Influence of Target Location, Size, and Patient Age on Normal Tissue Sparing- Proton and Photon Therapy in Paediatric Brain Tumour Patient-Specific Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
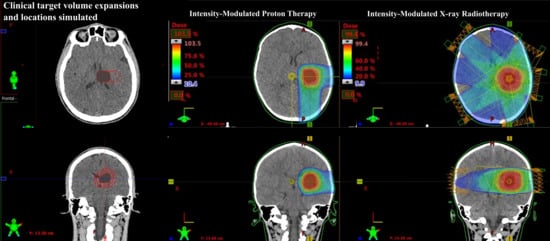
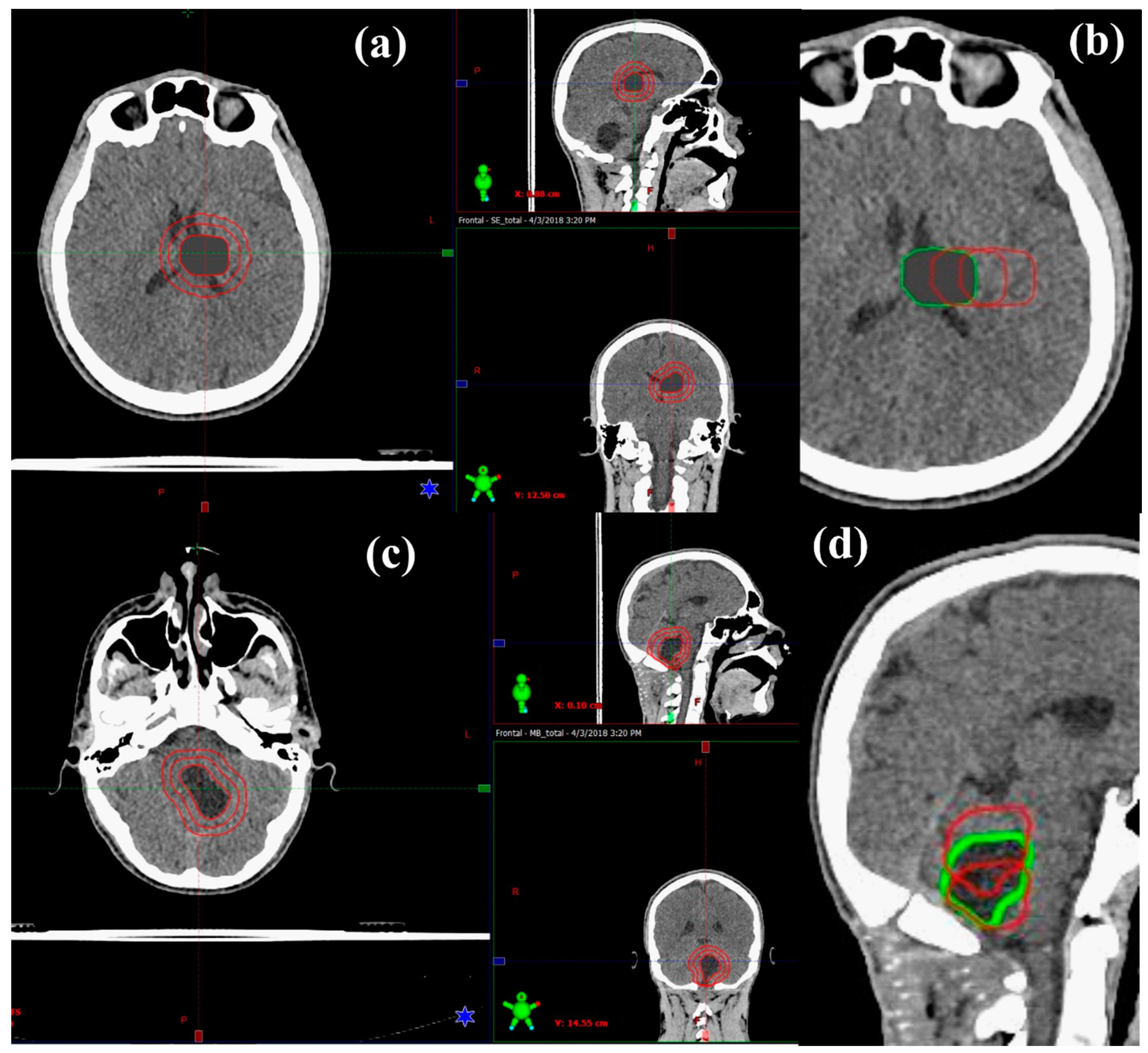
2.1. Patient Data and Target Volume Simulation
2.2. Treatment Planning
2.2.1. Prescribed Dose and Beam Arrangement for Intensity-Modulated Proton Therapy (IMPT)
2.2.2. Prescribed Dose and Beam Arrangement for Intensity-Modulated Photon Therapy (IMRT)
2.3. Dose, Volume, and Statistical Analysis
2.4. Reliability and Validity
3. Results
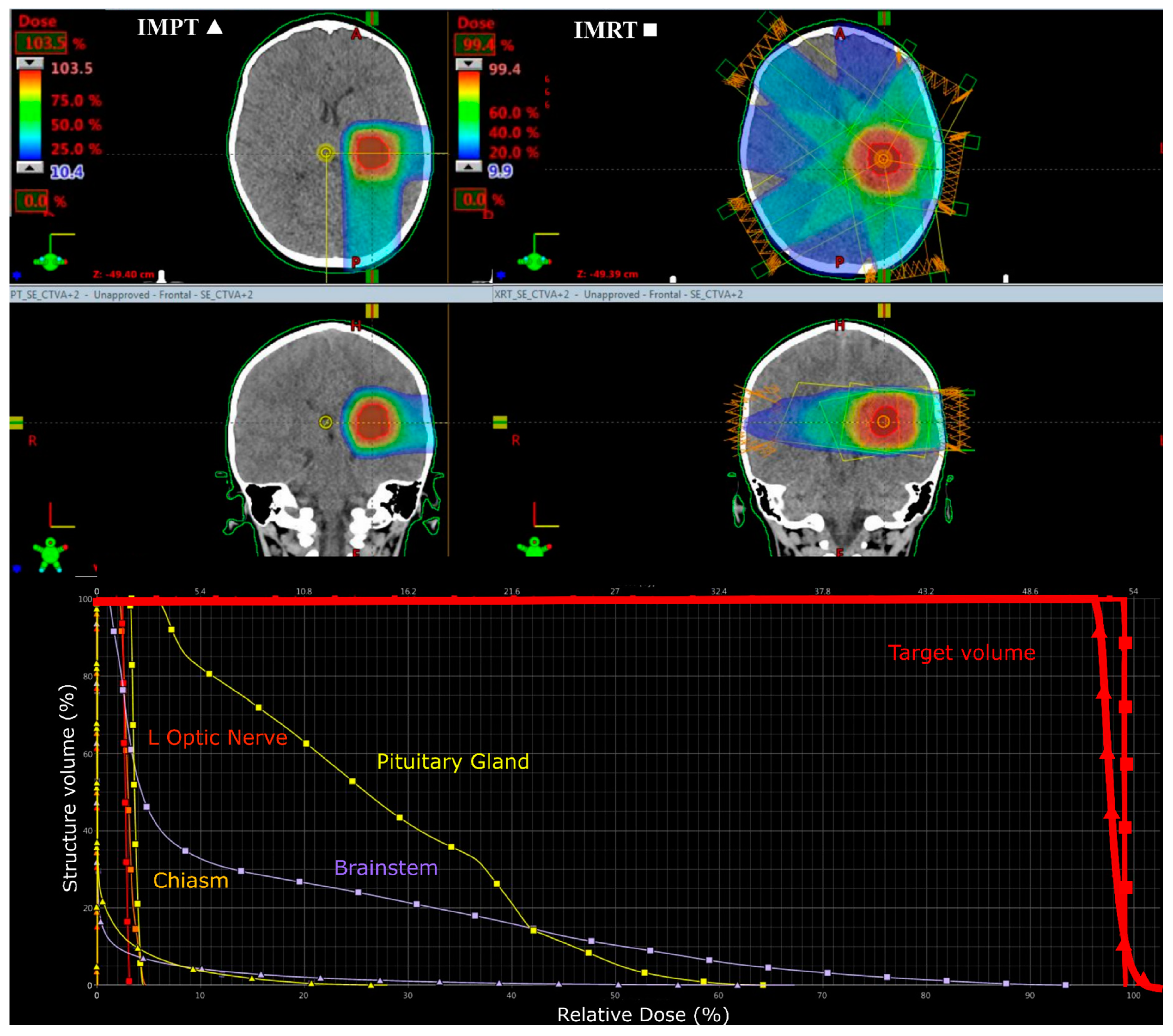
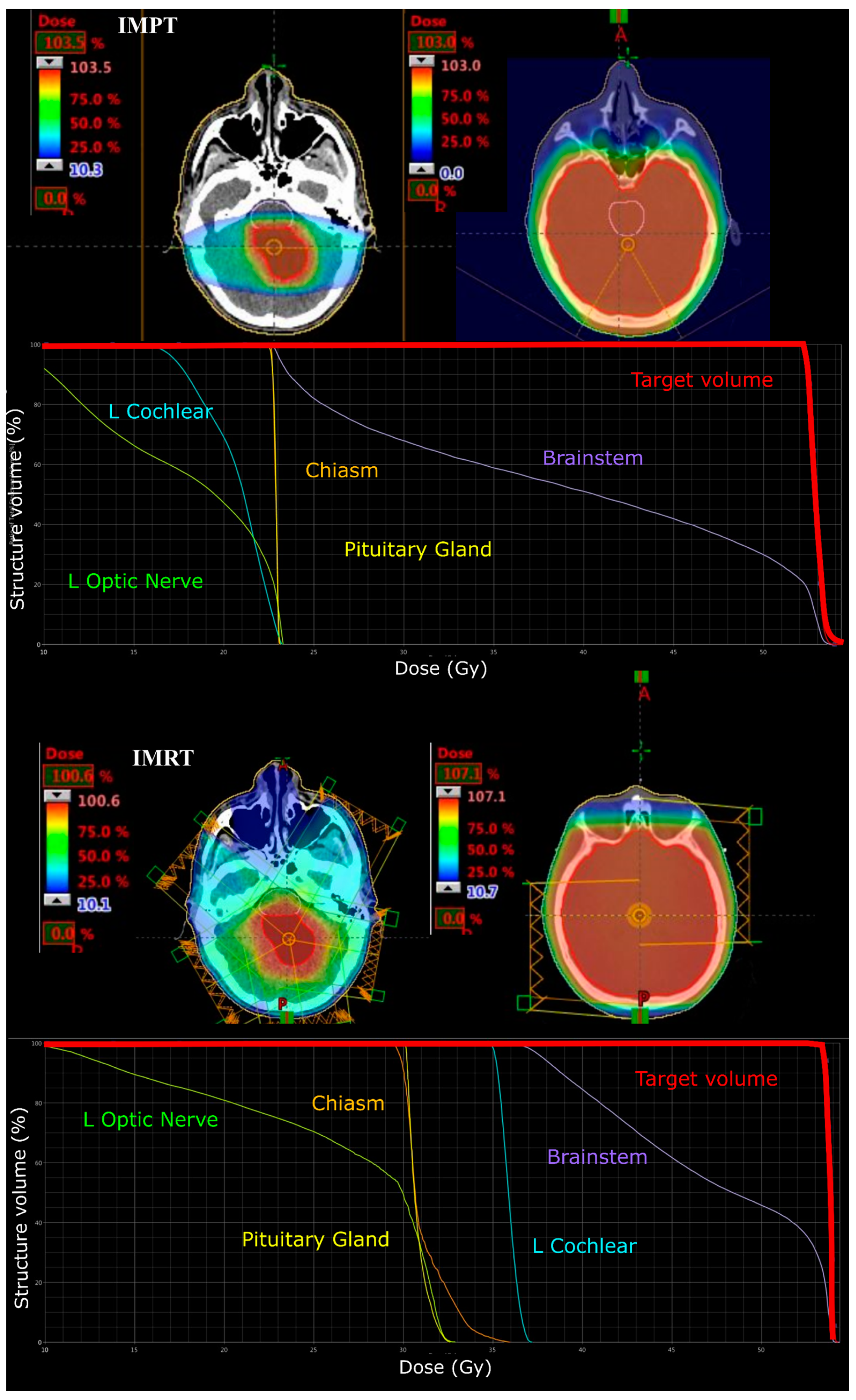
3.1. Medulloblastoma
3.2. Supratentorial Ependymoma
4. Discussion
4.1. Study Significance
4.2. Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Council. Cancer Council and Childhood Cancers. Available online: https://www.cancer.org.au/about-cancer/types-of-cancer/childhood-cancers/cancer-council-and-childhood-cancers.html (accessed on 13 February 2020).
- Mizumoto, M.; Oshiro, Y.; Yamamoto, T.; Kohzuki, H.; Sakurai, H. Proton Beam Therapy for Pediatric Brain Tumor. Neurol. Med. Chir. 2017, 57, 343–355. [Google Scholar] [CrossRef]
- Armoogum, K.; Thorp, N. Dosimetric Comparison and Potential for Improved Clinical Outcomes of Paediatric CNS Patients Treated with Protons or IMRT. Cancers 2015, 7, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; Macdonald, T.; Ebb, D.; Macdonald, S.M.; Tarbell, N.J.; et al. Clinical Outcomes Among Children with Standard-Risk Medulloblastoma Treated with Proton and Photon Radiation Therapy: A Comparison of Disease Control and Overall Survival. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Giaccia, A. Radiobiology for the Radiologist, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Lim, H. Radiation Therapy against Pediatric Malignant Central Nervous System Tumors: Embryonal Tumors and Proton Beam Therapy. J. Korean Neurosurg. Soc. 2018, 61, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Hua, C.-H.; Shukla, H.; Ying, X.; Nill, S.; Oelfke, U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr. Blood Cancer 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Lawrence, Y.R.; Li, X.A.; El Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation Dose–Volume Effects in the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef]
- Bavle, A.; Tewari, S.; Sisson, A.; Chintagumpala, M.; Anderson, M.; Paulino, A. Meta-analysis of the incidence and patterns of second neoplasms after photon craniospinal irradiation in children with medulloblastoma. Pediatr. Blood Cancer 2018, 65, e27095. [Google Scholar] [CrossRef]
- Rombi, B.; Vennarini, S.; Vinante, L.; Ravanelli, D.; Amichetti, M. Proton radiotherapy for pediatric tumors: Review of first clinical results. Ital. J. Pediatr. 2014, 40, 2308. [Google Scholar] [CrossRef]
- Stokkevåg, C.H.; Indelicato, D.J.; Herfarth, K.; Magelssen, H.; Evensen, M.E.; Ugland, M.; Nordberg, T.; Nystad, T.A.; Hægeland, C.; Alsaker, M.D.; et al. Normal tissue complication probability models in plan evaluation of children with brain tumors referred to proton therapy. Acta Oncol. 2019, 58, 1416–1422. [Google Scholar] [CrossRef]
- Huynh, M.; Marcu, L.G.; Giles, E.; Short, M.; Matthews, D.; Bezak, E. Current status of proton therapy outcome for paediatric cancers of the central nervous system—Analysis of the published literature. Cancer Treat. Rev. 2018, 70, 272–288. [Google Scholar] [CrossRef]
- Thomas, H.; Timmermann, B. Paediatric proton therapy. Br. J. Radiol. 2020, 93, 20190601. [Google Scholar] [CrossRef] [PubMed]
- Ludmir, E.B.; Grosshans, D.R.; Woodhouse, K.D. Radiotherapy Advances in Pediatric Neuro-Oncology. Bioengineering 2018, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Sakurai, H.; Mizumoto, M.; Kamizawa, S.; Murayama, S.; Yamashita, H.; Takao, S.; Suzuki, R.; Shirato, H.; Ito, Y.M. Lifetime attributable risk of radiation-induced secondary cancer from proton beam therapy compared with that of intensity-modulated X-ray therapy in randomly sampled pediatric cancer patients. J. Radiat. Res. 2016, 58, 363–371. [Google Scholar] [CrossRef]
- Nystrom, H.; Jensen, M.F.; Nyström, P.W. Treatment planning for proton therapy: What is needed in the next 10 years? Br. J. Radiol. 2020, 93, 20190304. [Google Scholar] [CrossRef] [PubMed]
- Kuhlthau, K.A.; Pulsifer, M.B.; Yeap, B.Y.; Morales, D.R.; Delahaye, J.; Hill, K.S.; Ebb, D.; Abrams, A.N.; Macdonald, S.M.; Tarbell, N.J.; et al. Prospective Study of Health-Related Quality of Life for Children with Brain Tumors Treated with Proton Radiotherapy. J. Clin. Oncol. 2012, 30, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Pulsifer, M.B.; Sethi, R.V.; Kuhlthau, K.A.; Macdonald, S.M.; Tarbell, N.J.; Yock, T.I. Early Cognitive Outcomes Following Proton Radiation in Pediatric Patients with Brain and Central Nervous System Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, D.J.; Flampouri, S.; Rotondo, R.L.; Bradley, J.A.; Morris, C.G.; Aldana, P.R.; Sandler, E.; Mendenhall, N.P. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014, 53, 1298–1304. [Google Scholar] [CrossRef]
- Stokkevåg, C.H.; Engeseth, G.-M.; Ytre-Hauge, K.S.; Rohrich, D.; Odland, O.H.; Muren, L.P.; Brydøy, M.; Hysing, L.B.; Szostak, A.; Palmer, M.B.; et al. Estimated risk of radiation-induced cancer following paediatric cranio-spinal irradiation with electron, photon and proton therapy. Acta Oncol. 2014, 53, 1048–1057. [Google Scholar] [CrossRef]
- Brodin, N.P.; Rosenschold, P.M.A.; Aznar, M.C.; Kiil-Berthelsen, A.; Vogelius, I.; Nilsson, P.; Lannering, B.; Björk-Eriksson, T. Radiobiological risk estimates of adverse events and secondary cancer for proton and photon radiation therapy of pediatric medulloblastoma. Acta Oncol. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Sato, M.; Gunther, J.R.; Mahajan, A.; Jo, E.; Paulino, A.C.; Adesina, A.M.; Jones, J.Y.; Ketonen, L.M.; Su, J.M.; Okcu, M.F.; et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 2017, 123, 2570–2578. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Merchant, T.E.; Bendel, A.E.; Sabin, N.D.; Burger, P.C.; Shaw, D.W.; Chang, E.; Wu, S.; Zhou, T.; Eisenstat, D.D.; Foreman, N.K.; et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J. Clin. Oncol. 2019, 37, 974–983. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Boil. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Vatner, R.E.; Niemierko, A.; Misra, M.; Weyman, E.A.; Goebel, C.P.; Ebb, D.H.; Jones, R.M.; Huang, M.S.; Mahajan, A.; Grosshans, D.R.; et al. Endocrine Deficiency as a Function of Radiation Dose to the Hypothalamus and Pituitary in Pediatric and Young Adult Patients with Brain Tumors. J. Clin. Oncol. 2018, 36, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Rose, S.R.; Bosley, C.; Wu, S.; Xiong, X.; Lustig, R.H. Growth Hormone Secretion after Conformal Radiation Therapy in Pediatric Patients with Localized Brain Tumors. J. Clin. Oncol. 2011, 29, 4776–4780. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.D.; Collins, J.S.; Holden, K.R. United States Head Circumference Growth Reference Charts: Birth to 21 Years. J. Pediatr. 2010, 156, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Peterson, R.; Ris, M.D.; Janzen, L.; Okcu, M.F.; Grosshans, D.R.; Ramaswamy, V.; Paulino, A.C.; Hodgson, D.; Mahajan, A.; et al. Superior Intellectual Outcomes After Proton Radiotherapy Compared with Photon Radiotherapy for Pediatric Medulloblastoma. J. Clin. Oncol. 2020, 38, 454–461. [Google Scholar] [CrossRef]
- Toussaint, L.; Indelicato, D.J.; Stokkevåg, C.H.; Lassen-Ramshad, Y.; Pedro, C.; Mikkelsen, R.; Di Pinto, M.; Li, Z.; Flampouri, S.; Vestergaard, A.; et al. Radiation doses to brain substructures associated with cognition in radiotherapy of pediatric brain tumors. Acta Oncol. 2019, 58, 1457–1462. [Google Scholar] [CrossRef]
- Cochran, D.M.; Yock, T.I.; Adams, J.A.; Tarbell, N.J. Radiation Dose to the Lens during Craniospinal Irradiation—An Improvement in Proton Radiotherapy Technique. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1336–1342. [Google Scholar] [CrossRef]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef]
- Eaton, B.R.; Esiashvili, N.; Kim, S.; Patterson, B.; Weyman, E.A.; Thornton, L.T.; Mazewski, C.; Macdonald, T.J.; Ebb, D.; Macdonald, S.M.; et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2015, 18, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.; Jones, R.M.; Macdonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef]
- Indelicato, D.; Bradley, J.; Rotondo, R.; Nanda, R.; Logie, N.; Sandler, E.; Aldana, P.; Ranalli, N.; Beier, A.; Morris, C.; et al. Outcomes following proton therapy for pediatric ependymoma. Acta Oncol. 2018, 57, 644–648. [Google Scholar] [CrossRef]
- Beltran, C.; Roca, M.; Merchant, T.E. On the Benefits and Risks of Proton Therapy in Pediatric Craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e281–e287. [Google Scholar] [CrossRef]
- Boehling, N.S.; Grosshans, D.R.; Bluett, J.B.; Palmer, M.T.; Song, X.; Amos, R.A.; Sahoo, N.; Meyer, J.J.; Mahajan, A.; Woo, S.Y. Dosimetric Comparison of Three-Dimensional Conformal Proton Radiotherapy, Intensity-Modulated Proton Therapy, and Intensity-Modulated Radiotherapy for Treatment of Pediatric Craniopharyngiomas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, Y.; Lee, S.U.; Kim, T.; Choi, Y.-K.; Kim, J.-Y. Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors. Radiat. Oncol. 2015, 10, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Brodin, N.P.; Rosenschold, P.M.A.; Blomstrand, M.; Kiil-Berthlesen, A.; Hollensen, C.; Vogelius, I.; Lannering, B.; Bentzen, S.M.; Björk-Eriksson, T. Hippocampal sparing radiotherapy for pediatric medulloblastoma: Impact of treatment margins and treatment technique. Neuro Oncol. 2013, 16, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.M.; Safai, S.; Trofimov, A.; Wolfgang, J.; Fullerton, B.; Yeap, B.Y.; Bortfeld, T.; Tarbell, N.J.; Yock, T. Proton Radiotherapy for Childhood Ependymoma: Initial Clinical Outcomes and Dose Comparisons. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 979–986. [Google Scholar] [CrossRef]
- Giantsoudi, D.; Sethi, R.V.; Yeap, B.Y.; Eaton, B.R.; Ebb, D.H.; Caruso, P.A.; Rapalino, O.; Chen, Y.-L.E.; Adams, J.A.; Yock, T.I.; et al. Incidence of CNS Injury for a Cohort of 111 Patients Treated with Proton Therapy for Medulloblastoma: LET and RBE Associations for Areas of Injury. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 287–296. [Google Scholar] [CrossRef]
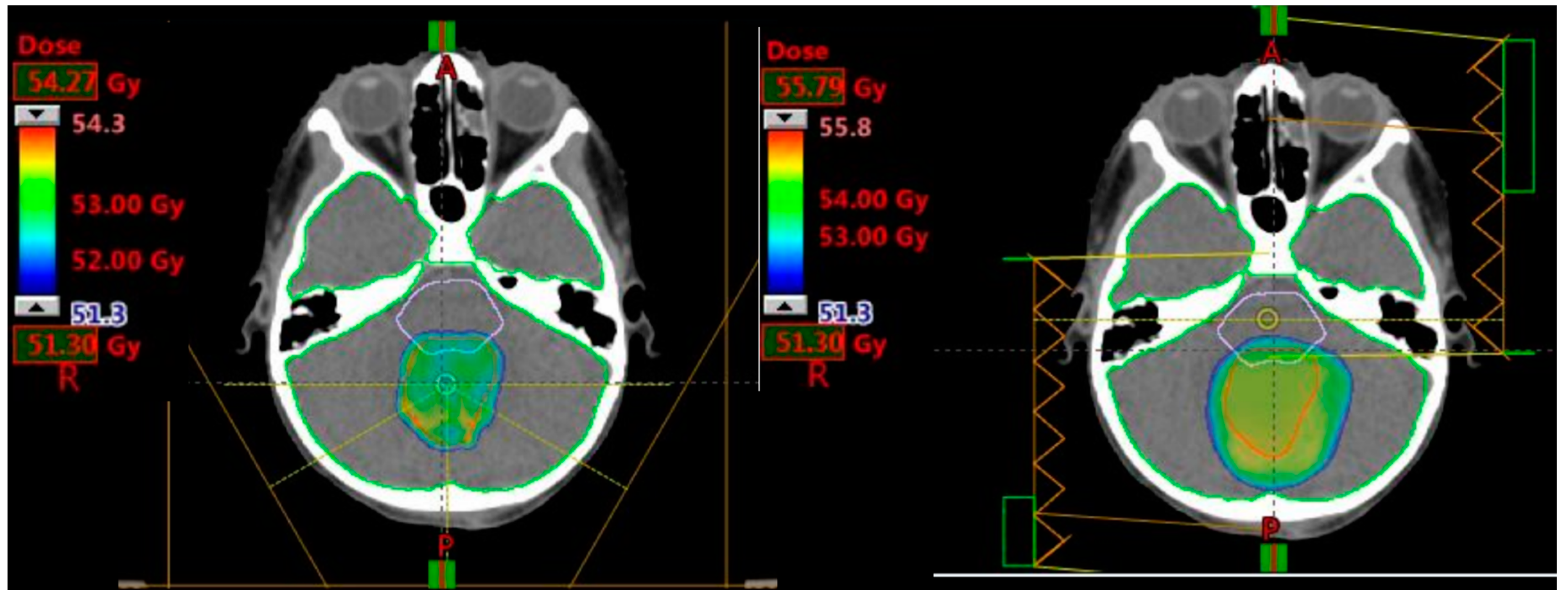

| Tumour Site | CTV Location | CTV Size |
|---|---|---|
| Infratentorial Medulloblastoma | Close to midline | Original post-surgical target |
| −5 mm | ||
| +5 mm | ||
| 1 cm superior shift | Original post-surgical target | |
| −5 mm | ||
| +5 mm | ||
| 1 cm inferior shift | Original post-surgical target | |
| −5 mm | ||
| +5 mm | ||
| Supratentorial Ependymoma | Close to midline | 15 mm diameter post-surgical target |
| +5 mm | ||
| +10 mm | ||
| 1 cm lateral shift | 15 mm diameter post-surgical target | |
| +5 mm | ||
| +10 mm | ||
| 2 cm lateral shift | 15 mm diameter post-surgical target | |
| +5 mm | ||
| +10 mm |
| Region of Interest | Dosimetric Goals |
|---|---|
| CTV | D95 ≥ 95% |
| PTV (IMRT plans) | D90 ≥ 98% |
| Cochlea | D50 < 20 Gy |
| Optic Globes | D10 < 35 Gy |
| D50 < 10 Gy | |
| Optic Chiasm/Nerves | D10 < 56 Gy |
| D50 < 54 Gy | |
| Brainstem | D10 < 52.9 Gy |
| D50 < 52.4 Gy | |
| D0.1 < 53.4 Gy |
| Structure | Dose Parameter (Gy) | 1 cm Diameter | 2 cm Diameter | 3 cm Diameter | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Central | 1 cm LAT | 2 cm LAT | Central | 1 cm LAT | 2 cm LAT | Central | 1 cm LAT | 2 cm LAT | ||
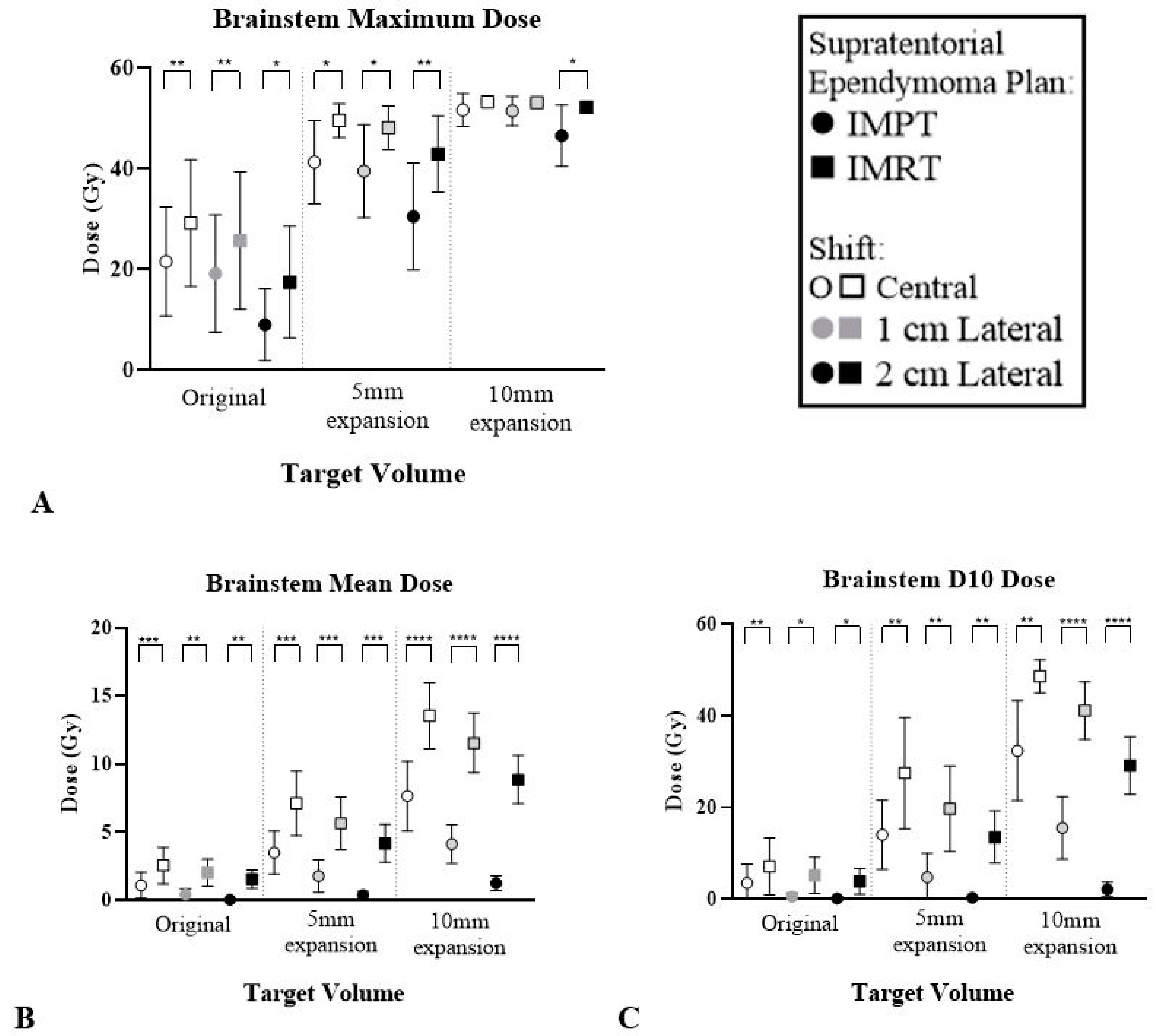
| Brainstem | ∆ Mean | 1.4 (1.0–2.1) *** | 1.5 (1.0–2.7) ** | 1.5 (0.9–2.7) ** | 3.6 (2.1–4.8) *** | 3.8 (2.2–4.9) *** | 3.8 (2.3–5.7) *** | 5.8 (4.1–6.6) **** | 7.4 (6.0–8.9) **** | 7.6 (5.9–9.8) **** |
| ∆ D10 | 3.6 (2.1–7.8) ** | 4.6 (2.4–10.9) * | 3.7 (1.9–9.3) * | 13.4 (4.5–19.1) ** | 14.9 (6–20.4) ** | 13.2 (6.1–22.5) ** | 16.2 (5.3–26.2) ** | 25.5 (23.4–28.5) **** | 27.0 (21.6–34.8) **** | |
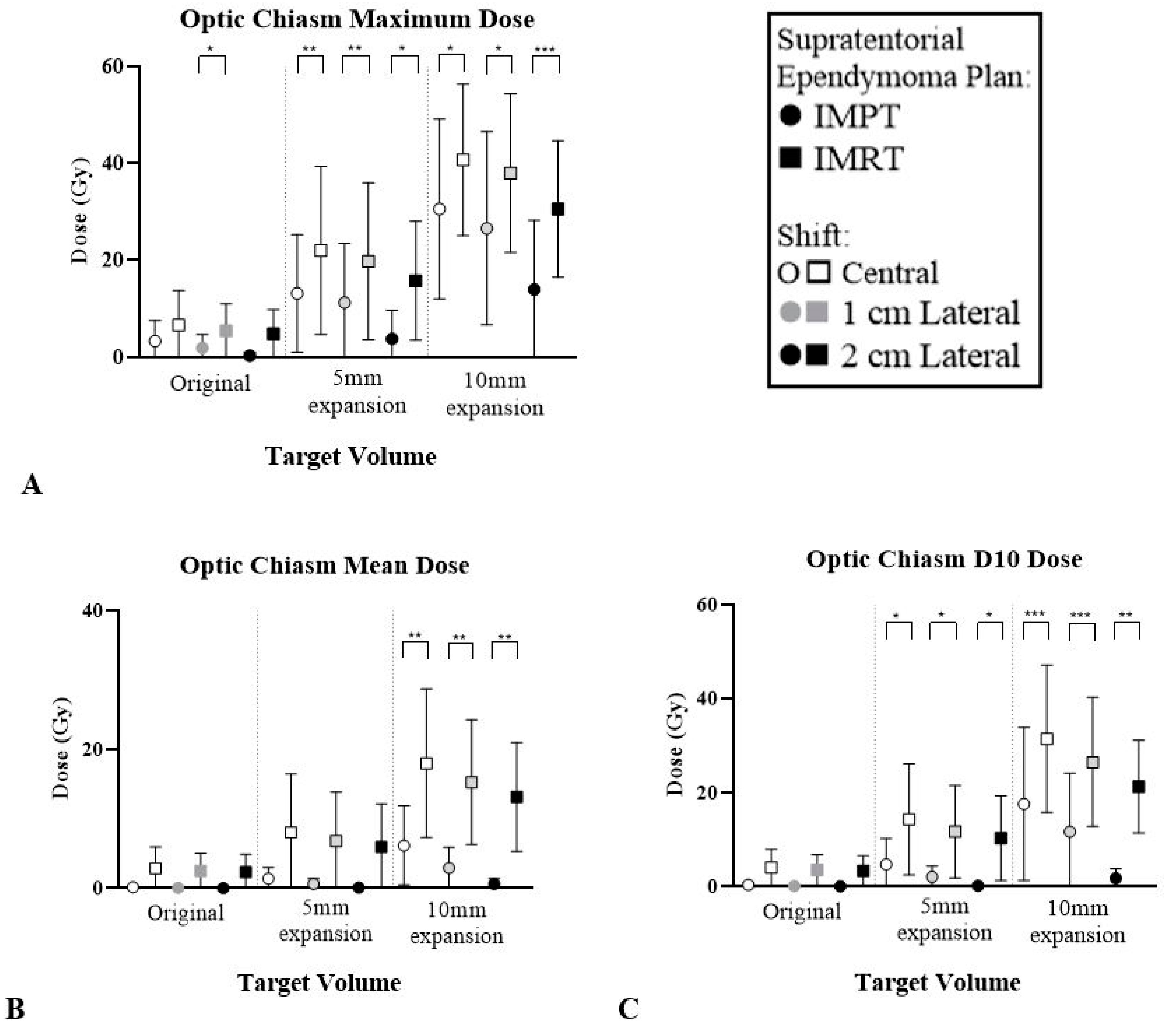
| Optic Chiasm | ∆ Mean | 2.7 (1.2–8.6) | 2.4 (0.9–7.4) | 2.3 (0.8–7.4) | 6.6 (1.9–21.0) | 6.1 (1.7–18.9) | 5.8 (1.6–17.8) | 11.8 (5.6–22.5) ** | 12.3 (4.5–22.6) ** | 12.5 (3.9–24.5) ** |
| ∆ D10 | 3.6 (1.3–10.8) | 3.3 (1.2–9.8) | 3.2 (1.1–9.6) | 9.5 (2.6–22.2) * | 9.6 (2.2–23.7) * | 10.1 (2–25.8) * | 13.9 (9.3–17.5) *** | 14.8 (7.7–18.6) *** | 19.5 (6.2–27.9) ** | |
| Ipsilateral Cochlea | ∆ Mean | 0.5 (0.5–0.6) **** | 0.5 (0.5–0.6) **** | 0.5 (0.5–0.6) **** | 0.9 (0.8–0.9) **** | 0.9 (0.8–1.0) **** | 0.9 (0.8–1.0) **** | 1.3 (1.2–1.5) **** | 1.4 (1.2–1.5) **** | 1.4 (1.2–1.5) **** |
| ∆ D10 | 0.5 (0.5–0.6) **** | 0.5 (0.5–0.6) **** | 0.5 (0.5–0.6) **** | 0.9 (0.9–1.0) **** | 0.9 (0.9–1.1) **** | 0.9 (0.9–1.1) **** | 1.4 (1.3–1.6) **** | 1.5 (1.3–1.7) **** | 1.5 (1.3–1.7) **** | |
| Ipsilateral Eye | ∆ Mean | 0.9 (0.3–3.6) | 0.9 (0.3–3.6) | 1.3 (0.3–5.4) | 2.1 (0.5–9.1) | 1.8 (0.5–6.5) | 1.9 (0.6–6.8) | 3.8 (0.9–15.4) | 3.1 (0.9–10.3) | 2.8 (0.9–7.3) |
| ∆ D10 | 2.1 (0.4–10.0) | 1.5 (0.4–6.5) | 1.8 (0.4–7) | 3.7 (0.8–17) | 2.7 (0.8–8.2) | 2.8 (0.8–7.4) | 5.7 (1.1–21.9) | 5.4 (1.2–19.7) | 3.9 (1.2–8.1) * | |
| Ipsilateral Lens | ∆ Mean | 0.5 (0.3–1.2) * | 0.9 (0.3–3.8) | 1.3 (0.3–6.0) | 1.2 (0.5–4.0) | 1.6 (0.5–6.2) | 1.8 (0.5–6.4) | 3.2 (0.7–13.9) | 2.4 (0.8–7.0) | 2.6 (0.7–6.7) |
| ∆ D10 | 0.5 (0.3–1.5) * | 1.2 (0.3–5.5) | 1.4 (0.3–6.3) | 1.4 (0.5–6.3) | 1.7 (0.5–6.4) | 2.1 (0.5–6.6) | 4.1 (0.8–18.9) | 2.8 (0.8–7.5) | 2.8 (0.8–6.9) | |
| Ipsilateral Optic Nerve | ∆ Mean | 1.1 (0.5–3.7) | 1.5 (0.5–5.9) | 1.6 (0.5–6.7) | 3.1 (0.9–13.5) | 2.2 (0.9–8.4) | 2.2 (0.9–8.2) | 4.9 (1.7–20.7) | 3.5 (1.7–10.7) | 3.1 (1.4–9.6) |
| ∆ D10 | 1.5 (0.7–5.5) | 1.8 (0.7–7.3) | 1.8 (0.7–7.2) | 3.9 (1.3–16.8) | 2.6 (1.3–9.2) | 2.5 (1.2–9) | 5.6 (2.2–21.8) * | 4.5 (2.0–12.2) | 4.1 (1.9–12) | |
| Pituitary Gland | ∆ Mean | 1.0 (0.7–1.6) *** | 0.9 (0.7–1.4) *** | 0.8 (0.6–1.3) *** | 1.9 (1.3–4.3) ** | 1.7 (1.2–3.5) ** | 1.5 (1.0–3.3) ** | 4.2 (2.0–13.3) | 4.1 (1.8–13.7) | 3.7 (1.7–12.4) |
| ∆ D10 | 1.1 (0.8–1.9) ** | 1.0 (0.8–1.7) *** | 0.8 (0.7–1.5) *** | 2.4 (1.4–6.5) * | 2.1 (1.3–5.4) * | 1.9 (1.2–5.2) * | 5.4 (2.3–18.6) | 5.3 (2.1–19) | 4.7 (1.9–17.2) | |
| Structure | Dose Parameter (Gy) | 1 cm Diameter | 0.5 cm Diameter | 1.5 cm Diameter | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Central | 1 cm SUP | 1 cm INF | Central | 1 cm SUP | 1 cm INF | Central | 1 cm SUP | 1 cm INF | ||
| Brainstem | ∆ Mean | 6.6 (4.8–8.2) **** | 9.3 (5.1–12.1) *** | 8.5 (5.3–11.8) *** | 12.1 (9.4–15.6) **** | 12.5 (10.9–14.3) **** | 9.5 (5.6–14.2) *** | 6.4 (5.3–8.6) **** | 6.8 (4.4–8.9) *** | 7.9 (6.3–9.7) **** |
| ∆ D10 | 0.9 (0.3–1.5) ** | 1.5 (0.5–4.3) * | 1.4 (0.4–2.3) ** | 7.9 (3.3–11.8) ** | 10.1 (5.5–15.8) ** | 8.0 (1.9–14.2) ** | 0.5 (−0.7–1.5) | 0.6 (0.1–1.6) * | 1.5 (0.3–3.5) * | |
| Optic Chiasm | ∆ Mean | 7.8 (2.4–10.4) ** | 9.5 (6.9–11.4) **** | 6.0 (1.9–9.8) ** | 6.1 (1.9–8.8) ** | 7.0 (3.8–8.7) *** | 4.1 (1.5–7.7) * | 10.5 (3.8–14.8) ** | 12.6 (9.4–15.1) **** | 8.5 (2.1–13.0) ** |
| ∆ D10 | 9.4 (2.8–12.9) ** | 11.8 (9.0–13.9) **** | 7.5 (2.1–12.7) ** | 7.5 (2.2–12.6) ** | 8.5 (4.9–12.3) *** | 5.2 (1.5–10.7) * | 12.4 (4.9–17.5) *** | 14.7 (13–17.7) **** | 10.2 (2.4–14.1) ** | |
| Ipsilateral Cochlea | ∆ Mean | 12.7 (10.6–15.2) **** | 12.3 (11.0–13.3) **** | 12.6 (12.2–13.3) **** | 13.3 (11.6–14.4) **** | 10.2 (3.8–13.9) ** | 13.0 (11.7–14.3) **** | 12.5 (10.7–15.1) **** | 13.1 (10.6–14.6) **** | 12.7 (11.8–13.7) **** |
| ∆ D10 | 12.1 (8.3–13.9) **** | 12.0 (10.9–13.6) **** | 12.3 (11.1–13.2) **** | 12.8 (11.4–13.7) **** | 10.2 (3.9–13.2) ** | 11.0 (3.1–13.1) ** | 12.1 (9.8–14.1) **** | 12.8 (10.3–14) **** | 12.7 (11–13.7) **** | |
| Ipsilateral Eye | ∆ Mean | 5.2 (2.1–7.3) *** | 5.0 (2.3–7.3) ** | 5.3 (2.0–7.4) *** | 3.9 (2.0–5.8) *** | 3.5 (1.4–5.6) ** | 3.8 (1.9–5.6) *** | 7.6 (2.2–10.8) ** | 7.4 (3.1–10.3) ** | 6.8 (2.1–10.5) ** |
| ∆ D10 | 7.4 (3.0–10.1) *** | 7.4 (3.0–11.5) ** | 7.3 (2.8–9.5) *** | 6.2 (2.8–8.6) *** | 5.7 (2.9–9.3) ** | 6.1 (2.8–8.4) *** | 9.7 (3.0–12.9) ** | 10.0 (3.3–15.4) ** | 8.9 (2.9–12.3) ** | |
| Ipsilateral Lens | ∆ Mean | 3.4 (1.9–5.3) ** | 3.1 (0.6–5.1) ** | 3.5 (2.2–5.0) *** | 2.7 (0.2–4.6) ** | 2.6 (0.0–4.6) ** | 2.6 (0.0–4.5) ** | 5.9 (2.3–8.0) ** | 5.1 (2.6–7.6) ** | 5.2 (2.2–8.7) ** |
| ∆ D10 | 4.4 (2.3–7.1) ** | 3.8 (0.6–6.9) * | 5.0 (2.2–6.9) *** | 3.3 (–0.1–6.4) * | 3.3 (–0.4–6.3) * | 3.3 (–0.3–6.2) * | 7.2 (2.4–10.4) ** | 6.3 (2.6–9.7) ** | 6.6 (2.3–11.1) ** | |
| Ipsilateral Optic Nerve | ∆ Mean | 6.9 (0.0–9.3) ** | 7.6 (0.9–10.6) ** | 6.1 (0.0–9.6) ** | 4.2 (–0.1–6.1) ** | 4.0 (0.0–5.9) ** | 3.5 (–0.2–5.3) ** | 10.5 (0.3–13.6) ** | 11.5 (3.4–14.9) ** | 8.5 (0.0–13.3) ** |
| ∆ D10 | 8.2 (2.2–11.3) ** | 9.6 (4.2–11.6) *** | 7.1 (2.0–22.3) ** | 5.4 (2.0–8.0) ** | 5.4 (2.1–7.0) *** | 3.9 (1.6–7.5) ** | 11.2 (2.5–14.6) ** | 12.3 (7–14.9) *** | 9.5 (2–14.4) ** | |
| Pituitary Gland | ∆ Mean | 8.4 (5.2–11.2) *** | 9.2 (7.9–11.2) **** | 6.9 (1.9–11.6) ** | 6.5 (2.9–8.8) *** | 7.0 (6.7–7.2) **** | 5.2 (1.5–7.7) ** | 11.7 (7.4–14.8) *** | 13.1 (11.1–15.4) **** | 9.4 (3.2–13.1) ** |
| ∆ D10 | 9.3 (6.0–12.2) *** | 10.6 (9.0–12.8) **** | 7.7 (2.0–12.7) ** | 7.1 (3.8–11.3) *** | 7.1 (6.9–7.6) **** | 5.4 (1.5–8.9) ** | 12.7 (8.7–15.2) **** | 14.2 (12.4–17.2) **** | 10.0 (4.0–13.2) ** | |
| Structure | Dose Parameter (Gy) | Infratentorial Medulloblastoma (p Value) | Supratentorial Ependymoma (p Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Shift | Age (years) | Shift | ||||||
| 5 vs. 9 | 9 vs. 13 | 5 vs. 13 | 5 vs. 9 | 9 vs. 13 | 5 vs. 13 | ||||
| Brainstem | ∆ Mean | − | − | − | − | − | − | − | − |
| ∆ D10 | − | − | − | − | − | − | − | − | |
| Optic Chiasm | ∆ Mean | − | − | − | 0.0197 * | − | 0.0009 | 0.0006 | − |
| ∆ D10 | − | − | − | 0.0231 * | − | − | 0.0245 | − | |
| Ipsilateral Cochlea | ∆ Mean | − | − | − | − | − | − | − | − |
| ∆ D10 | − | − | − | − | − | − | − | − | |
| Ipsilateral Eye | ∆ Mean | − | 0.0020 | 0.0172 | − | 0.0293 | 0.0164 | − | − |
| ∆ D10 | − | 0.0002 | 0.0268 | − | 0.0257 | 0.0498 | − | − | |
| Ipsilateral Lens | ∆ Mean | − | 0.0197 | 0.0063 | − | − | 0.0019 | 0.0195 | − |
| ∆ D10 | − | 0.0095 | 0.0042 | − | − | 0.0109 | − | − | |
| Ipsilateral Optic Nerve | ∆ Mean | − | 0.0034 | − | − | − | 0.0264 | 0.0367 | − |
| ∆ D10 | − | − | − | − | − | 0.0264 | 0.0400 | − | |
| Pituitary Gland | ∆ Mean | − | − | − | − | 0.0010 | 0.0008 | 0.0009 | − |
| ∆ D10 | − | − | − | − | − | 0.0011 | 0.0009 | − | |
| Age and Gender | Circumference—Current Study | Circumference—Referenced Study |
|---|---|---|
| 5 Female | 47.7 cm | 50.5 cm |
| 5 Male | 49.0 cm | 51.5 cm |
| 9 Female | 51.5 cm | 52.0 cm |
| 9 Male | 52.0 cm | 53.0 cm |
| 13 Female | 53.5 cm | 53.5 cm |
| 13 Male | 51.0 cm | 54.5 cm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Oro, M.; Short, M.; Wilson, P.; Hua, C.-H.; Gargone, M.; Merchant, T.E.; Bezak, E. Influence of Target Location, Size, and Patient Age on Normal Tissue Sparing- Proton and Photon Therapy in Paediatric Brain Tumour Patient-Specific Approach. Cancers 2020, 12, 2578. https://doi.org/10.3390/cancers12092578
Dell’Oro M, Short M, Wilson P, Hua C-H, Gargone M, Merchant TE, Bezak E. Influence of Target Location, Size, and Patient Age on Normal Tissue Sparing- Proton and Photon Therapy in Paediatric Brain Tumour Patient-Specific Approach. Cancers. 2020; 12(9):2578. https://doi.org/10.3390/cancers12092578
Chicago/Turabian StyleDell’Oro, Mikaela, Michala Short, Puthenparampil Wilson, Chia-Ho Hua, Melissa Gargone, Thomas E. Merchant, and Eva Bezak. 2020. "Influence of Target Location, Size, and Patient Age on Normal Tissue Sparing- Proton and Photon Therapy in Paediatric Brain Tumour Patient-Specific Approach" Cancers 12, no. 9: 2578. https://doi.org/10.3390/cancers12092578
APA StyleDell’Oro, M., Short, M., Wilson, P., Hua, C.-H., Gargone, M., Merchant, T. E., & Bezak, E. (2020). Influence of Target Location, Size, and Patient Age on Normal Tissue Sparing- Proton and Photon Therapy in Paediatric Brain Tumour Patient-Specific Approach. Cancers, 12(9), 2578. https://doi.org/10.3390/cancers12092578