A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future?
Simple Summary
Abstract
1. Introduction
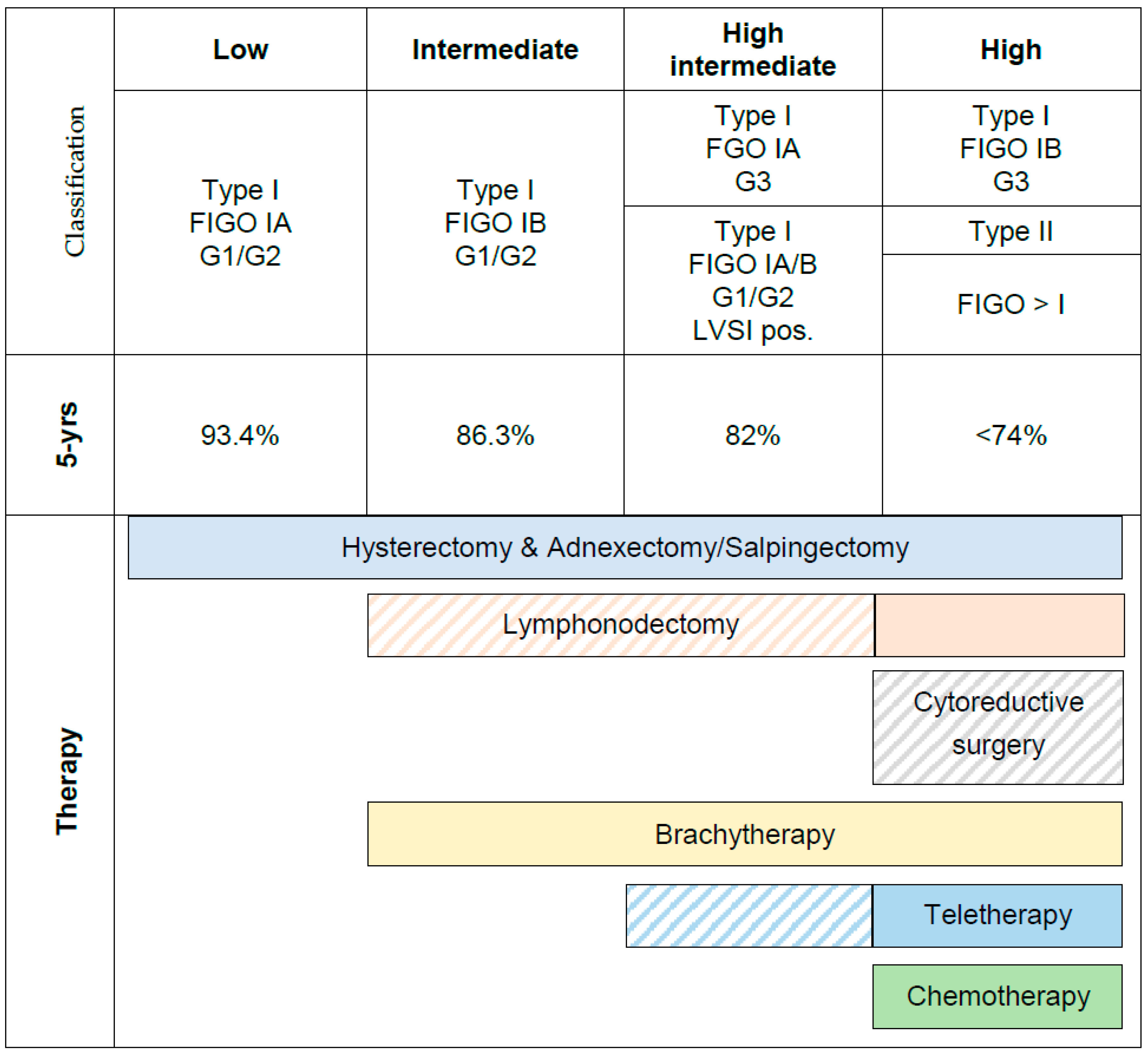
1.1. Current Risk Stratification
1.2. Limitations of Clinical Risk Factors
1.3. Current Recommendations for Adjuvant Treatment in Low-Risk Disease and Intermediate-Risk Parameters
1.3.1. Radiation Needed in Stage I?
1.3.2. Radiation, Chemotherapy or Both: Who Could Profit?
1.3.3. The Need for Additional Differentiation of EC to Predict Adjuvant Therapy
1.4. Response Predicition for Chemotherapy and Radiation
1.5. New Study Landscape
1.6. New Guidelines for Adjuvant Treatment
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Salerno, M.G.; Di Donato, V.; Marchetti, C.; Perniola, G.; Palagiano, A.; Perutelli, A.; Maneschi, F.; Lissoni, A.A.; et al. Secondary analyses from a randomized clinical trial: Age as the key prognostic factor in endometrial carcinoma. Am. J. Obstet. Gynecol. 2014, 210, 363.e1–363.e10. [Google Scholar] [CrossRef]
- Ureyen, I.; Karalok, A.; Turkmen, O.; Kimyon, G.; Akdas, Y.R.; Akyol, A.; Tasci, T.; Turan, T. Factors predicting recurrence in patients with stage IA endometrioid endometrial cancer: What is the importance of LVSI? Arch. Gynecol. Obstet. 2019, 301, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer 2016, 26. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Haddock, M.G.; Calori, G.; Podratz, K.C. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am. J. Obstet. Gynecol. 2000, 182, 1506–1519. [Google Scholar] [CrossRef]
- Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Creasman, W.T.; Hornback, N.B.; Kurman, R.; Thigpen, J.T. Doxorubicin as an Adjuvant Following Surgery and Radiation-Therapy in Patients with High-Risk Endometrial Carcinoma, Stage-I and Occult Stage-Ii: A Gynecologic Oncology Group-Study. Gynecol. Oncol. 1990, 36, 166–171. [Google Scholar] [CrossRef]
- Mariani, A.; Webb, M.J.; Keeney, G.L.; Aletti, G.; Podratz, K.C. Assessment of prognostic factors in stage IIIA endometrial cancer. Gynecol. Oncol. 2002, 86, 38–44. [Google Scholar] [CrossRef]
- Giglio, A.; Miller, B.; Curcio, E.; Kuo, Y.H.; Erler, B.; Bosscher, J.; Hicks, V.; ElSahwi, K. Challenges to Intraoperative Evaluation of Endometrial Cancer. J. Soc. Laparosc. Robot. Surg. 2020, 24. [Google Scholar] [CrossRef]
- de Boer, S.M.; Wortman, B.G.; Bosse, T.; Powell, M.E.; Singh, N.; Hollema, H.; Wilson, G.; Chowdhury, M.N.; Mileshkin, L.; Pyman, J.; et al. Clinical consequences of upfront pathology review in the randomised PORTEC-3 trial for high-risk endometrial cancer. Ann. Oncol. 2017. [Google Scholar] [CrossRef]
- Kumar, S.; Medeiros, F.; Dowdy, S.C.; Keeney, G.L.; Bakkum-Gamez, J.N.; Podratz, K.C.; Cliby, W.A.; Mariani, A. A prospective assessment of the reliability of frozen section to direct intraoperative decision making in endometrial cancer. Gynecol. Oncol. 2012, 127, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Grevenkamp, F.; Kommoss, F.; Kommoss, F.; Lax, S.; Fend, F.; Wallwiener, D.; Schonfisch, B.; Kramer, B.; Brucker, S.Y.; Taran, F.A.; et al. Second Opinion Expert Pathology in Endometrial Cancer: Potential Clinical Implications. Int. J. Gynecol. Cancer 2017, 27, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic Pelvic Lymphadenectomy vs No Lymphadenectomy in Early-Stage Endometrial Carcinoma: Randomized Clinical Trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- Naumann, R.W. The role of lymphadenectomy in endometrial cancer: Was the ASTEC trial doomed by design and are we destined to repeat that mistake? Gynecol. Oncol. 2012, 126, 5–11. [Google Scholar] [CrossRef]
- Creasman, W.T.; Mutch, D.E.; Herzog, T.J. ASTEC lymphadenectomy and radiation therapy studies: Are conclusions valid? Gynecol. Oncol. 2010, 116, 293–294. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Plante, M.; Stanleigh, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Gregoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef]
- Ignatov, A.; Lebius, C.; Ignatov, T.; Ivros, S.; Knueppel, R.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Lymph node micrometastases and outcome of endometrial cancer. Gynecol. Oncol. 2019, 154, 475–479. [Google Scholar] [CrossRef]
- Piedimonte, S.; Richer, L.; Souhami, L.; Arseneau, J.; Fu, L.; Gilbert, L.; Alfieri, J.; Jardon, K.; Zeng, X.Z. Clinical significance of isolated tumor cells and micrometastasis in low-grade, stage I endometrial cancer. J. Surg. Oncol. 2018, 118, 1194–1198. [Google Scholar] [CrossRef]
- Gomez-Hidalgo, N.R.; Ramirez, P.T.; Ngo, B.; Perez-Hoyos, S.; Coreas, N.; Sanchez-Iglesias, J.L.; Cabrera, S.; Franco, S.; Benavente, A.P.; Gil-Moreno, A. Oncologic impact of micrometastases or isolated tumor cells in sentinel lymph nodes of patients with endometrial cancer: A meta-analysis. Clin. Transl. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Kato, H.; Okamoto, K.; Minobe, S.; Yamashiro, K.; Sakuragi, N. Isolated tumor cells and micrometastases in regional lymph nodes in stage I to II endometrial cancer. J. Gynecol. Oncol. 2016, 27, e1. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Ballester, M.; Bendifallah, S.; Darai, E. European guidelines (ESMO-ESGO-ESTRO consensus conference) for the management of endometrial cancer. Bull. Cancer 2017, 104, 1032–1038. [Google Scholar] [CrossRef]
- Kapucuoglu, N.; Bulbul, D.; Tulunay, G.; Temel, M.A. Reproducibility of grading systems for endometrial endometrioid carcinoma and their relation with pathologic prognostic parameters. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2008, 18, 790–796. [Google Scholar] [CrossRef]
- Clarke, B.A.; Gilks, C.B. Endometrial carcinoma: Controversies in histopathological assessment of grade and tumour cell type. J. Clin. Pathol. 2010, 63, 410–415. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor inter-observer reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 2012, 91, 248A. [Google Scholar]
- Guan, H.; Semaan, A.; Bandyopadhyay, S.; Arabi, H.; Feng, J.; Fathallah, L.; Pansare, V.; Qazi, A.; Abdul-Karim, F.; Morris, R.T.; et al. Prognosis and reproducibility of new and existing binary grading systems for endometrial carcinoma compared to FIGO grading in hysterectomy specimens. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 654–660. [Google Scholar] [CrossRef]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Kobel, M. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jurgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef]
- Zeimet, A.G.; Reimer, D.; Huszar, M.; Winterhoff, B.; Puistola, U.; Azim, S.A.; Muller-Holzner, E.; Ben-Arie, A.; van Kempen, L.C.; Petru, E.; et al. L1CAM in early-stage type I endometrial cancer: Results of a large multicenter evaluation. J. Natl. Cancer Inst. 2013, 105, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in human cancer. Int. J. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; Stelloo, E.; Dreef, E.; Nijman, H.W.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Creutzberg, C.L.; Smit, V.T. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: Pooled PORTEC trial results. Eur. J. Cancer 2014, 50, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, T.H.; Smith, D.D.; Ouyang, C.; Warden, C.D.; Williams, J.C.; Han, E.S. L1CAM is an independent predictor of poor survival in endometrial cancer—An analysis of The Cancer Genome Atlas (TCGA). Gynecol. Oncol. 2016, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, L.J.; Visser, N.C.; van de Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef]
- Kommoss, F.; Kommoss, F.; Grevenkamp, F.; Bunz, A.K.; Taran, F.A.; Fend, F.; Brucker, S.Y.; Wallwiener, D.; Schonfisch, B.; Greif, K.; et al. L1CAM: Amending the “low-risk” category in endometrial carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 255–262. [Google Scholar] [CrossRef]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Leung, S.; Magrill, J.; McConechy, M.K.; Yang, W.; Chow, C.; Kobel, M.; Lee, C.H.; Huntsman, D.G.; Talhouk, A.; et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J. Pathol. Clin. Res. 2017, 3, 279–293. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Stelloo, E.; Nout, R.A.; Nijman, H.W.; Edmondson, R.J.; Church, D.N.; MacKay, H.J.; Leary, A.; Powell, M.E.; Mileshkin, L.; et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod. Pathol. 2016, 29, 174–181. [Google Scholar] [CrossRef]
- Kommoss, F.K.; Karnezis, A.N.; Kommoss, F.; Talhouk, A.; Taran, F.A.; Staebler, A.; Gilks, C.B.; Huntsman, D.G.; Kramer, B.; Brucker, S.Y.; et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer 2018, 119, 480–486. [Google Scholar] [CrossRef]
- Aalders, J.; Abeler, V.; Kolstad, P.; Onsrud, M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet. Gynecol. 1980, 56, 419–427. [Google Scholar]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef]
- Kong, A.; Johnson, N.; Kitchener, H.C.; Lawrie, T.A. Adjuvant Radiotherapy for Stage I Endometrial Cancer: An Updated Cochrane Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2012, 104, 1625–1634. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van de Steen-Banasik, E.; et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000, 355, 1404–1411. [Google Scholar] [CrossRef]
- Maggi, R.; Lissoni, A.; Spina, F.; Melpignano, M.; Zola, P.; Favalli, G.; Colombo, A.; Fossati, R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br. J. Cancer 2006, 95, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Susumu, N.; Sagae, S.; Udagawa, Y.; Niwa, K.; Kuramoto, H.; Satoh, S.; Kudo, R.; Japanese Gynecologic Oncology, G. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 108, 226–233. [Google Scholar] [CrossRef]
- Randall, M.E.; Spirtos, N.M.; Dvoretsky, P. Whole abdominal radiotherapy versus combination chemotherapy with doxorubicin and cisplatin in advanced endometrial carcinoma (phase III): Gynecologic Oncology Group Study No. 122. J. Natl. Cancer Inst. Monogr. 1995, 19, 13–15. [Google Scholar]
- Randall, M.E.; Filiaci, V.L.; Muss, H.; Spirtos, N.M.; Mannel, R.S.; Fowler, J.; Thigpen, J.T.; Benda, J.A.; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2006, 24, 36–44. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016, 17, 1114–1126. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.L.; Randall, M.; Steinhoff, M.; DiSilvestro, P.; Moxley, K.M.; Kim, B.; Powell, M.A.; O’Malley, D.M.; Spirtos, N.M.; et al. A randomized phase III trial of cisplatin and tumor volume directed irradiation followed by carboplatin and paclitaxel vs. carboplatin and paclitaxel for optimally debulked, advanced endometrial carcinoma. J. Clin. Oncol. 2017, 35, 5505. [Google Scholar] [CrossRef]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef]
- Reijnen, C.; Kusters-Vandevelde, H.V.N.; Prinsen, C.F.; Massuger, L.F.; Snijders, M.P.; Kommoss, S.; Brucker, S.Y.; Kwon, J.S.; McAlpine, J.N.; Pijnenborg, J.M.A. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol. Oncol. 2019, 154, 124–130. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Rayner, E.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Tomlinson, I.P.M.; Church, D.N.; Smit, V.; de Wind, N.; Bosse, T.; et al. Adjuvant Treatment for POLE Proofreading Domain-Mutant Cancers: Sensitivity to Radiotherapy, Chemotherapy, and Nucleoside Analogues. Clin. Cancer Res. 2018, 24, 3197–3203. [Google Scholar] [CrossRef]
- Mohammadi, H.; Prince, A.; Figura, N.B.; Peacock, J.S.; Fernandez, D.C.; Montejo, M.E.; Chon, H.S.; Wenham, R.M.; Eschrich, S.A.; Torres-Roca, J.F.; et al. Using the Radiosensitivity Index (RSI) to Predict Pelvic Failure in Endometrial Cancer Treated With Adjuvant Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 496–502. [Google Scholar] [CrossRef]
- Wortman, B.G.; Bosse, T.; Nout, R.A.; Lutgens, L.; van der Steen-Banasik, E.M.; Westerveld, H.; van den Berg, H.; Slot, A.; De Winter, K.A.J.; Verhoeven-Adema, K.W.; et al. Molecular-integrated risk profile to determine adjuvant radiotherapy in endometrial cancer: Evaluation of the pilot phase of the PORTEC-4a trial. Gynecol. Oncol. 2018, 151, 69–75. [Google Scholar] [CrossRef]
- Krämer, P.; Talhouk, A.; Brett, M.A.; Chiu, D.S.; Cairns, E.S.; Scheunhage, D.A.; Hammond, R.F.; Farnell, D.; Nazeran, T.M.; Grube, M.; et al. Endometrial Cancer Molecular Risk Stratification is Equally Prognostic for Endometrioid Ovarian Carcinoma. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
| Risk Group | Molecular Classification Un-Known | Molecular Classification Known |
|---|---|---|
| Low |
|
|
| Intermediate |
|
|
| High-Intermediate |
|
|
| High |
|
|
| Advanced Metastatic |
|
|
| New Risk Groups According to Figure 1 | Future Adjuvant Treatment Recommendations (Suggestions) | ||
|---|---|---|---|
| Brachytherapy | External Beam Radiation | Chemotherapy | |
| Low Risk | no | no | no |
| Intermediate Risk | yes | no | no |
| High-Intermediate Risk pN0 (surgical staging or SLN neg.) | yes | If substantial LVSI | no |
| High-Intermediate Risk cN0/pNX (no surgical staging nor SLN) | yes | If substantial LVSI | Especially for NSMP grade 3 (see PORTEC3 protocol) |
| High Risk p53+ regardless of stage; stage IIIC1 NSMP/MMRd, no residual tumor | no | Concurrent chemoradiation +chemo (see PORTEC3 protocol) In case of contra-indications against chemo: EBRT recommended | |
| High Risk stage III C2 | no | Extended field radiation: Concurrent chemoradiation + chemo (see PORTEC3 protocol) In case of contra-indications against chemo: EBRT recommended | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marnitz, S.; Walter, T.; Schömig-Markiefka, B.; Engler, T.; Kommoss, S.; Brucker, S.Y. A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future? Cancers 2020, 12, 2577. https://doi.org/10.3390/cancers12092577
Marnitz S, Walter T, Schömig-Markiefka B, Engler T, Kommoss S, Brucker SY. A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future? Cancers. 2020; 12(9):2577. https://doi.org/10.3390/cancers12092577
Chicago/Turabian StyleMarnitz, Simone, Till Walter, Birgid Schömig-Markiefka, Tobias Engler, Stefan Kommoss, and Sara Yvonne Brucker. 2020. "A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future?" Cancers 12, no. 9: 2577. https://doi.org/10.3390/cancers12092577
APA StyleMarnitz, S., Walter, T., Schömig-Markiefka, B., Engler, T., Kommoss, S., & Brucker, S. Y. (2020). A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future? Cancers, 12(9), 2577. https://doi.org/10.3390/cancers12092577





