, J.-M. Differential Expression of Gl Cyclins and Cyclin-Dependent Kinase Inhibitors in Normal and Transformed Melanocytes. Investig. Ophthalmol. Vis. Sci. 1998, 39, 876–884. [Google Scholar]
- Mouriaux, F.; Maurage, C.; science, P.L. Cyclin-dependent kinase inhibitory protein expression in human choroidal melanoma tumors. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2837–2843. [Google Scholar]
- Bronkhorst, I.H.G.; Jager, M.J. Uveal melanoma: The inflammatory microenvironment. J. Innate Immun. 2012, 4, 454–462. [Google Scholar] [CrossRef]
- Ly, L.; Baghat, A.; Versluis, M.; Jordanova, E.; Immunol, G.L.-J.; Luyten, G.P.M.; van Rooijen, N.; van Hall, T.; van der Velden, P.A.; Jager, M.J. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J. Immunol. 2010, 185, 3481–3488. [Google Scholar] [CrossRef]
- Ward, J.; Gubin, M.; Schreiber, R.D. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef]
- Turcotte, S.; Gros, A.; Hogan, K.; Tran, E.; Hinrichs, C.S.; Wunderlich, J.R.; Dudley, M.E.; Rosenberg, S.A. Phenotype and Function of T Cells Infiltrating Visceral Metastases from Gastrointestinal Cancers and Melanoma: Implications for Adoptive Cell Transfer Therapy. J. Immunol. 2013, 191, 2217–2225. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Sutmuller, R.P.M.; Schurmans, L.R.H.M.; van Duivenvoorde, L.M.; Tine, J.A.; van der Voort, E.I.H.; Toes, R.E.M.; Melief, C.J.M.; Jager, M.J.; Offringa, R. Adoptive T Cell Immunotherapy of Human Uveal Melanoma Targeting gp100. J. Immunol. 2000, 165, 7308–7315. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.M.; Sommer, J.; Fischer, A.; Britting, S.; Karg, M.M.; Bock, B.; Atreya, I.; Heindl, L.M.; Mackensen, A.; Bosch, J.J. Characterization of CD4+ T cells primed and boosted by MHCII primary uveal melanoma cell-based vaccines. Oncotarget 2019, 10, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.; Chen, L.; Chen, J.; Lu, X.; Li, Z.; Wu, B.; Yin, L.; Guan, Y.-Q. Long-term G 1 cell cycle arrest in cervical cancer cells induced by co-immobilized TNF-α plus IFN-γ polymeric drugs. J. Mater. Chem. B 2018, 6, 327–336. [Google Scholar] [CrossRef]
- Kortylewski, M.; Komyod, W.; Kauffmann, M.E.; Bosserhoff, A.; Heinrich, P.C.; Behrmann, I. Interferon-γ-Mediated Growth Regulation of Melanoma Cells: Involvement of STAT1-Dependent and STAT1-Independent Signals. J. Investig. Dermatol. 2004, 122, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Philippidou, D.; Reinsbach, S.E.; Margue, C.; Wienecke-Baldacchino, A.; Nashan, D.; Behrmann, I.; Kreis, S. Interferon-γ-induced activation of Signal Transducer and Activator of Transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell Commun. Signal. 2012, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Search of: NCT01082887—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01082887&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Huang, Y.; Yuan, J.; Righi, E.; Kamoun, W.S.; Ancukiewicz, M.; Nezivar, J.; Santosuosso, M.; Martin, J.D.; Martin, M.R.; Vianello, F.; et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 2012, 109, 17561–17566. [Google Scholar] [CrossRef]
- Grossniklaus, H.E. Progression of ocular melanoma metastasis to the liver: The 2012 Zimmerman lecture. JAMA Ophthalmol. 2013, 131, 462–469. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Zhang, Q.; You, S.; McCarthy, C.; Heegaard, S.; Coupland, S.E. Metastatic ocular melanoma to the liver exhibits infiltrative and nodular growth patterns. Hum. Pathol. 2016, 57, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Angi, M.; Kalirai, H.; Prendergast, S.; Simpson, D.; Hammond, D.E.; Madigan, M.C.; Beynon, R.J.; Coupland, S.E. In-depth proteomic profiling of the uveal melanoma secretome. Oncotarget 2016, 7, 49623–49635. [Google Scholar] [CrossRef]
- Almog, N.; Ma, L.; Raychowdhury, R.; Schwager, C.; Erber, R.; Short, S.; Hlatky, L.; Vajkoczy, P.; Huber, P.E.; Folkman, J.; et al. Transcriptional Switch of Dormant Tumors to Fast-Growing Angiogenic Phenotype. Cancer Res. 2009, 69, 836–880. [Google Scholar] [CrossRef]
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Chen, G.; Wang, H.; Wang, S.; Xing, H.; Gao, Q.; Lu, Y.; He, Y.; Ma, D. Inhibition of ovarian cancer metastasis by adeno-associated virus-mediated gene transfer of nm23H1 in an orthotopic implantation model. Cancer Gene Ther. 2006, 13, 266–272. [Google Scholar] [CrossRef]
- Liu, F.; Qi, H.-L.; Chen, H.-L. Effects of all-trans retinoic acid and epidermal growth factor on the expression of nm23-H1 in human hepatocarcinoma cells. J. Cancer Res. Clin. Oncol. 2000, 126, 85–90. [Google Scholar]
- Search of: NCT03572387—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03572387&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Jiang, Y.; Berk, M.; Singh, L.S.; Tan, H.; Yin, L.; Powell, C.T.; Xu, Y. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin. Exp. Metastasis 2005, 22, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Takino, T.; Koshikawa, N.; Miyamori, H.; Tanaka, M.; Sasaki, T.; Okada, Y.; Seiki, M.; Sato, H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene 2003, 22, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- Theodorescu, D.; Sapinoso, L.M.; Conaway, M.R.; Oxford, G.; Hampton, G.M.; Frierson, H.F. Reduced Expression of Metastasis Suppressor RhoGDI2 Is Associated with Decreased Survival for Patients with Bladder Cancer. Clin. Cancer Res. 2004, 10, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M.; Danke, J.R.; Henry, M.D. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol. Ther. 2010, 9, 607–614. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Creel, P.; Turnbull, J.; Moore, C.; Jaffe, T.A.; Haley, S.; Petros, W.; Yenser, S.; Gockerman, J.P.; Sleep, D.; et al. A Phase I-II Study of Docetaxel and Atrasentan in Men with Castration-Resistant Metastatic Prostate Cancer. Clin. Cancer Res. 2008, 14, 6270–6276. [Google Scholar] [CrossRef]
- Quinn, D.I.; Tangen, C.M.; Hussain, M.; Lara, P.N.; Goldkorn, A.; Moinpour, C.M.; Garzotto, M.G.; Mack, P.C.; Carducci, M.A.; Monk, J.P.; et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Witteveen, P.; van der Mijn, K.; Neoplasia, M.L.; Los, M.; Kronemeijer, R.H.; Groenewegen, G.; Voest, E.E. Phase 1/2 study of atrasentan combined with pegylated liposomal doxorubicin in platinum-resistant recurrent ovarian cancer. Neoplasia 2010, 12, 941-IN20. [Google Scholar] [CrossRef]
- Carducci, M.A.; Manola, J.; Nair, S.; Liu, G.; Rousey, S.; Dutcher, J.P.; Wilding, G. Atrasentan in Patients With Advanced Renal Cell Carcinoma: A Phase 2 Trial of the ECOG-ACRIN Cancer Research Group (E6800). Clin. Genitourin. Cancer 2015, 13, 531–539.e1. [Google Scholar] [CrossRef]
- Santini, V.; Gozzini, A.; Ferrari, G. Histone deacetylase inhibitors: Molecular and biological activity as a premise to clinical application. Current Drug Metab. 2007, 8, 383–394. [Google Scholar] [CrossRef]
- Baradaran, P.C.; Kozovska, Z.; Furdova, A.; Smolková, B. Targeting Epigenetic Modifications in Uveal Melanoma. Int. J. Mol. Sci. 2020, 21, 5314. [Google Scholar] [CrossRef]
- Van Der Kooij, M.K.; Speetjens, F.M.; Van Der Burg, S.H.; Kapiteijn, E. Uveal Versus Cutaneous Melanoma; Same Origin, Very Distinct Tumor Types. Cancers 2019, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Homsi, J.; Bedikian, A.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.-J.; Mahoney, S.L.; Hwu, P. Phase 2 open-label study of weekly docosahexaenoic acid–paclitaxel in patients with metastatic uveal melanoma. Melanoma Res. 2010, 20, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, T.T.; Suciu, S.; Hansson, J.; Kruit, W.H.J.; Vuoristo, M.-S.; Kloke, O.; Gore, M.; Hahka-Kemppinen, M.; Parvinen, L.-M.; Kumpulainen, E.; et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur. J. Cancer 2003, 39, 1115–1120. [Google Scholar] [CrossRef]
- Schmittel, A.; Scheulen, M.E.; Bechrakis, N.E.; Strumberg, D.; Baumgart, J.; Bornfeld, N.; Foerster, M.H.; Thiel, E.; Keilholz, U. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005, 15, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Leyvraz, S.; Piperno-Neumann, S. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): A multicentric randomized. Ann. Oncol. 2014, 25, 742–746. [Google Scholar] [CrossRef]
- Pingpank, J.F.; Hughes, M.S.; Alexander, H.R.; Faries, M.B.; Zager, J.S.; Royal, R.; Whitman, E.D.; Nutting, C.W.; Siskin, G.P.; Agarwala, S.S. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J. Clin. Oncol. 2010, 28, LBA8512. [Google Scholar] [CrossRef]
- Gonsalves, C.F.; Eschelman, D.J.; Sullivan, K.L.; Anne, P.R.; Doyle, L.; Sato, T. Radioembolization as Salvage Therapy for Hepatic Metastasis of Uveal Melanoma: A Single-Institution Experience. Am. J. Roentgenol. 2011, 196, 468–473. [Google Scholar] [CrossRef]
- Klingenstein, A.; Haug, A.; Zech, C.J.; Schaller, U.C. Radioembolization as Locoregional Therapy of Hepatic Metastases in Uveal Melanoma Patients. Cardiovasc. Interv. Radiol. 2012, 36, 158–165. [Google Scholar] [CrossRef]
- Bedikian, A.; Papadopoulos, N.; Plager, C.; Eton, O.; Ring, S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003, 13, 303–306. [Google Scholar] [CrossRef]
- Spagnolo, F.; Grosso, M.; Picasso, V.; Tornari, E.; Pesce, M.; Queirolo, P. Treatment of metastatic uveal melanoma with intravenous fotemustine. Melanoma Res. 2013, 23, 196–198. [Google Scholar] [CrossRef]
- O’Neill, P.; Butt, M.; Eswar, C.; Gillis, P.; Marshall, E. A prospective single arm phase II study of dacarbazine and treosulfan as first-line therapy in metastatic uveal melanoma. Melanoma Res. 2006, 16, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Schinzari, G.; Rossi, E.; Cassano, A.; Dadduzio, V.; Quirino, M.; Pagliara, M.; Blasi, M.A.; Barone, C. Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: A single institution phase II study. Melanoma Res. 2017, 27, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Piperno-Neumann, S.; Servois, V.; Berry, M.; Dorval, T.; Plancher, C.; Couturier, J.; Levy-Gabriel, C.; Rouic, L.L.-L.; Desjardins, L.; et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur. J. Surg. Oncol. (EJSO) 2009, 35, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Nir, I.; Hendler, K.; Lotem, M.; Eid, A.; Jurim, O.; Pe’Er, J. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br. J. Ophthalmol. 2009, 93, 1042–1046. [Google Scholar] [CrossRef]
- Akyuz, M.; Yazici, P.; Dural, A.C.; Yigitbas, H.; Okoh, A.; Bucak, E.; McNamara, M.; Singh, A.; Berber, E. Laparoscopic management of liver metastases from uveal melanoma. Surg. Endosc. 2015, 30, 2567–2571. [Google Scholar] [CrossRef]
- Rivoire, M.; Kodjikian, L.; Baldo, S.; Kaemmerlen, P.; Négrier, S.; Grange, J.-D. Treatment of Liver Metastases From Uveal Melanoma. Ann. Surg. Oncol. 2005, 12, 422–428. [Google Scholar] [CrossRef]
- Augsburger, J.J.; Correa, Z.M.; Shaikh, A.H. Effectiveness of Treatments for Metastatic Uveal Melanoma. Am. J. Ophthalmol. 2009, 148, 119–127. [Google Scholar] [CrossRef]
- Ripley, R.T.; Davis, J.L.; Klapper, J.A.; Mathur, A.; Kammula, U.; Royal, R.E.; Yang, J.C.; Sherry, R.M.; Hughes, M.S.; Libutti, S.K.; et al. Liver Resection for Metastatic Melanoma with Postoperative Tumor-Infiltrating Lymphocyte Therapy. Ann. Surg. Oncol. 2009, 17, 163–170. [Google Scholar] [CrossRef]
- Salmon, R.; Levy, C.; Plancher, C.; Dorval, T.; Desjardins, L.; Leyvrazi, S.; Pouillart, P.; Schlienger, P.; Servois, V.; Asselain, B. Treatment of liver metastases from uveal melanoma by combined surgery—chemotherapy. Eur. J. Surg. Oncol. (EJSO) 1998, 24, 127–130. [Google Scholar] [CrossRef]
- Aoyama, T.; Mastrangelo, M.J.; Berd, D.; Nathan, F.E.; Shields, C.L.; Shields, J.A.; Rosato, E.L.; Rosato, F.E.; Sato, T. Protracted survival after resection of metastatic uveal melanoma. Cancer 2000, 89, 1561–1568. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Zorzi, D.; Abdalla, E.K.; Clary, B. Hepatic Resection for Metastatic Melanoma: Distinct Patterns of Recurrence and Prognosis for Ocular Versus Cutaneous Disease Endomicroscopy View project geografical disparities in liver transplant View project. Ann. Surg. Oncol. 2006, 13, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Chiche, L.; Aloia, T.; Elias, D.; Salmon, R.; Rivoire, M.; Jaeck, D.; Saric, J.; Le Treut, Y.P.; Belghiti, J.; et al. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1452 patients and development of a prognostic model. Ann. Surg. 2006, 244, 524. [Google Scholar] [CrossRef]
- de Ridder, J.A.M. Liver Metastases Incidence, Treatment & Prognostic Factors. Ph.D. Thesis, Radboud University of Nijmegen, Nijmegen, The Netherlands, 19 May 2017. [Google Scholar]
- Groeschl, R.T.; Nachmany, I.; Steel, J.L.; Reddy, S.K.; Glazer, E.S.; De Jong, M.C.; Pawlik, T.M.; Geller, D.A.; Tsung, A.; Marsh, J.W.; et al. Hepatectomy for Noncolorectal Non-Neuroendocrine Metastatic Cancer: A Multi-Institutional Analysis. J. Am. Coll. Surg. 2012, 214, 769–777. [Google Scholar] [CrossRef]
- Mariani, P.; Almubarak, M.M.; Kollen, M.; Wagner, M.; Plancher, C.; Audollent, R.; Piperno-Neumann, S.; Cassoux, N.; Servois, V. Radiofrequency ablation and surgical resection of liver metastases from uveal melanoma. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Derek, E.; Matsuoka, L.; Alexopoulos, S.; Fedenko, A.A.; Genyk, Y.; Selby, R. Combined surgical resection and radiofrequency ablation as treatment for metastatic ocular melanoma. Surg. Today 2012, 43, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Servois, V.; Bouhadiba, T.; Dureau, S.; Da Costa, D.; Almubarak, M.M.; Foucher, R.; Savignoni, A.; Cassoux, N.; Pierron, G.; Mariani, P. Iterative treatment with surgery and radiofrequency ablation of uveal melanoma liver metastasis: Retrospective analysis of a series of very long-term survivors. Eur. J. Surg. Oncol. 2019, 45, 1717–1722. [Google Scholar] [CrossRef]
- Olofsson Bagge, R.; Cahlin, C.; All-Ericsson, C.; Hashimi, F. Isolated Hepatic Perfusion for Ocular Melanoma Metastasis: Registry Data Suggests a Survival Benefit Reducing radiation-induced side effects in organs at risk View project PDX v2.0 View project. Artic. Ann. Surg. Oncol. 2013, 21, 466–472. [Google Scholar] [CrossRef]
- Search of: NCT01785316—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01785316&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT02678572—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02678572&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Bedikian, A.Y.; Legha, S.S.; Mavligit, G.; Carrasco, C.H.; Khorana, S.; Plager, C.; Papadopoulos, N.; Benjamin, R.S. Treatment of uveal melanoma metastatic to the liver. A review of the M. D. Anderson cancer center experience and prognostic factors. Cancer 1995, 76, 1665–1670. [Google Scholar] [CrossRef]
- Feun, L.G.; Reddy, K.R.; Yrizarry, J.M.; Savaraj, N.; Guerra, J.J.; Purser, R.K.; Waldman, S.; Levi, J.U.; Moffatt, F.; Morrell, L.; et al. A Phase I Study of Chemoembolization with Cisplatin and Lipiodol for Primary and Metastatic Liver Cancer. Am. J. Clin. Oncol. 1994, 17, 405–410. [Google Scholar] [CrossRef]
- Mavligit, G.; Charnsangavej, C.; Carrasco, C.; Jama, Y.P.; Benjamin, R.S.; Wallace, S. Regression of ocular melanoma metastatic to the liver after hepatic arterial chemoembolization with cisplatin and polyvinyl sponge. JAMA 1988, 260, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kim, K.B.; Papadopoulos, N.E.; Hwu, W.-J.; Hwu, P.; Prieto, V.G.; Bar-Eli, M.; Zigler, M.; Dobroff, A.; Bronstein, Y.; et al. A phase II study of gefitinib in patients with metastatic melanoma. Melanoma Res. 2011, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bedikian, A.; Ahrar, J.; Ensor, J.; Ahrar, K.; Madoff, D.C.; Wallace, M.J.; Murthy, R.; Tam, A.; Hwu, P. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: Response, survival, and prognostic factors. Am. J. Clin. Oncol. 2010, 33, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Eichler, K.; Zangos, S.; Herzog, C.; Hammerstingl, R.; Balzer, J.; Gholami, A. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: Local tumor control and survival. J. Cancer Res. Clin. Oncol. 2006, 133, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Huppert, P.; Fierlbeck, G.; Pereira, P.L.; Schanz, S.; Duda, S.; Wietholtz, H.; Rozeik, C.; Claussen, C.D. Transarterial chemoembolization of liver metastases in patients with uveal melanoma. Eur. J. Radiol. 2010, 74, e38–e44. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Panikkar, R.; Kirkwood, J.M. Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with. Melanoma Res. 2004, 14, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, C.F.; Eschelman, D.J.; Adamo, R.D.; Anne, P.R.; Orloff, M.M.; Terai, M.; Hage, A.N.; Yi, M.; Chervoneva, I.; Sato, T. A Prospective Phase II Trial of Radioembolization for Treatment of Uveal Melanoma Hepatic Metastasis. Radiology 2019, 293, 223–231. [Google Scholar] [CrossRef]
- Arulananda, S.; Parakh, S.; Palmer, J.; Goodwin, M.; Andrews, M.C.; Cebon, J. A pilot study of intrahepatic yttrium-90 microsphere radioembolization in combination with intravenous cisplatin for uveal melanoma liver-only metastases. Cancer Rep. 2019, 2, e1183. [Google Scholar] [CrossRef]
- Search of: NCT01893099—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01893099+&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Valsecchi, M.; Terai, M.; Eschelman, D.J.; Gonsalves, C.F.; Chervoneva, I.; Shields, J.A.P.; Shields, C.L.; Yamamoto, A.; Sullivan, K.L.; Laudadio, M.; et al. Double-Blinded, Randomized Phase II Study Using Embolization with or without Granulocyte–Macrophage Colony-Stimulating Factor in Uveal Melanoma with. J. Vasc. Interv. Radiol. 2015, 26, 523–532. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Šimon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kämpgen, E.; et al. Phase II DeCOG-Study of Ipilimumab in Pretreated and Treatment-Naïve Patients with Metastatic Uveal Melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.P.; De Olza, M.O.; Codes, M.; Lopez-Martin, J.A.; Berrocal, A.; García, M.; Gurpide, A.; Homet, B.; Martin-Algarra, S. Phase II study evaluating ipilimumab as a single agent in the first-line treatment of adult patients (Pts) with metastatic uveal melanoma (MUM): The GEM-1 trial. J. Clin. Oncol. 2014, 32, 9033. [Google Scholar] [CrossRef]
- Joshua, A.M.; Monzon, J.G.; Mihalcioiu, C.; Hogg, D.; Smylie, M.; Cheng, T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 2015, 25, 342–347. [Google Scholar] [CrossRef]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef]
- Mignard, C.; Huvier, A.D.; Gillibert, A.; Modeste, A.B.D.; Dutriaux, C.; Khammari, A.; Avril, M.-F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Johnson, D.B.; Bao, R.; Ancell, K.K.; Daniels, A.B.; Wallace, D.; Sosman, J.A.; Luke, J.J. Response to Anti–PD-1 in Uveal Melanoma Without High-Volume Liver Metastasis. J. Natl. Compr. Cancer Netw. 2019, 17, 114–117. [Google Scholar] [CrossRef]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Fiévet, A.; Gardrat, S.; Barnhill, R.L.; Popova, T.; Servois, V.; Rampanou, A.; Mouton, A.; et al. Outlier response to anti-PD1 in uveal melanoma reveals germline MBD4 mutations in hypermutated tumors. Nat. Commun. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Search of: NCT02626962—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02626962+&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT01585194—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01585194&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Pelster, M.; Gruschkus, S.K.; Bassett, R.; Gombos, D.S.; Shephard, M.; Posada, L.; Glover, M.; Diab, A.; Hwu, P.; Patel, S.P. Phase II study of ipilimumab and nivolumab (ipi/nivo) in metastatic uveal melanoma (UM). J. Clin. Oncol. 2019, 37, 9522. [Google Scholar] [CrossRef]
- Search of: NCT02697630—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02697630&cntry=&state=&city=&dist= (accessed on 11 September 2020).
- Jespersen, H.; Bagge, R.O.; Ullenhag, G.; Carneiro, A.; Helgadottir, H.; Ljuslinder, I.; Levin, M.; All-Eriksson, C.; Andersson, B.; Stierner, U.; et al. Concomitant use of pembrolizumab and entinostat in adult patients with metastatic uveal melanoma (PEMDAC study): Protocol for a multicenter phase II open label study. BMC Cancer 2019, 19, 415. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Prevoo, W.; Meier, M.A.; Sikorska, K.; Van, T.M.; Van De Wiel, B.A.; Van Der Wal, J.E.; Mallo, H.A.; Grijpink-Ongering, L.G.; Broeks, A.; et al. Phase Ib/II trial testing combined radiofrequency ablation and ipilimumab in uveal melanoma (SECIRA-UM). Melanoma Res. 2020, 30, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Search of: NCT04283890—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT04283890+&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT03472586—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03472586&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT02913417—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02913417&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Chandran, S.; Somerville, R.; Yang, J.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
- van Loenen, M.M.; de Boer, R.; Hagedoorn, R.S.; Jankipersadsing, V.; Amir, A.L.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Multi-cistronic vector encoding optimized safety switch for adoptive therapy with T-cell receptor-modified T cells. Gene Ther. 2013, 20, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, E.M.V.; Lindberg, M.F.; Jespersen, H.; Alsén, S.; Bagge, R.O.; Donia, M.; Svane, I.M.; Nilsson, O.; Ny, L.; Nilsson, L.M.; et al. HER2 CAR-T Cells Eradicate Uveal Melanoma and T-cell Therapy–Resistant Human Melanoma in IL2 Transgenic NOD/SCID IL2 Receptor Knockout Mice. Cancer Res. 2019, 79, 899–904. [Google Scholar] [CrossRef]
- Tavera, R.J.; Forget, M.A.; Kim, Y.U.; Sakellariou-Thompson, D.; Creasy, C.A.; Bhatta, A.; Fulbright, O.J.; Ramachandran, R.; Thorsen, S.T.; Flores, E.; et al. Utilizing T-cell Activation Signals 1, 2, and 3 for Tumor-infiltrating Lymphocytes (TIL) Expansion: The Advantage over the Sole Use of Interleukin-2 in Cutaneous and Uveal Melanoma. J. Immunother. 2018, 41, 399–405. [Google Scholar] [CrossRef]
- Search of: NCT03467516—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03467516&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT03068624—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03068624&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Damato, B.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef]
- Middleton, M.; Steven, N.M.; Evans, T.J.; Infante, J.R.; Sznol, M.; Mulatero, C.; Hamid, O.; Shoushtari, A.N.; Shingler, W.; Johnson, A.; et al. Safety, pharmacokinetics and efficacy of IMCgp100, a first-in-class soluble TCR-antiCD3 bispecific t cell redirector with solid tumour activity: Results from the FIH study in melanoma. J. Clin. Oncol. 2016, 34, 3016. [Google Scholar] [CrossRef]
- Search of: NCT02570308—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02570308&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT03070392—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03070392&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT03635632—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03635632&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Bol, K.F.; Mensink, H.W.; Aarntzen, E.H.; Schreibelt, G.; Keunen, J.E.; Coulie, P.G.; De Klein, A.; Punt, C.J.; Paridaens, D.; Figdor, C.G.; et al. Long Overall Survival After Dendritic Cell Vaccination in Metastatic Uveal Melanoma Patients. Am. J. Ophthalmol. 2014, 158, 939–947. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Yu, B.; Yu, D.; Morelli, D.; Hall, M.; Bogle, D.; Yan, L.; Targan, S.; Solomon, J.; Nichol, G.; et al. Extended Dose Ipilimumab with a Peptide Vaccine: Immune Correlates Associated with Clinical Benefit in Patients with Resected High-Risk Stage IIIc/IV Melanoma. Clin. Cancer Res. 2010, 17, 896–906. [Google Scholar] [CrossRef]
- Lesterhuis, W.J.; Schreibelt, G.; Scharenborg, N.M.; Brouwer, H.M.H.; Gerritsen, M.P.; Croockewit, S.; Coulie, P.G.; Torensma, R.; Adema, G.J.; Figdor, C.G.; et al. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol. Immunother. 2011, 60, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.J.; Iheagwara, U.K.; Reid, S.; Srivastava, M.K.; Wolf, J.; Lotem, M.; Ksander, B.R.; Ostrand-Rosenberg, S. Uveal melanoma cell-based vaccines express MHC II molecules that traffic via the endocytic and secretory pathways and activate CD8+ cytotoxic, tumor-specific T cells. Cancer Immunol. Immunother. 2009, 59, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Agarwala, S.S.; Smithers, B.; Ross, M.I.; Scoggins, C.R.; Coventry, B.J.; Neuhaus, S.J.; Minor, D.R.; Singer, J.M.; Wachter, E.A. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann. Surg. Oncol. 2014, 22, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
- Search of: NCT00219843—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT00219843&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2008, 457, 599–602. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Griewank, K.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations inGNA11in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion-Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Flaherty, K.T.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; et al. Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur. J. Cancer 2020, 126, 33–44. [Google Scholar] [CrossRef]
- Onken, M.; Makepeace, C. Targeting nucleotide exchange to inhibit constitutively active G protein α subunits in cancer cells. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef]
- Lapadula, D.; Farias, E.; Randolph, C.E.; Purwin, T.J.; McGrath, D.; Charpentier, T.H.; Zhang, L.; Wu, S.; Terai, M.; Sato, T.; et al. Effects of Oncogenic Gαq and Gα11 Inhibition by FR900359 in Uveal Melanoma. Mol. Cancer Res. 2018, 17, 963–973. [Google Scholar] [CrossRef]
- Xiong, X.-F.; Zhang, H.; Boesgaard, M.W.; Underwood, C.R.; Bräuner-Osborne, H.; Strømgaard, K. Structure–Activity Relationship Studies of the Natural Product G q/11 Protein Inhibitor YM-254890. ChemMedChem 2019, 14, 865–870. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Qiu, C.; Shang, Q.; Qian, G.; Fan, X.; Ge, S.; Jia, R. The oncolytic virus H101 combined with GNAQ siRNA-mediated knockdown reduces uveal melanoma cell viability. J. Cell. Biochem. 2018, 120, 5766–5776. [Google Scholar] [CrossRef] [PubMed]
- Posch, C.; Latorre, A.; Crosby, M.B.; Celli, A.; Latorre, A.; Vujic, I.; Sanlorenzo, M.; Green, G.A.; Weier, J.; Zekhtser, M.; et al. Detection of GNAQ mutations and reduction of cell viability in uveal melanoma cells with functionalized gold nanoparticles. Biomed. Microdevices 2015, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Piperno-Neumann, S.; Kapiteijn, E.; Chapman, P.B.; Frank, S.; Joshua, A.M.; Piulats, J.M.; Wolter, P.; Cocquyt, V.; Chmielowski, B. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: A phase III, multicentre, randomised trial (SUMIT). J. Clin. Oncol. 2018, 36, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Musi, E.; Ho, A.L.; De Stanchina, E.; Schwartz, G.K. Inhibition of Mutant GNAQ Signaling in Uveal Melanoma Induces AMPK-Dependent Autophagic Cell Death. Mol. Cancer Ther. 2013, 12, 768–776. [Google Scholar] [CrossRef]
- Search of: NCT01979523—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01979523&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Bhatia, S.; Moon, J.; Margolin, K.A.; Weber, J.S.; Lao, C.D.; Othus, M.; Aparicio, A.M.; Ribas, A.; Sondak, V.K. Phase II Trial of Sorafenib in Combination with Carboplatin and Paclitaxel in Patients with Metastatic Uveal Melanoma: SWOG S0512. PLoS ONE 2012, 7, e48787. [Google Scholar] [CrossRef]
- Search of: NCT02601378—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=+NCT02601378&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Zeldis, J.; Heller, C.; Seidel, G.; Yuldasheva, N.; Stirling, D.; ShutackS, Y.; Libutti, K. A randomized phase II trial comparing two doses of lenalidomide for the treatment of stage IV ocular melanoma. J. Clin. Oncol. 2009, 27, e20012. [Google Scholar] [CrossRef]
- Luke, J.J.; Olson, D.J.; Allred, J.B.; Strand, C.A.; Bao, R.; Zha, Y.; Carll, T.; Labadie, B.W.; Bastos, B.R.; Butler, M.O.; et al. Randomized Phase II Trial and Tumor Mutational Spectrum Analysis from Cabozantinib versus Chemotherapy in Metastatic Uveal Melanoma (Alliance A091201). Clin. Cancer Res. 2019, 26, 804–811. [Google Scholar] [CrossRef]
- Search of: NCT01413191—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01413191&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT01252251—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01252251&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Shoushtari, A.N.; Ong, L.T.; Schoder, H.; Singh-Kandah, S.; Abbate, K.T.; Postow, M.A.; Callahan, M.K.; Wolchok, J.; Chapman, P.B.; Panageas, K.S.; et al. A phase 2 trial of everolimus and pasireotide long-acting release in patients with metastatic uveal melanoma. Melanoma Res. 2016, 26, 272–277. [Google Scholar] [CrossRef]
- Daud, A.I.; Kluger, H.M.; Kurzrock, R.; Schimmoller, F.; Weitzman, A.L.; Samuel, T.A.; Moussa, A.H.; Gordon, M.S.; Shapiro, G. I Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br. J. Cancer 2017, 116, 432–440. [Google Scholar] [CrossRef]
- Shah, S.; Luke, J.J.; Jacene, H.A.; Chen, T.; Giobbie-Hurder, A.; Ibrahim, N.; Buchbinder, E.I.; McDermott, D.F.; Flaherty, K.T.; Sullivan, R.J.; et al. Results from phase II trial of HSP90 inhibitor, STA-9090 (ganetespib), in metastatic uveal melanoma. Melanoma Res. 2018, 28, 605–610. [Google Scholar] [CrossRef]
- Guenterberg, K.; Grignol, V.; ROlenckielekar, K.V.; Varker, K.A.; Chen, H.X.; Kendra, K.L.; Olencki, T.L.; Carson, W.E. A pilot study of bevacizumab and interferon-α2b in ocular melanoma. Am. J. Clin. Oncol. 2011, 34, 87. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Servois, V.; Bidard, F.-C.; Mariani, P.; Plancher, C.; Diallo, A.; Vago-Ady, N.; Desjardins, L. BEVATEM: Phase II study of bevacizumab (B) in combination with temozolomide (T) in patients (pts) with first-line metastatic uveal melanoma (MUM): Final results. J. Clin. Oncol. 2013, 31, 9057. [Google Scholar] [CrossRef]
- Spitler, L.E.; Boasberg, P.; Day, S.O.; Hamid, O.; Cruickshank, S.; Mesko, S.; Weber, R.W. Phase II Study of Nab-Paclitaxel and Bevacizumab as First-line Therapy for Patients with Unresectable Stage III and IV Melanoma. Am. J. Clin. Oncol. 2015, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Frankel, P.; Margolin, K.A.; Christensen, S.; Ruel, C.; Shipe-Spotloe, J.; Gandara, D.R.; Chen, A.; Kirkwood, J.M. Aflibercept (VEGF Trap) in Inoperable Stage III or Stage IV Melanoma of Cutaneous or Uveal Origin. Clin. Cancer Res. 2011, 17, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.B.; Kauczok-Vetter, C.S.; Houben, R.; Becker, J.C. Overexpression of the KIT/SCF in Uveal Melanoma Does Not Translate into Clinical Efficacy of Imatinib Mesylate. Clin. Cancer Res. 2009, 15, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Penel, N.; Delcambre, C.; Durando, X.; Clisant, S.; Hebbar, M.; Négrier, S.; Fournier, C.; Isambert, N.; Mascarelli, F.; Mouriaux, F. O-Mel-Inib: A Cancéro-pôle Nord-Ouest multicenter phase II trial of high-dose Imatinib mesylate in metastatic uveal melanoma. Investig. New Drugs 2008, 26, 561–565. [Google Scholar] [CrossRef]
- Mahipal, A.; Tijani, L.; Chan, K.; Laudadio, M.; Mastrangelo, M.J.; Sato, T. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res. 2012, 22, 440–446. [Google Scholar] [CrossRef]
- Haas, N.B.; Quirt, I.; Hotte, S.; McWhirter, E.; Polintan, R.; Litwin, S.; Adams, P.D.; McBryan, T.; Wang, L.; Martin, L.P.; et al. Phase II trial of vorinostat in advanced melanoma. Investig. New Drugs 2014, 32, 526–534. [Google Scholar] [CrossRef]
- Search of: NCT03417739—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03417739&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT02743611—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02743611+&cntry=&state=&city=&dist= (accessed on 22 August 2020).
- Search of: NCT01835145—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01835145&cntry=&state=&city=&dist= (accessed on 22 August 2020).
| Mnemonic | Feature | N of Features | Choroidal Nevus Growth into Melanoma (%) | Monitoring |
|---|---|---|---|---|
| To Find Small Ocular Melanoma Using helpful Hints Daily | Thickness > 2 mm | None | 4% | Every 6 months  Once a year (if stability persists) Once a year (if stability persists) |
| Fluid (subretinal) | ||||
| Symptoms - decreased vision - flashes/floaters | 1 Feature | 36% | Every 4–6 months | |
| Orange pigment | ||||
| Margin ≤ 3 mm to disc | 2 Features | 45% | Every 4–6 months | |
| Ultrasonographic hollowness | ||||
| Halo absence Drusen absence | 3 or more Features | 50% | Referral to Experienced Center Primary treatment Prognosis |
| Clinical Trials N | Tested Agent and Mechanism of Action | Phase | Status |
|---|---|---|---|
| NCT02223819 | Crizotinib (c-Met inhibitor) | II | Recruiting |
| NCT02068586 | Sunitinib (c-Kit inhibitor) vs. Valproic acid (HDAC inhibitor) | II | Recruiting |
| NCT00489944 | Suntinib (c-Kit inhibitor) + Tamoxifen (estrogen receptor modulator) + Cisplatin (alkylating agent) | II | Unknown |
| NCT01983748 | Dendritic cell vaccination (immunotherapy) | III | Recruiting |
| NCT00929019 | Dendritic cell vaccination (immunotherapy) | I/II | Terminated, slow accrual |
| NCT02519322 | Nivolumab (anti-PD1) with or without Ipilimumab (anti-CTLA4) or Relatlimab (anti-LAG3) | II | Recruiting |
| NCT03528408 | Ipilimumab (anti-CTLA4) + Nivolumab(anti-PD1) | II | Recruiting |
| NCT02336763 | Prophylactic External-Beam Radiation Therapy to the liver | II | Terminated, lack of accrual |
| Clinical Trials N | Tested Agent and Mechanism of Action | Phase | Status |
|---|---|---|---|
| NCT01785316 | IHP with melphalan or best alternative care | III | Recruiting |
| NCT02678572 | PHP with melphalan or best alternative care | III | Recruiting |
| NCT01893099 | 90Y-labelled microspheres and sorafenib (inhibitor of RAF/MEK/ERK and VEGFR/PDGFR) | I | Complete, no results |
| NCT02626962 | Nivolumab (anti-PD1) + ipilimumab (anti-CTLA4) | II | Active, not recruiting |
| NCT01585194 | Nivolumab (anti-PD1) + ipilimumab (anti-CTLA4) | II | Active, not recruiting |
| NCT02697630 | Pembrolizumab (anti-PD1) + Entinostat (HDAC inhibitor) | II | Active, not recruiting |
| NCT04283890 | PHP with melphalan + ipilimumab (anti-CTLA4) and nivolumab (anti-PD1) | I/II | Recruiting |
| NCT02913417 | 90Y-labelled microspheres + Ipilimumab (anti-CTLA4) and nivolumab (anti-PD1) | I/II | Recruiting |
| NCT03472586 | Immunoembolization + Ipilimumab (anti-CTLA4) and nivolumab (anti-PD1) | II | Recruiting |
| NCT03467516 | TILs | II | Recruiting |
| NCT03068624 | TILs + cyclophosphamide (alkylating agent), aldesleukin (human recombinant IL-2), and ipilimumab (anti-CTLA4) | Ib | Active, not recruiting |
| NCT02570308 | ImmTAC molecule (IMCgp100) targeting gp100 | I/II | Active, not recruiting |
| NCT03070392 | ImmTAC molecule (IMCgp100) targeting gp100Vs. investigator’s choice | II | Active, not recruiting. |
| NCT03635632 | C7R-GD2.CAR T cells | I | Recruiting |
| NCT00219843 | Intralesional (IL) PV-10 chemoablation(rose bengal disodium, 10%) | I | Complete, no results |
| NCT01979523 | Trametinib (MEK inhibitor) ± AKT inhibition | II | Complete, has results |
| NCT02601378 | LXS196 (PKC inhibitor) ± HDM201 (MDM2 inhibitor) | I | Active, not recruiting |
| NCT01413191 | Cixutumumab (IGF1R inhibitor) | II | Complete, has results |
| NCT01252251 | Everoliumus (mTOR inhibitor) and pasireotide (somatostatin analog) | II | Complete, has results |
| NCT03417739 | BVD-523(ERK1/ERK2 inhibitor) | II | Active, not recruiting |
| NCT02743611 | BPX-701 (PRAME-targeting T-cell receptor) | I/II | Active, not recruiting |
| NCT01835145 | Carbozantinib (c-MET, c-KIT, VEGFR2 inhibitor) vs. temozolomide (alkylating agent) or dacarbazine (alkylating agent) | II | Active, not recruiting, has results |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallone, F.; Sacchetti, M.; Lambiase, A.; Moramarco, A. Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma. Cancers 2020, 12, 2761. https://doi.org/10.3390/cancers12102761
Mallone F, Sacchetti M, Lambiase A, Moramarco A. Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma. Cancers. 2020; 12(10):2761. https://doi.org/10.3390/cancers12102761
Chicago/Turabian StyleMallone, Fabiana, Marta Sacchetti, Alessandro Lambiase, and Antonietta Moramarco. 2020. "Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma" Cancers 12, no. 10: 2761. https://doi.org/10.3390/cancers12102761
APA StyleMallone, F., Sacchetti, M., Lambiase, A., & Moramarco, A. (2020). Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma. Cancers, 12(10), 2761. https://doi.org/10.3390/cancers12102761