Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Study Cohort
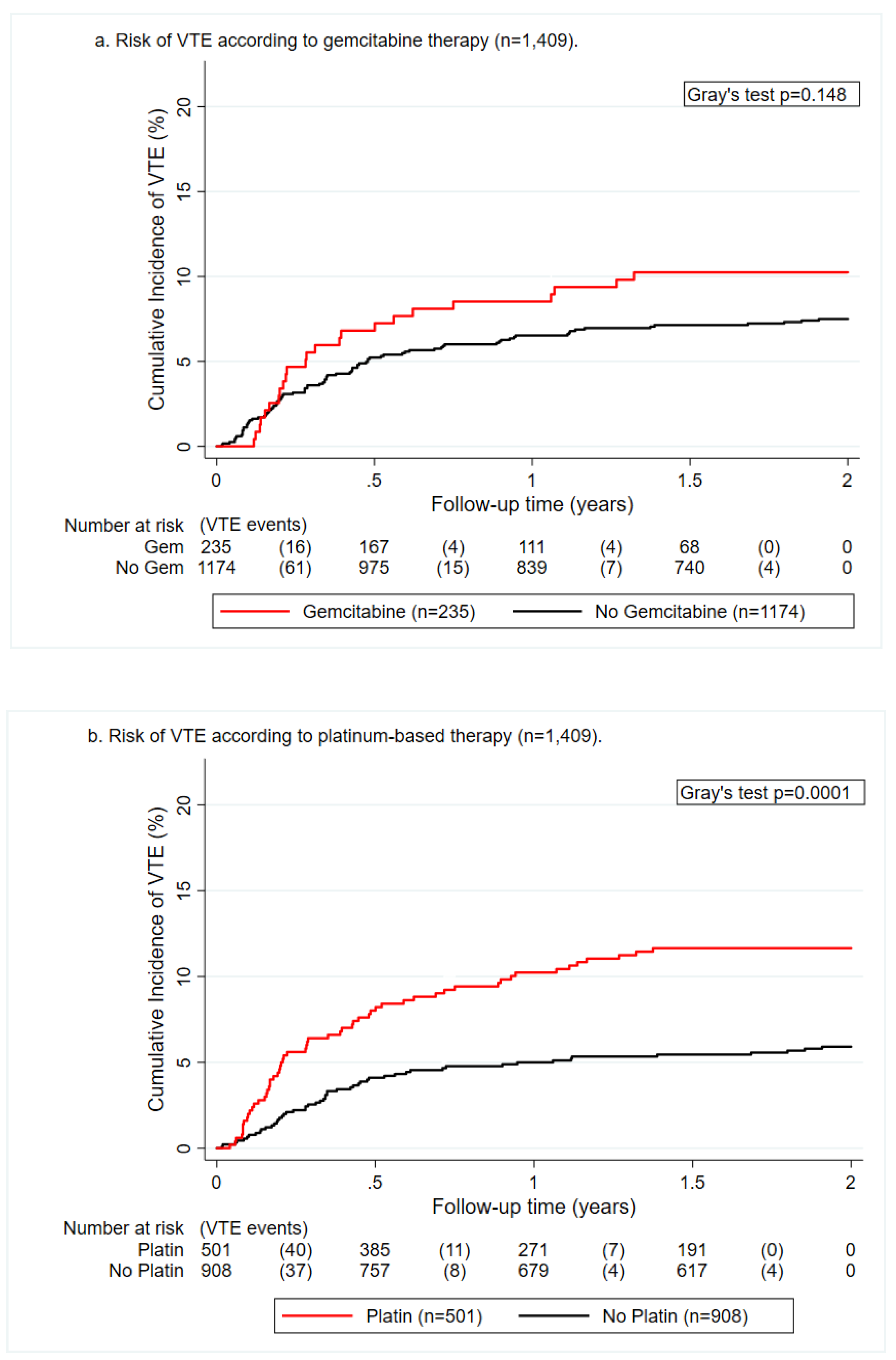
2.2. Crude VTE Incidence According to Chemotherapeutic Agent
2.3. Prediction of Cancer-Associated Thrombosis Beyond the CATS Score
2.4. Sensitivity Analysis
3. Discussion
4. Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Seng, S.; Liu, Z.; Chiu, S.K.; Proverbs-Singh, T.; Sonpavde, G.; Choueiri, T.K.; Tsao, C.-K.; Yu, M.; Hahn, N.M.; Oh, W.K.; et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: A systematic review and meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 4416–4426. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.A.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-X.; Lin, F.; Sun, Y.-J.; Tang, L.-N.; Shen, Z.; Yao, Y. Risk of venous and arterial thromboembolic events in cancer patients treated with gemcitabine: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2013, 76, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Agnelli, G.; Barni, S.; Gasparini, G.; Labianca, R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern. Emerg. Med. 2012, 7, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, M.; Van Es, N.; Rotunno, L.; Anzoletti, N.; Falcone, L.; De Tursi, M.; Natoli, C.; Tinari, N.; Cavallo, I.; Valeriani, E.; et al. Long-term performance of risk scores for venous thromboembolism in ambulatory cancer patients. J. Thromb. Thrombolysis 2019, 48, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Van Es, N.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Otten, H.-M.; Mahé, I.; Wilts, I.T.; Twint, D.C.; Porreca, E.; Arrieta, O.; et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: A prospective cohort study. Haematologica 2017, 102, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, I.; Van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.-M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef]
- Ahlbrecht, J.; Dickmann, B.; Ay, C.; Dunkler, D.; Thaler, J.; Schmidinger, M.; Quehenberger, P.; Haitel, A.; Zielinski, C.; Pabinger, I. Tumor grade is associated with venous thromboembolism in patients with cancer: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3870–3875. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Vormittag, R.; Dunkler, D.; Simanek, R.; Chiriac, A.-L.; Drach, J.; Quehenberger, P.; Wagner, O.; Zielinski, C.; Pabinger, I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2009, 27, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Yang, E.H.; Iliescu, C.A.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular toxicities of cancer therapies. Circulation 2016, 133, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Han, J.; Cui, P.; Dai, M.; Li, H.; Zhang, J.; Xiu, R. Cisplatin up-regulates ICAM-1 expression in endothelial cell via a NF-κB dependent pathway. Cancer Sci. 2008, 99, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, S.; Shinozaki, R.; Fukue, K.; Kougo, T. Release of cytokines from human umbilical vein endothelial cells treated with platinum compounds in vitro. Jpn. J. Cancer Res. 1998, 89, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Witucka, A.; Pakuła, M.; Uruski, P.; Begier-Krasińska, B.; Niklas, A.; Tykarski, A.; Książek, A. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 2018, 76, 681–697. [Google Scholar] [CrossRef]
- Dasanu, C.A. Gemcitabine: Vascular toxicity and prothrombotic potential. Expert Opin. Drug Saf. 2008, 7, 703–716. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet (Lond. Engl.) 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Simanek, R.; Vormittag, R.; Dunkler, D.; Alguel, G.; Koder, S.; Kornek, G.; Marosi, C.; Wagner, O.; Zielinski, C.; et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008, 112, 2703. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Posch, F.; Kaider, A.; Zielinski, C.; Pabinger, I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J. Thromb. Haemost. 2015, 13, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
| Variable | Overall (n = 1409) | No Gemcitabine (n = 1174) | Gemcitabine (n = 235) | p1 | No Platinum (n = 908) | Platinum (n = 501) | p1 |
|---|---|---|---|---|---|---|---|
| Clinical Variables | |||||||
| Age at entry (years) | 62.9 (54.2–68·9) | 63.0 (53.6–68·9) | 62.6 (56.1–69.6) | 0.256 | 63.7 (53.9–70.4) | 61.2 (54·4–67.2) | 0.003 |
| BMI (kg/m2) | 25.1 (22.1–28.3) | 25.3 (22.5–28.5) | 24.0 (20.9–26.5) | <0.0001 | 25.4 (22.5–28.7) | 24.5 (21.6–27.3) | <0.0001 |
| Male sex | 760 (54%) | 640 (55%) | 120 (51%) | 0.333 | 472 (52%) | 288 (57%) | 0.047 |
| Tumor site | |||||||
| Low/intermediate risk of VTE | 378 (27%) | 366 (31%) | 12 (5%) | <0.0001 | 373 (41%) | 5 (1%) | <0.0001 |
| Breast | 226 (16%) | 215 (18%) | 11 (5%) | <0.0001 | 224 (25%) | 2 (0%) | <0.0001 |
| Prostate | 153 (11%) | 151 (13%) | 1 (<1%) | <0.0001 | 149 (16%) | 3 (1%) | <0.0001 |
| High Risk of VTE | 854 (61%) | 733 (62%) | 121 (51%) | <0.0001 | 467 (51%) | 387 (77%) | <0.0001 |
| Lung | 289 (21%) | 213 (18%) | 76 (32%) | <0.0001 | 88 (10%) | 201 (40%) | <0.0001 |
| Colorectal | 171 (12%) | 170 (14%) | 1 (<1%) | <0.0001 | 68 (7%) | 103 (21%) | <0.0001 |
| Kidney | 42 (3%) | 33 (3%) | 9 (4%) | 0.401 | 39 (4%) | 3 (1%) | <0.0001 |
| Lymphoma | 247 (18%) | 241 (21%) | 6 (3%) | <0.0001 | 227 (25%) | 20 (4%) | <0.0001 |
| Other sites | 105 (7%) | 76 (6%) | 29 (12%) | 0.002 | 45 (5%) | 60 (12%) | <0.0001 |
| Very high risk of VTE | 177 (13%) | 75 (6%) | 102 (43%) | <0.0001 | 68 (7%) | 109 (22%) | <0.0001 |
| Pancreas | 116 (8%) | 14 (1%) | 102 (43%) | <0.0001 | 49 (5%) | 67 (13%) | <0.0001 |
| Stomach | 61 (4%) | 61 (5%) | 0 (0%) | <0.0001 | 19 (2%) | 42 (8%) | <0.0001 |
| Tumor characteristics | |||||||
| Newly diagnosed cancer | 997 (71%) | 819 (70%) | 178 (76%) | 0.066 | 606 (67%) | 391 (78%) | <0.0001 |
| Tumor grade G3/G4 | 518 (38%) | 418 (36%) | 100 (43%) | 0.05 | 315 (36%) | 203 (41%) | 0.04 |
| Tumor stage (UICC/AnnArbor) | / | / | / | <0.0001 | / | / | <0.0001 |
| Stage I | 138 (10%) | 133 (12%) | 5 (2%) | / | 116 (14%) | 22 (4%) | / |
| Stage II | 309 (23%) | 278 (25%) | 31 (13%) | / | 264 (31%) | 45 (9%) | / |
| Stage III | 221 (16%) | 184 (17%) | 37 (16%) | / | 124 (15%) | 97 (19%) | / |
| Stage IV | 672 (50%) | 511 (46%) | 161 (69%) | / | 336 (40%) | 336 (67%) | / |
| Biomarker levels | |||||||
| D-dimer (µg/mL) | 0.7 (0.4–1.5) | 0.7 (0.3–1.3) | 1.2 (0.6–2.6) | <0.0001 | 0.6 (0.3–1.2) | 1.0 (0.5–2.0) | <0.0001 |
| VTE prediction model | |||||||
| CATS score: predicted 6-month VTE risk (%) 2 | 5.0 (3.3–6.3] | 4.8 (2.8–5.70 | 7.4 (5.2–11.0) | <0.0001 | 4.6 (2.6–5.5) | 5.8 (5.0–8.6) | <0.0001 |
| Outcomes | |||||||
| Mortality | 532 (37.8%) | 377 (32.1%) | 155 (66.0%) | / | 253 (27.9%) | 279 (55.7%) | / |
| VTE events | 111 (7.9%) | 87 (7.4%) | 24 (10.2%) | / | 53 (5.9%) | 58 (11.6%) | / |
| Model | Variable | SHR | 95%CI | p |
|---|---|---|---|---|
| #1 | Gemcitabine | 0.83 | 0.53–1.31 | 0.432 |
| Tumor type: Low/moderate VTE risk | Ref. | Ref. | Ref. | |
| Tumor type: High VTE risk | 2.48 | 1.37–4.48 | 0.003 | |
| Tumor type: Very high VTE risk | 5.48 | 2.82–10.68 | <0.0001 | |
| #2 | Gemcitabine | 1.18 | 0.75–1.85 | 0.481 |
| D-dimer (per doubling) | 1.44 | 1.25–1.66 | <0.0001 | |
| #3 | Gemcitabine | 0.82 | 0.53–1.28 | 0.390 |
| Tumor type: Low/moderate VTE risk | Ref. | Ref. | Ref. | |
| Tumor type: High VTE risk | 2.26 | 1.24–4.10 | 0.008 | |
| Tumor type: Very high VTE risk | 4.27 | 2.10–8.66 | <0.0001 | |
| D-dimer (per doubling) | 1.31 | 1.11–1.53 | 0.001 | |
| #4 | Platinum-based therapy | 1.46 | 0.97–2.21 | 0.073 |
| Tumor type: Low/moderate VTE risk | Ref. | Ref. | Ref. | |
| Tumor type: High VTE risk | 2.03 | 1.09–3.77 | 0.025 | |
| Tumor type: Very high VTE risk | 3.89 | 1.87–8.11 | <0.001 | |
| #5 | Platinum-based therapy | 1.84 | 1.26–2.69 | 0.002 |
| D-dimer (per doubling) | 1.40 | 1.20–1.63 | <0.0001 | |
| #6 | Platinum-based therapy | 1.44 | 0.96–2.17 | 0.080 |
| Tumor type: Low/moderate VTE risk | Ref. | Ref. | Ref. | |
| Tumor type: High VTE risk | 1.85 | 0.99–3.46 | 0.055 | |
| Tumor type: Very high VTE risk | 3.08 | 1.45–6.57 | 0.004 | |
| D-dimer (per doubling) | 1.31 | 1.1–1.53 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moik, F.; van Es, N.; Posch, F.; Di Nisio, M.; Fuereder, T.; Preusser, M.; Pabinger, I.; Ay, C. Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Cancers 2020, 12, 2493. https://doi.org/10.3390/cancers12092493
Moik F, van Es N, Posch F, Di Nisio M, Fuereder T, Preusser M, Pabinger I, Ay C. Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Cancers. 2020; 12(9):2493. https://doi.org/10.3390/cancers12092493
Chicago/Turabian StyleMoik, Florian, Nick van Es, Florian Posch, Marcello Di Nisio, Thorsten Fuereder, Matthias Preusser, Ingrid Pabinger, and Cihan Ay. 2020. "Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study" Cancers 12, no. 9: 2493. https://doi.org/10.3390/cancers12092493
APA StyleMoik, F., van Es, N., Posch, F., Di Nisio, M., Fuereder, T., Preusser, M., Pabinger, I., & Ay, C. (2020). Gemcitabine and Platinum-Based Agents for the Prediction of Cancer-Associated Venous Thromboembolism: Results from the Vienna Cancer and Thrombosis Study. Cancers, 12(9), 2493. https://doi.org/10.3390/cancers12092493






