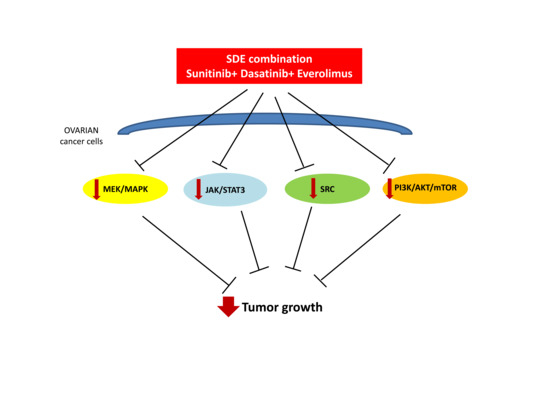
Synergistic Anti-Tumor Activity by Targeting Multiple Signaling Pathways in Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Results
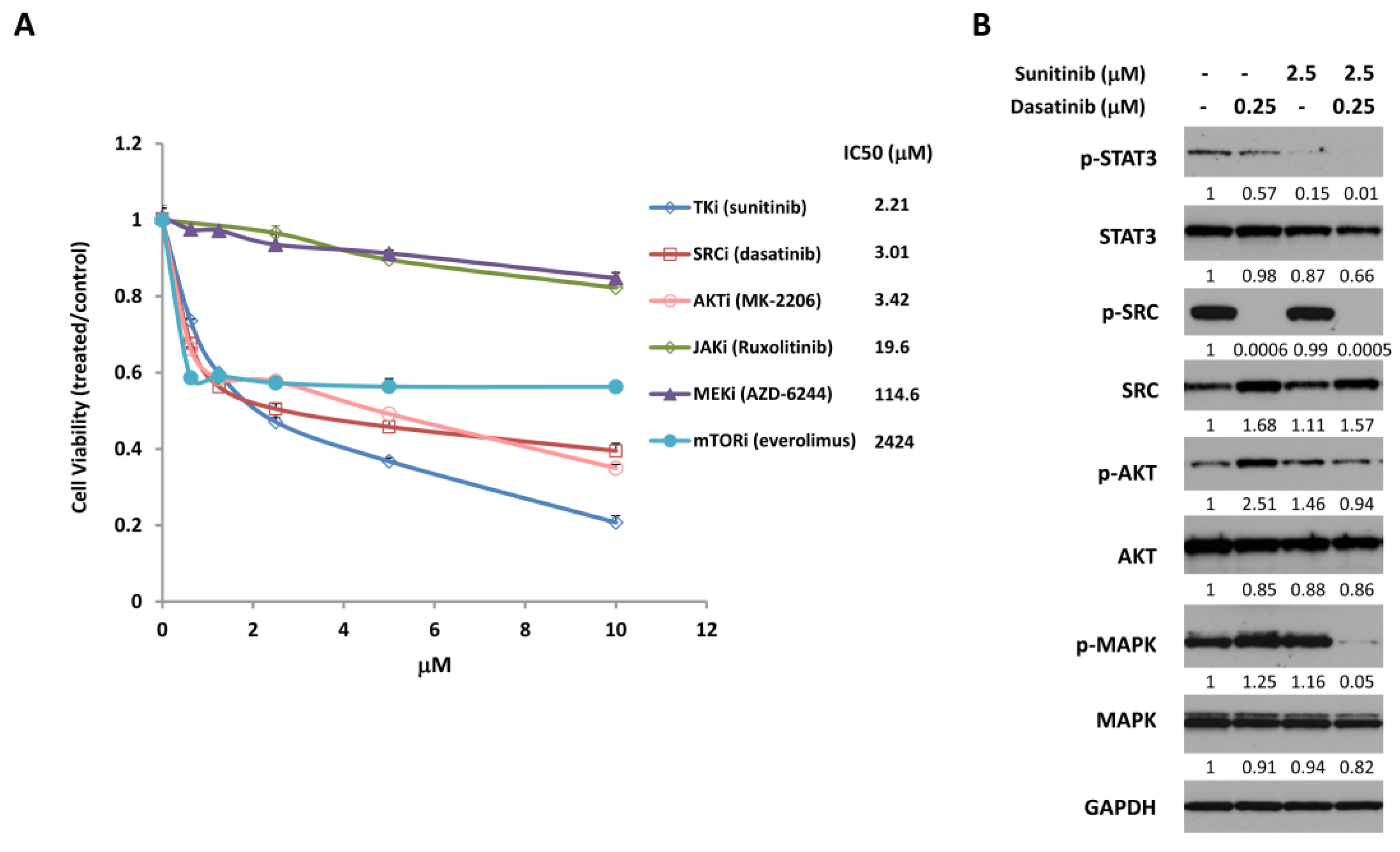
2.1. Combined Treatment with Sunitinib and Dasatinib Leads to Inhibition of Multiple Signaling Pathways
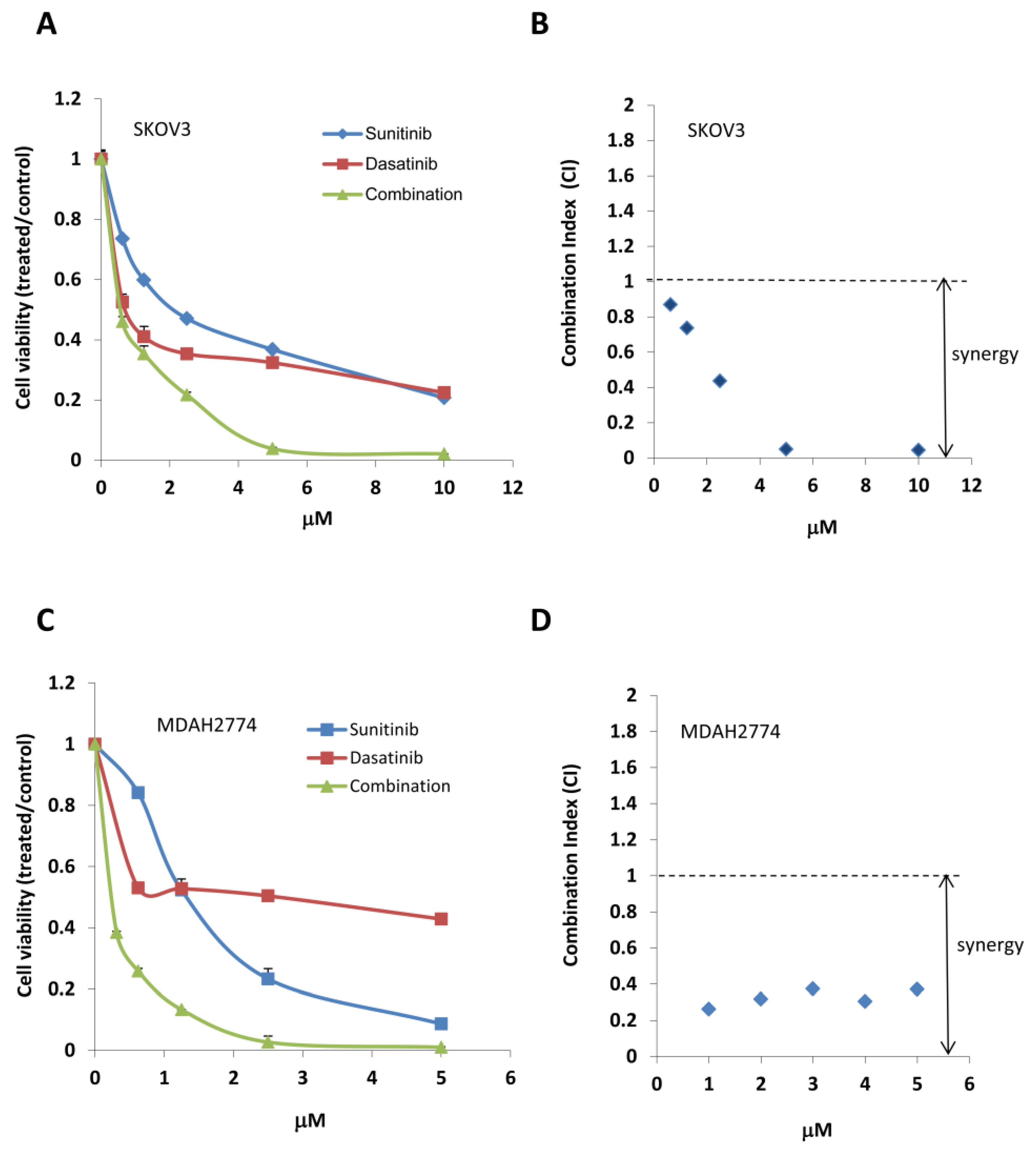
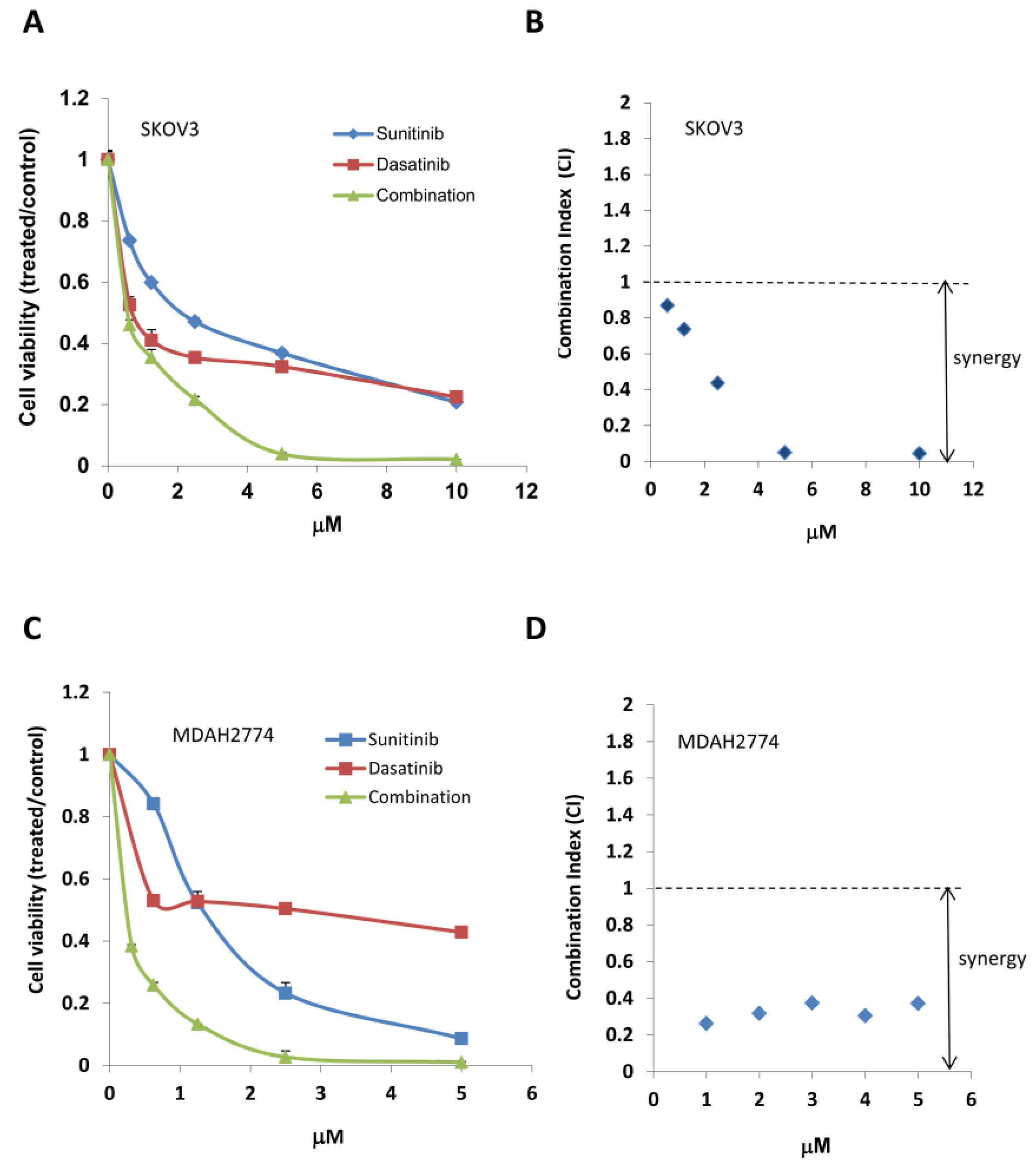
2.2. Combined Treatment with Sunitinib and Dasatinib Results in a Synergistic Inhibition of Cell Growth/Survival
2.3. Additional Suppression of the PI3K/AKT/mTOR Pathway Leads to Further Inhibition of Cell Growth
2.4. Combination Treatment with Sunitinib, Dasatinib, and Everolimus Results in a Synergistic Inhibition of Human Ovarian Tumor Growth in Mice
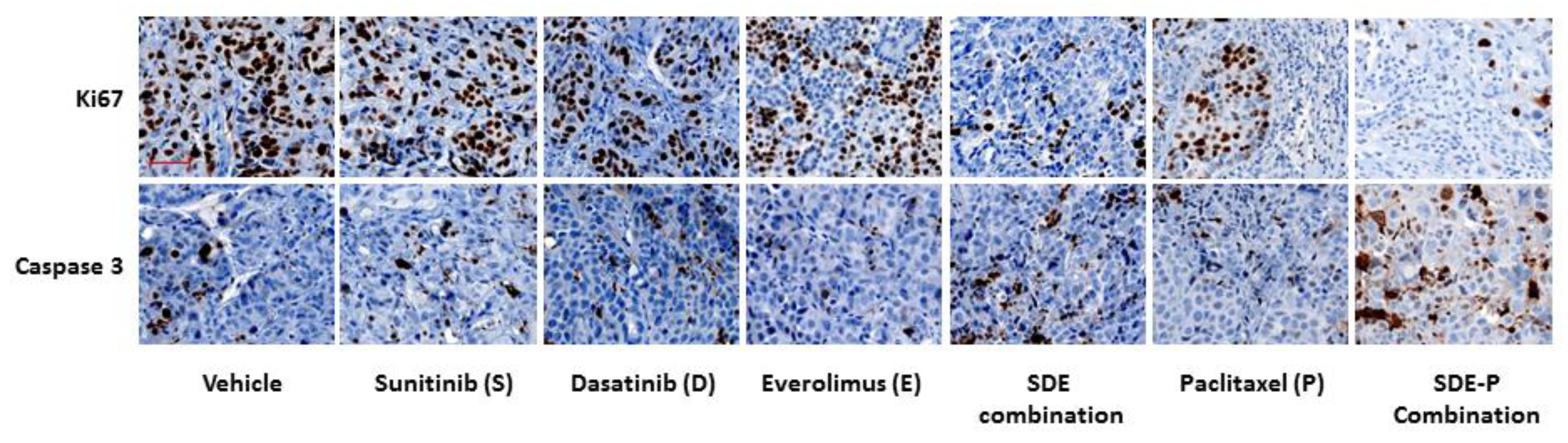
2.5. SDE Triple Combination Synergistically Increases the Anti-Tumor Activity of Paclitaxel, Both In Vitro and In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Viability Assays
4.4. Determination of Combination Index
4.5. Western Blot Analysis
4.6. Animal Models
4.7. Immunohistochemistry (IHC)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cannistra, S.A. Cancer of the ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Bast, R.C., Jr. Minireview: Human ovarian cancer: Biology, current management, and paths to personalizing therapy. Endocrinology 2012, 153, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.J., Jr.; Alvarez, R.D.; Armstrong, D.K.; Boston, B.; Burger, R.A.; Chen, L.M. Epithelial ovarian cancer. J. Nat. Compr. Cancer Netw. JNCCN 2011, 9, 82–113. [Google Scholar] [CrossRef]
- Cristea, M.; Han, E.; Salmon, L.; Morgan, R.J. Practical considerations in ovarian cancer chemotherapy. Ther. Adv. Med Oncol. 2010, 2, 175–187. [Google Scholar] [CrossRef]
- Maoz, A.; Matsuo, K.; Ciccone, M.A.; Matsuzaki, S.; Klar, M.; Roman, L.D.; Sood, A.K.; Gershenson, D.M. Molecular Pathways and Targeted Therapies for Malignant Ovarian Germ Cell Tumors and Sex Cord-Stromal Tumors: A Contemporary Review. Cancers 2020, 12, 1398. [Google Scholar] [CrossRef]
- Landrum, L.M.; Java, J.; Mathews, C.A.; Lanneau, G.S., Jr.; Copeland, L.J.; Armstrong, D.K.; Walker, J.L. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol. Oncol. 2013, 130, 12–18. [Google Scholar] [CrossRef]
- Narod, S. Can advanced-stage ovarian cancer be cured? Nat. Rev. Clin. Oncol. 2016, 13, 255–261. [Google Scholar] [CrossRef]
- Yap, T.A.; Carden, C.P.; Kaye, S.B. Beyond chemotherapy: Targeted therapies in ovarian cancer. Nat. Rev. Cancer 2009, 9, 167–181. [Google Scholar] [CrossRef]
- Burger, R.A. Experience with Bevacizumab in the Management of Epithelial Ovarian Cancer. J. Clin. Oncol. 2007, 25, 2902–2908. [Google Scholar] [CrossRef]
- Liu, J.; Matulonis, U.A. New Strategies in Ovarian Cancer: Translating the Molecular Complexity of Ovarian Cancer into Treatment Advances. Clin. Cancer Res. 2014, 20, 5150–5156. [Google Scholar] [CrossRef] [PubMed]
- Brasseur, K.; Gevry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2017, 8, 4008–4042. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Liang, W.; Wu, J.; Kowolik, C.M.; Buettner, R.; Scuto, A.; Hsieh, M.-Y.; Hong, H.; Brown, C.E.; Forman, S.J.; et al. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol. Cancer Ther. 2014, 13, 3037–3048. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Wu, J.; Liu, L.; Tian, Y.; Buettner, R.; Hsieh, M.Y.; Horne, D.; Dellinger, T.; Han, E.S.; Jove, R.; et al. Synergistic anti-tumor effect of combined inhibition of EGFR and JAK/STAT3 pathways in human ovarian cancer. Mol. Cancer 2015, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Han, E.S.; Dellinger, T.H.; Wu, J.; Guo, Y.; Buettner, R.; Horne, D.A.; Jove, R.; Yim, J.H. Increasing Antitumor Activity of JAK Inhibitor by Simultaneous Blocking Multiple Survival Signaling Pathways in Human Ovarian Cancer. Transl. Oncol. 2019, 12, 1015–1025. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef]
- Atkins, M.; Jones, C.A.; Kirkpatrick, P. Sunitinib maleate. Nat. Rev. Drug Discov. 2006, 5, 279–280. [Google Scholar] [CrossRef]
- Mendel, D.B.; Laird, A.D.; Xin, X.; Louie, S.G.; Christensen, J.G.; Li, G.; Schreck, R.E.; Abrams, T.J.; Ngai, T.J.; Lee, L.B.; et al. In vivo Antitumor Activity of SU11248, a Novel Tyrosine Kinase Inhibitor Targeting Vascular Endothelial Growth Factor and Platelet-derived Growth Factor Receptors. Clin. Cancer Res. 2003, 9, 327–337. [Google Scholar]
- Chow, L.Q.M.; Eckhardt, S.G. Sunitinib: From Rational Design to Clinical Efficacy. J. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef]
- Yang, F.; Jove, V.; Xin, H.; Hedvat, M.; Van Meter, T.E.; Yu, H. Sunitinib Induces Apoptosis and Growth Arrest of Medulloblastoma Tumor Cells by Inhibiting STAT3 and AKT Signaling Pathways. Mol. Cancer Res. 2010, 8, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Zhang, C.; Herrmann, A.; Du, Y.; Figlin, R.; Yu, H. Sunitinib Inhibition of Stat3 Induces Renal Cell Carcinoma Tumor Cell Apoptosis and Reduces Immunosuppressive Cells. Cancer Res. 2009, 69, 2506–2513. [Google Scholar] [CrossRef]
- Ammanamanchi, S.; Brattain, M.G. Restoration of Transforming Growth Factor-β Signaling through Receptor RI Induction by Histone Deacetylase Activity Inhibition in Breast Cancer Cells. J. Biol. Chem. 2004, 279, 32620–32625. [Google Scholar] [CrossRef] [PubMed]
- Brave, M.; Goodman, V.; Kaminskas, E.; Farrell, A.; Timmer, W.; Pope, S.; Harapanhalli, R.; Saber, H.; Morse, D.; Bullock, J.; et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin. Cancer Res. 2008, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Boil. 1997, 13, 513–609. [Google Scholar] [CrossRef]
- Wiener, J.R.; Windham, T.C.; Estrella, V.C.; Parikh, N.U.; Thall, P.F.; Deavers, M.T.; Bast, R.C.; Mills, G.B.; Gallick, G.E. Activated Src Protein Tyrosine Kinase is Overexpressed in Late-Stage Human Ovarian Cancers. Gynecol. Oncol. 2003, 88, 73–79. [Google Scholar] [CrossRef]
- Konecny, G.E.; Glas, R.; Dering, J.; Manivong, K.; Qi, J.; Finn, R.S.; Yang, G.R.; Hong, K.-L.; Ginther, C.; Winterhoff, B.; et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br. J. Cancer 2009, 101, 1699–1708. [Google Scholar] [CrossRef]
- Wiener, J.R.; Nakano, K.; Kruzelock, R.P.; Bucana, C.D.; Bast, R.C.; Gallick, G.E. Decreased Src Tyrosine Kinase Activity Inhibits Malignant Human Ovarian Cancer Tumor Growth in a Nude Mouse Model. Clin. Cancer Res. 1999, 5, 2164–2170. [Google Scholar]
- Han, L.Y.; Landen, C.N.; Trevino, J.G.; Halder, J.; Lin, Y.G.; Kamat, A.A.; Kim, T.-J.; Merritt, W.M.; Coleman, R.L.; Gershenson, D.M.; et al. Antiangiogenic and Antitumor Effects of Src Inhibition in Ovarian Carcinoma. Cancer Res. 2006, 66, 8633–8639. [Google Scholar] [CrossRef]
- Liang, W.; Kujawski, M.; Wu, J.; Lu, J.; Herrmann, A.; Loera, S.; Yen, Y.; Lee, F.; Yu, H.; Wen, W.; et al. Antitumor activity of targeting SRC kinases in endothelial and myeloid cell compartments of the tumor microenvironment. Clin. Cancer Res. 2010, 16, 924–935. [Google Scholar] [CrossRef]
- Schilder, R.J.; Brady, W.E.; Lankes, H.A.; Fiorica, J.V.; Shahin, M.S.; Zhou, X.C.; Mannel, R.S.; Pathak, H.B.; Hu, W.; Alpaugh, R.K.; et al. Phase II evaluation of dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2012, 127, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Secord, A.A.; Teoh, D.K.; Barry, W.T.; Yu, M.; Broadwater, G.; Havrilesky, L.J.; Lee, P.S.; Berchuck, A.; Lancaster, J.; Wenham, R.M. A Phase I Trial of Dasatinib, an Src-Family Kinase Inhibitor, in Combination with Paclitaxel and Carboplatin in Patients with Advanced or Recurrent Ovarian Cancer. Clin. Cancer Res. 2012, 18, 5489–5498. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; De Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grünwald, V.; Thompson, J.A.; Figlin, R.A.; Hollaender, N.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008, 372, 449–456. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2011, 366, 520–529. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Blumenschein, G.R., Jr. Defining biomarkers to predict sensitivity to PI3K/Akt/mTOR pathway inhibitors in breast cancer. Cancer Treat. Rev. 2013, 39, 313–320. [Google Scholar] [CrossRef]
- Grunt, T.W.; Mariani, G.L. Novel approaches for molecular targeted therapy of breast cancer: Interfering with PI3K/AKT/mTOR signaling. Curr. Cancer Drug Targets 2013, 13, 188–204. [Google Scholar] [CrossRef]
- Bartlett, J.M. Biomarkers and patient selection for PI3K/Akt/mTOR targeted therapies: Current status and future directions. Clin. Breast Cancer 2010, 10 (Suppl. S3), S86–S95. [Google Scholar] [CrossRef]
- McAuliffe, P.F.; Meric-Bernstam, F.; Mills, G.B.; Gonzalez-Angulo, A.M. Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin. Breast Cancer 2010, 10 (Suppl. S3), S59–S65. [Google Scholar] [CrossRef]
- Ganesan, P.; Moulder, S.; Lee, J.J.; Janku, F.; Valero, V.; Zinner, R.G.; Naing, A.; Fu, S.; Tsimberidou, A.; Hong, D.; et al. Triple-negative breast cancer patients treated at MD anderson cancer center in phase I trials: Improved outcomes with combination chemotherapy and targeted agents. Mol. Cancer Ther. 2014, 13, 3175–3184. [Google Scholar] [CrossRef]
- Wan, X.; Harkavy, B.; Shen, N.; Grohar, P.; Helman, L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yue, P.; Kim, Y.A.; Fu, H.; Khuri, F.R.; Sun, S.Y. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008, 68, 7409–7418. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E.; Chu, T.; Elvin, J.A.; Edwards, R.P.; Zorn, K.K. Phase II study of everolimus and bevacizumab in recurrent ovarian, peritoneal, and fallopian tube cancer. Gynecol. Oncol. 2020, 156, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Jabbour, E.; Grimley, J.; Kirkpatrick, P. Dasatinib. Nat. Rev. Drug Discov. 2006, 5, 717–718. [Google Scholar] [CrossRef]
- Duan, Z.; Foster, R.; Bell, D.A.; Mahoney, J.; Wolak, K.; Vaidya, A.; Hampel, C.; Lee, H.; Seiden, M.V. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin. Cancer Res. 2006, 12, 5055–5063. [Google Scholar] [CrossRef]
- Lee, H.-J.; Zhuang, G.; Cao, Y.; Du, P.; Kim, H.-J.; Settleman, J. Drug Resistance via Feedback Activation of Stat3 in Oncogene-Addicted Cancer Cells. Cancer Cell 2014, 26, 207–221. [Google Scholar] [CrossRef]
- Eichten, A.; Su, J.; Adler, A.P.; Zhang, L.; Ioffe, E.; Parveen, A.A.; Yancopoulos, G.D.; Rudge, J.; Lowy, I.; Lin, H.S.; et al. Resistance to Anti-VEGF Therapy Mediated by Autocrine IL6/STAT3 Signaling and Overcome by IL6 Blockade. Cancer Res. 2016, 76, 2327–2339. [Google Scholar] [CrossRef]
- Zhao, C.; Li, H.; Lin, H.J.; Yang, S.; Lin, J.; Liang, G. Feedback Activation of STAT3 as a Cancer Drug-Resistance Mechanism. Trends Pharmacol. Sci. 2016, 37, 47–61. [Google Scholar] [CrossRef]
- Kandala, P.K.; Srivastava, S.K. Diindolylmethane suppresses ovarian cancer growth and potentiates the effect of cisplatin in tumor mouse model by targeting signal transducer and activator of transcription 3 (STAT3). BMC Med. 2012, 10, 9. [Google Scholar] [CrossRef]
- Hart, S.; Goh, K.C.; Novotny-Diermayr, V.; Hu, C.Y.; Hentze, H.; Tan, Y.C.; Madan, B.; Amalini, C.; Loh, Y.K.; Ong, L.C.; et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia 2011, 25, 1751–1759. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Bristow, R.; Cha, M.S.; Wang, B.G.; Ho, C.-L.; Kurman, R.J.; Wang, T.-L.; Shih, L.M. Characterization of Active Mitogen-Activated Protein Kinase in Ovarian Serous Carcinomas. Clin. Cancer Res. 2004, 10, 6432–6436. [Google Scholar] [CrossRef] [PubMed]
- McGivern, N.; El-Helali, A.; Mullan, P.; McNeish, I.A.; Paul Harkin, D.; Kennedy, R.D.; McCabe, N. Activation of MAPK signalling results in resistance to saracatinib (AZD0530) in ovarian cancer. Oncotarget 2017, 9, 4722. [Google Scholar] [CrossRef] [PubMed]
- Simpkins, F.; Jang, K.; Yoon, H.; Hew, K.E.; Kim, M.; Azzam, D.J.; Sun, J.; Zhao, D.; Ince, T.A.; Liu, W.; et al. Dual Src and MEK Inhibition Decreases Ovarian Cancer Growth and Targets Tumor Initiating Stem-Like Cells. Clin. Cancer Res. 2018, 2, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Hanrahan, A.J.; Schultz, N.; Westfal, M.L.; Sakr, R.A.; Giri, D.D.; Scarperi, S.; Janikariman, M.; Olvera, N.; Stevens, E.V.; She, Q.-B.; et al. Genomic Complexity and AKT Dependence in Serous Ovarian Cancer. Cancer 2012, 2, 56–67. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Mahadevan, D.; Harris, W.B.; Hoff, D.D.V.; Younger, A.E.; Rensvold, D.M.; Shelton, C.F.; Hennessy, J.R.; Ramanathan, R.K. Phase I evaluation of SF1126, a vascular targeted PI3K inhibitor, administered twice weekly IV in patients with refractory solid tumors. J. Clin. Oncol. 2009, 27 (Suppl. S15), 2558. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Yap, T.A.; Fearen, I.; Taylor, A.; Carpenter, C.; Brunetto, A.T.; Beeram, M.; Papadopoulos, K.; Yan, L.; de Bono, J. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST). J. Clin. Oncol. 2009, 27 (Suppl. S15), 3503. [Google Scholar] [CrossRef]
- Shapiro, G.; Kwak, E.; Baselga, J.; Rodon, J.; Scheffold, C.; Laird, A.D.; Bedell, L.C.; Edelman, G. Phase I dose-escalation study of XL147, a PI3K inhibitor administered orally to patients with solid tumors. J. Clin. Oncol. 2009, 27 (Suppl. S15), 3500. [Google Scholar] [CrossRef]
- Kinross, K.M.; Brown, D.V.; Kleinschmidt, M.; Jackson, S.; Christensen, J.; Cullinane, C.; Hicks, R.J.; Johnstone, R.W.; McArthur, G.A. In Vivo Activity of Combined PI3K/mTOR and MEK Inhibition in a Kras G12D;Pten Deletion Mouse Model of Ovarian Cancer. Mol. Cancer Ther. 2011, 10, 1440–1449. [Google Scholar] [CrossRef]
- Giornelli, G.H. Management of relapsed ovarian cancer: A review. SpringerPlus 2016, 5, 1197. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Gong, D.; Han, Z.; Wei, X.; Yan, Y.; Ye, F.; Ding, W.; Wang, J.; Xia, X.; Li, F.; et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett. 2013, 341, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Miklja, Z.; Yadav, V.N.; Cartaxo, R.T.; Siada, R.; Thomas, C.C.; Cummings, J.R.; Mullan, B.; Stallard, S.; Paul, A.; Bruzek, A.K.; et al. Everolimus improves the efficacy of dasatinib in PDGFRα-driven glioma. J. Clin. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Sinai, P.; Kain, S.R. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal. Biochem. 1996, 241, 103–108. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Wen, W.; Marcinkowski, E.; Luyimbazi, D.; Luu, T.; Xing, Q.; Yan, J.; Wang, Y.; Wu, J.; Guo, Y.; Tully, D.; et al. Eribulin Synergistically Increases Anti-Tumor Activity of an mTOR Inhibitor by Inhibiting pAKT/pS6K/pS6 in Triple Negative Breast Cancer. Cells 2019, 8, 1010. [Google Scholar] [CrossRef]



| Ratio Sunitinib: Dasatinib | Fold Reduction (IC50) | Fold Reduction (IC75) | Combination Index (CI) | ||||
|---|---|---|---|---|---|---|---|
| Sunitinib | Dasatinib | Sunitinib | Dasatinib | ED50 | ED75 | ||
| SKOV3 | 1:1 | 3.06 | 0.98 | 5.50 | 5.52 | 1.35 | 0.36 |
| 2:1 | 2.52 | 1.61 | 4.20 | 8.41 | 0.71 | 0.35 | |
| 5:1 | 2.28 | 3.63 | 3.42 | 17.14 | 0.59 | 0.39 | |
| 10:1 | 2.21 | 7.04 | 2.82 | 28.24 | 1.02 | 0.36 | |
| MDAH2774 | 1:1 | 4.96 | 5.01 | 4.21 | 23.16 | 0.40 | 0.28 |
| 2:1 | 4.48 | 9.03 | 3.87 | 42.58 | 0.34 | 0.28 | |
| 5:1 | 8.02 | 15.22 | 2.67 | 73.33 | 0.40 | 0.39 | |
| 10:1 | 2.96 | 29.79 | 2.58 | 141.94 | 0.37 | 0.39 | |
| Cell | Combination | Fold Reduction (IC50) | Fold Reduction (IC75) | CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| S | D | M | S | D | M | ED50 | ED75 | ||
| MDAH2774 | SM (1:5) | 1.58 | NA | 7.79 | 1.05 | NA | 37.32 | 0.76 | 0.97 |
| DM (1:50) | NA | 8.43 | 37.9 | NA | 37.44 | 26.35 | 0.18 | 0.06 | |
| SD (10:1) | 4.21 | 9.56 | NA | 2.19 | 110.49 | NA | 0.34 | 0.46 | |
| SDM (10:1:50) | 6.83 | 15.51 | 33.68 | 3.71 | 186.76 | 131.44 | 0.24 | 0.28 | |
| SKOV3 | SM (1:1) | 5.04 | NA | 3.76 | 6.98 | NA | 26.22 | 0.46 | 0.18 |
| DM (1:10) | 4.62 | 4.40 | NA | NA | 24.56 | 17.68 | 0.44 | 0.10 | |
| SD (10:1) | 3.26 | 2.3 | NA | 2.95 | 15.37 | NA | 0.74 | 0.40 | |
| SDM (10:1:10) | 7.9 | 5.6 | 5.92 | 10.91 | 56.84 | 40.95 | 0.47 | 0.13 | |
| Cell | Combination | Fold Reduction (IC50) | Fold Reduction (IC75) | CI | |||||
|---|---|---|---|---|---|---|---|---|---|
| S | D | E | S | D | E | ED50 | ED75 | ||
| MDAH 2774 | SE (2:1) | 6.33 | NA | 200.1 | 1.04 | NA | 529271 | 0.32 | 0.097 |
| DE (1:5) | NA | 28.7 | 825.9 | NA | 13.01 | 131663 | 0.035 | 0.077 | |
| SD (10:1) | 4.21 | 9.56 | NA | 2.2 | 110.42 | NA | 0.37 | 0.46 | |
| SDE (10:1:5) | 12.8 | 28.7 | 814.8 | 3.2 | 161.12 | 1631020 | 0.11 | 0.32 | |
| SKOV3 | SE (10:1) | 3.51 | NA | 29.2 | 1.58 | NA | 2811 | 0.32 | 0.63 |
| DE (1:1) | NA | 6.2 | 72.3 | NA | 3.51 | 1201 | 0.18 | 0.29 | |
| SD (10:1) | 3.26 | 2.3 | NA | 2.95 | 15.37 | NA | 0.74 | 0.4 | |
| SDE (10:1:1) | 6.72 | 4.76 | 55.9 | 4.78 | 25.15 | 8591 | 0.38 | 0.25 | |
| A2780CR | SDE (40:1:2) | 26.93 | 15.06 | 3.39 | 5.78 | 4.59 | 18.05 | 0.37 | 0.44 |
| OVCAR8 | SDE (1000:10:1) | 5.64 | 4.95 | 6.93 | 2.55 | 2.53 | 26.98 | 0.51 | 0.82 |
| Cells | Combination (Ratio) | Fold Reduction | Combination Index |
|---|---|---|---|
| IC50 (Paclitaxel) | ED50 | ||
| MDAH2774 | P-S (0.01:2) | 1.79 | 0.72 |
| P-D (0.01:0.2) | 2.27 | 0.83 | |
| P-E (0.01:1) | 2.43 | 0.41 | |
| P-SD (0.01:2:0.2) | 2.4 | 0.74 | |
| P-SE (0.01:2:1) | 3.5 | 0.46 | |
| P-DE (0.01:0.2:1) | 6.3 | 0.42 | |
| P-SDE (0.01:2:0.2:1) | 6.5 | 0.51 | |
| SKOV3 | P-S (0.1:10) | 3.01 | 0.39 |
| P-D (0.1:1) | 9.48 | 0.28 | |
| P-E (0.1:1) | 4.15 | 0.29 | |
| P-SD (0.1:10:1) | 6.8 | 0.85 | |
| P-SE (0.1:10:1) | 11.8 | 0.62 | |
| P-DE (0.1:1:1) | 11.1 | 0.13 | |
| P-SDE (0.1:10:1:1) | 69.5 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, W.; Han, E.S.; Dellinger, T.H.; Lu, L.X.; Wu, J.; Jove, R.; Yim, J.H. Synergistic Anti-Tumor Activity by Targeting Multiple Signaling Pathways in Ovarian Cancer. Cancers 2020, 12, 2586. https://doi.org/10.3390/cancers12092586
Wen W, Han ES, Dellinger TH, Lu LX, Wu J, Jove R, Yim JH. Synergistic Anti-Tumor Activity by Targeting Multiple Signaling Pathways in Ovarian Cancer. Cancers. 2020; 12(9):2586. https://doi.org/10.3390/cancers12092586
Chicago/Turabian StyleWen, Wei, Ernest S. Han, Thanh H. Dellinger, Leander X. Lu, Jun Wu, Richard Jove, and John H. Yim. 2020. "Synergistic Anti-Tumor Activity by Targeting Multiple Signaling Pathways in Ovarian Cancer" Cancers 12, no. 9: 2586. https://doi.org/10.3390/cancers12092586
APA StyleWen, W., Han, E. S., Dellinger, T. H., Lu, L. X., Wu, J., Jove, R., & Yim, J. H. (2020). Synergistic Anti-Tumor Activity by Targeting Multiple Signaling Pathways in Ovarian Cancer. Cancers, 12(9), 2586. https://doi.org/10.3390/cancers12092586