A Pilot Study on Efficacy of Lipid Bubbles for Theranostics in Dogs with Tumors
Abstract
1. Introduction
2. Results
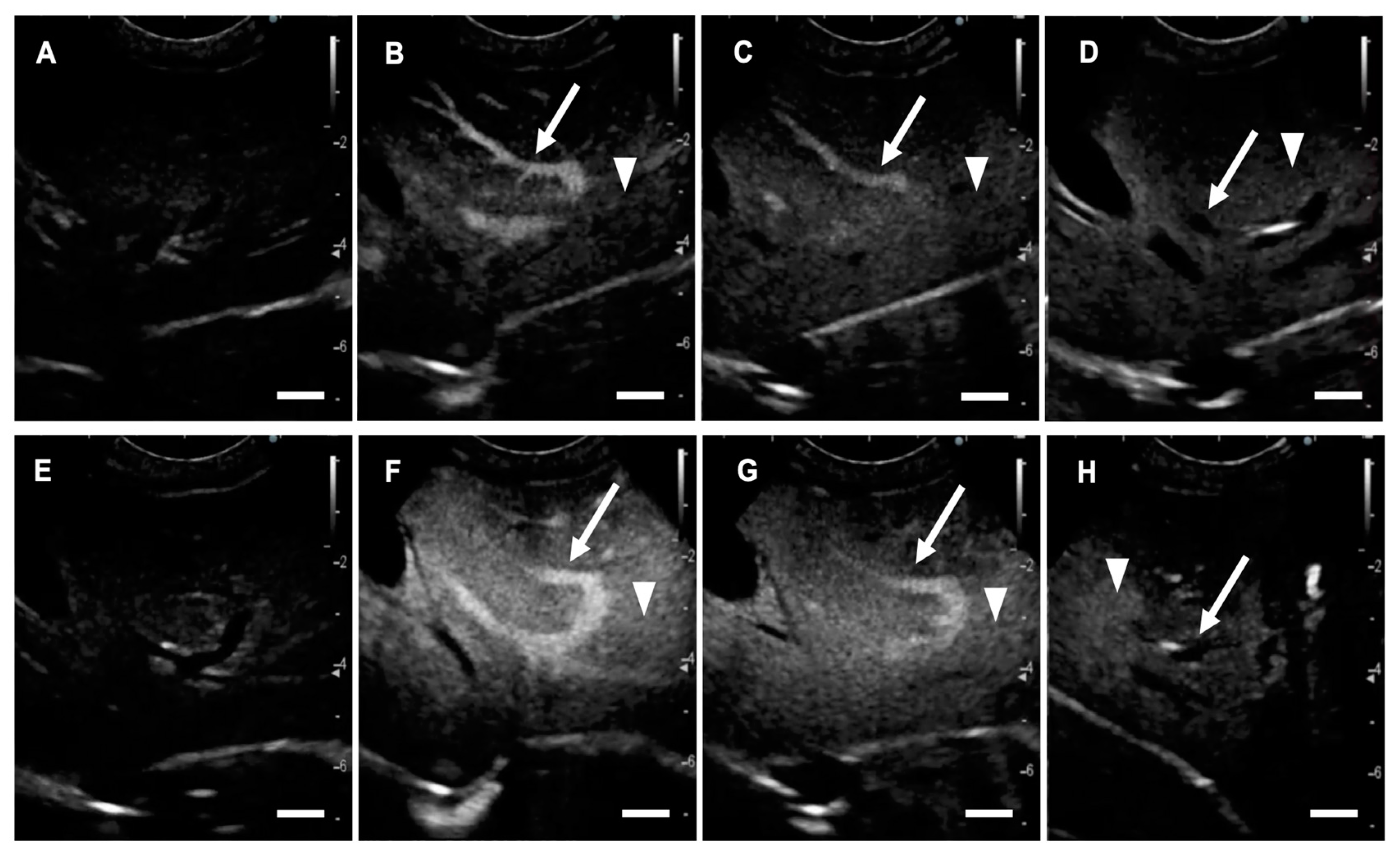
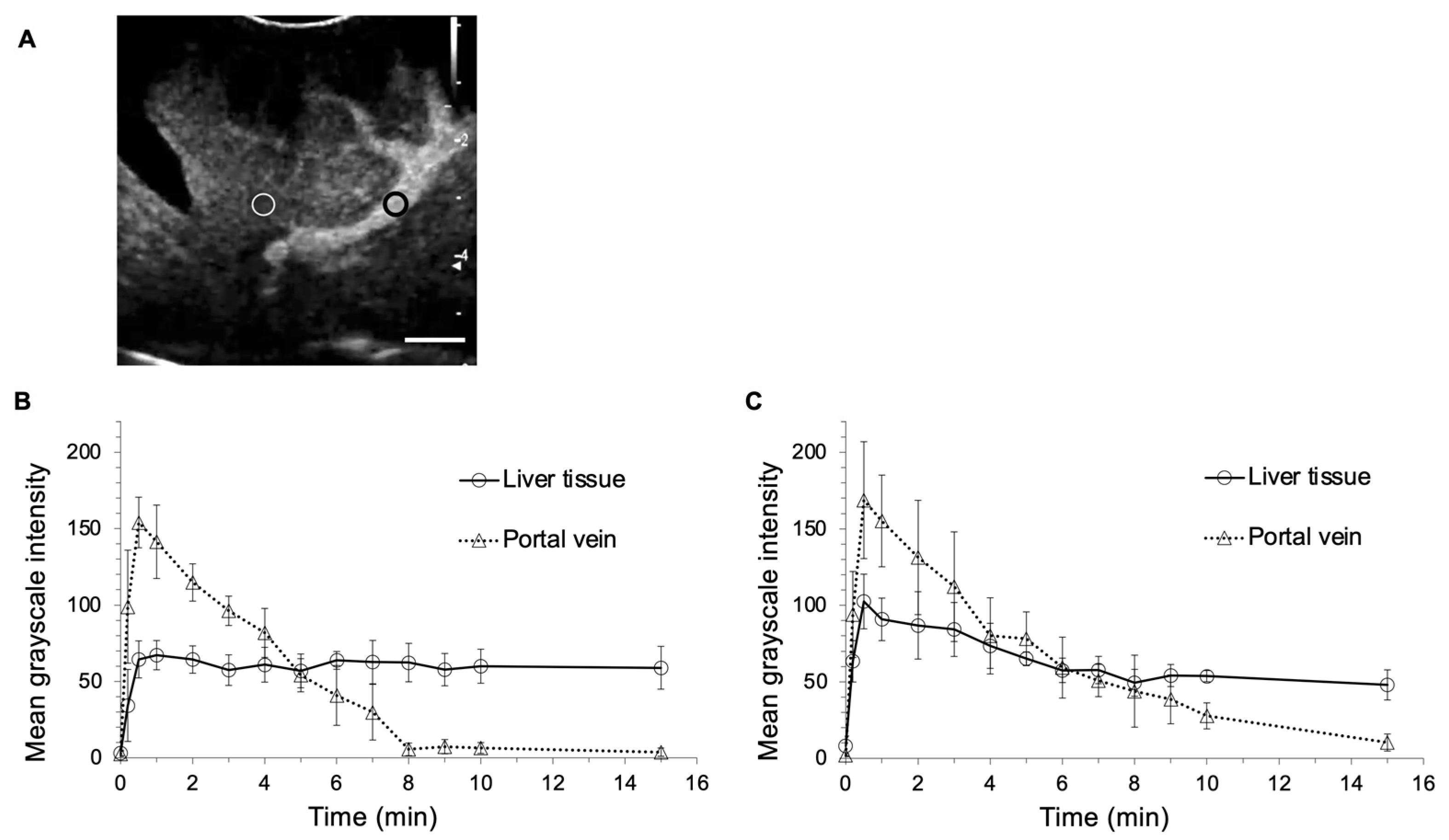
2.1. US Contrast Effect and Safety of LBs in Healthy Beagles
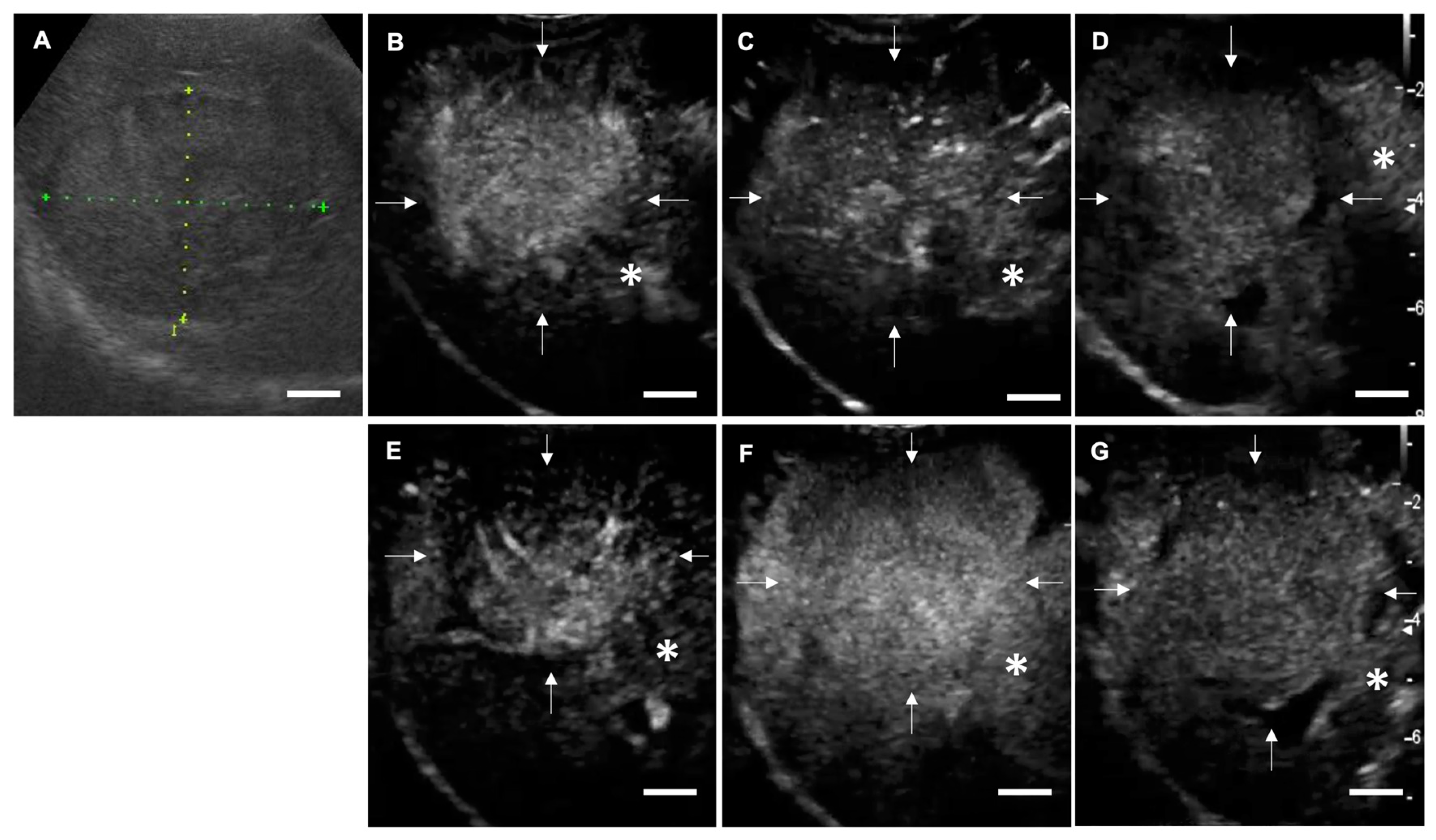
2.2. CEUS in Dogs with Focal Liver Lesions
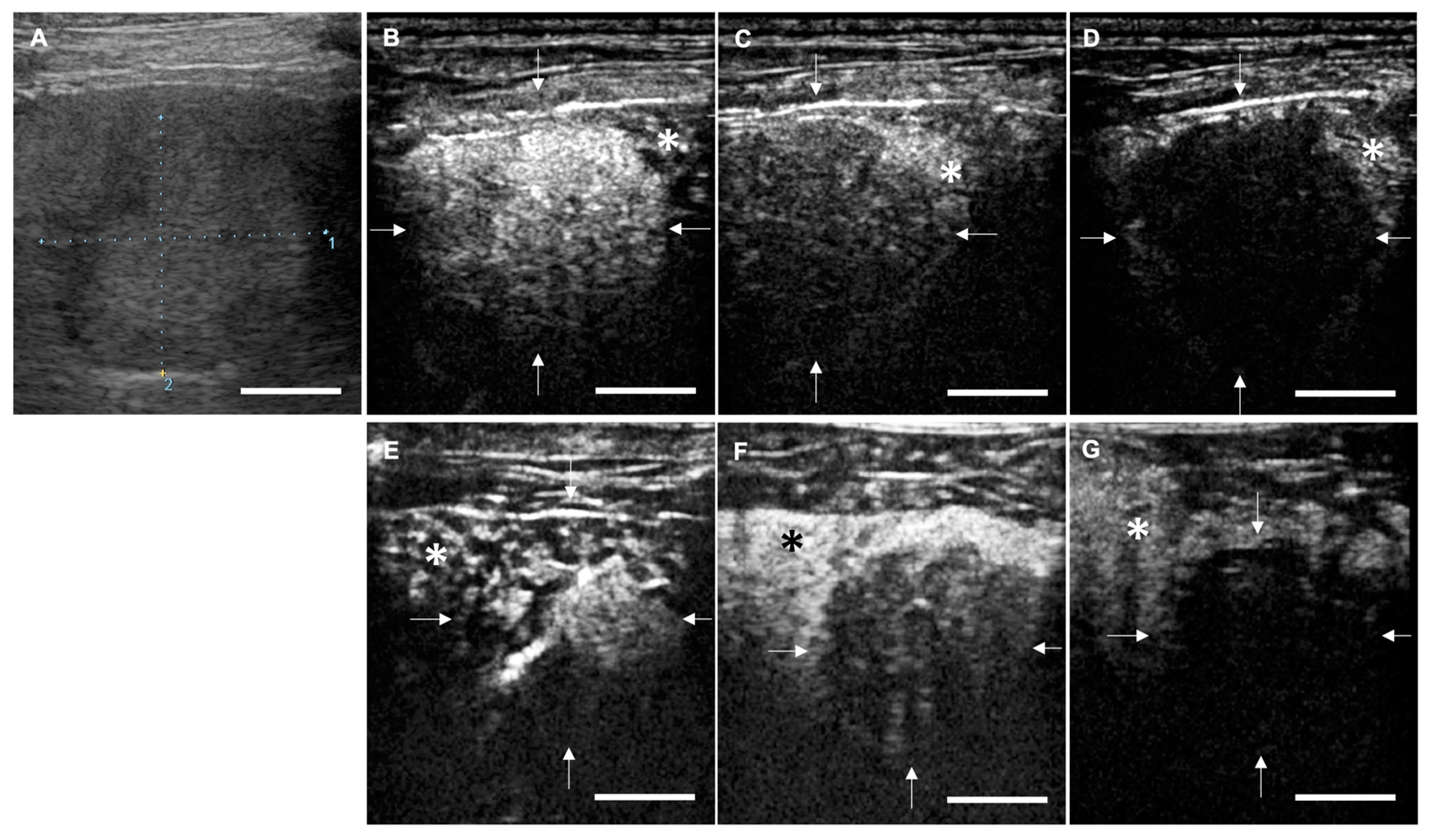
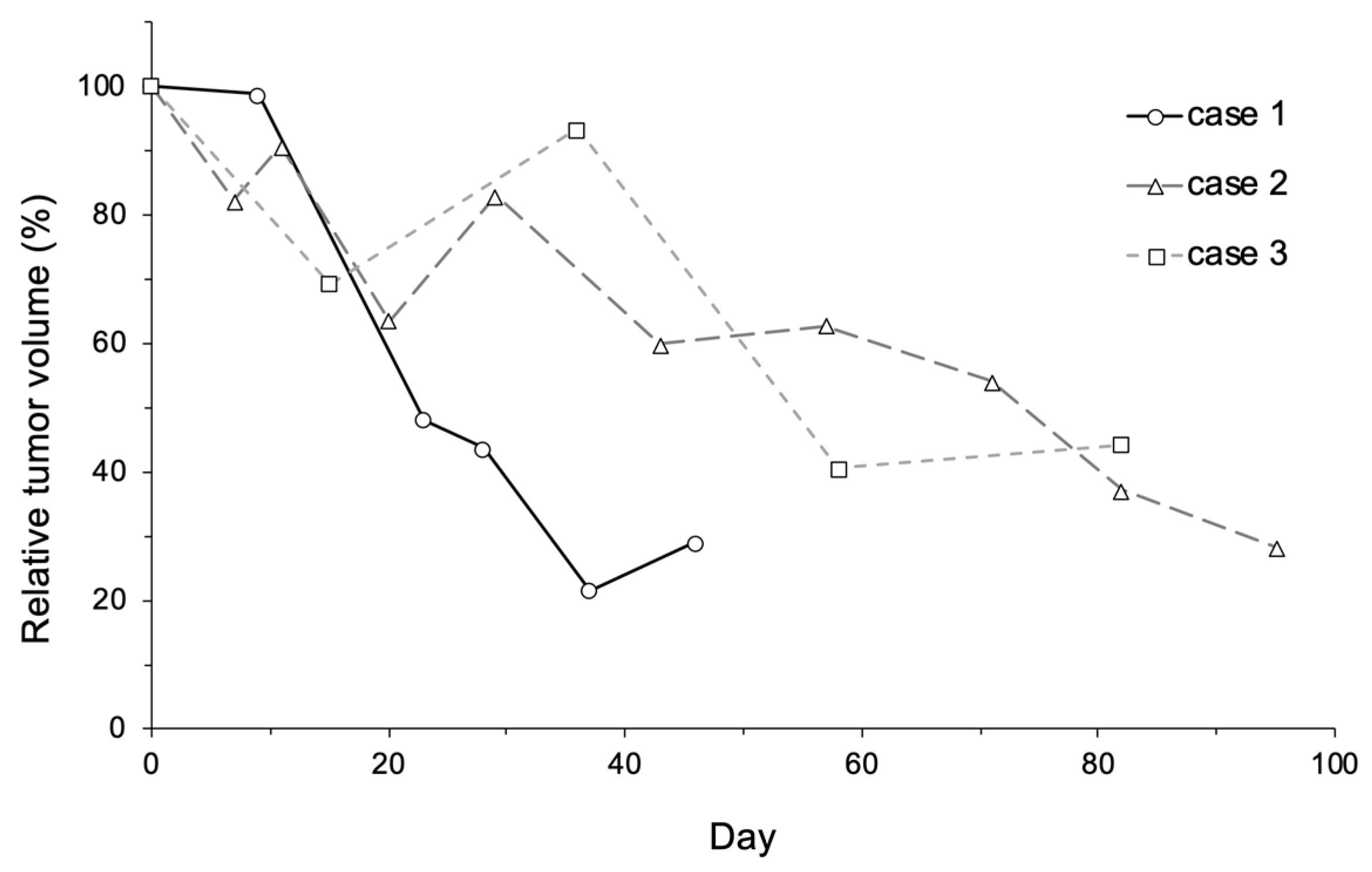
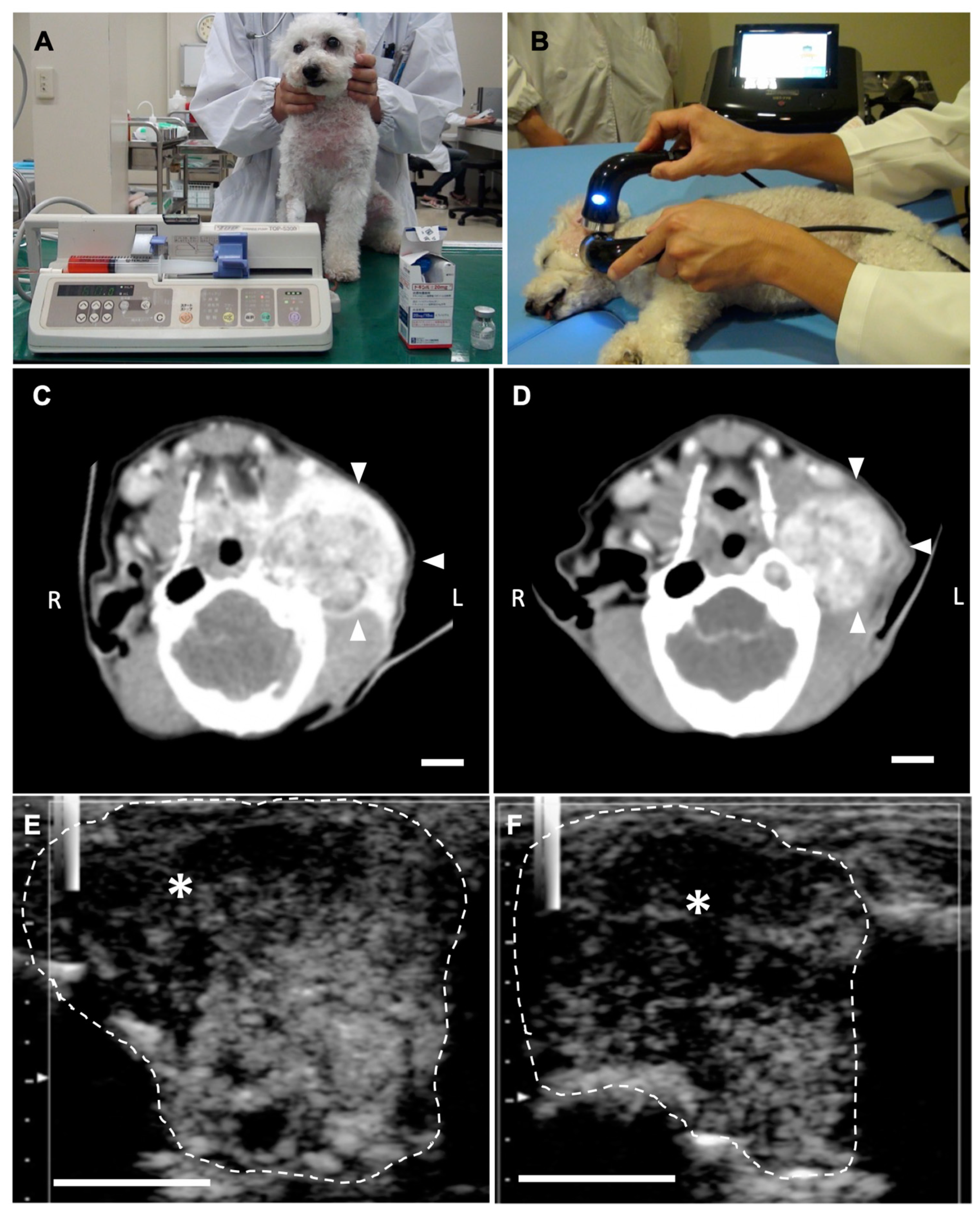
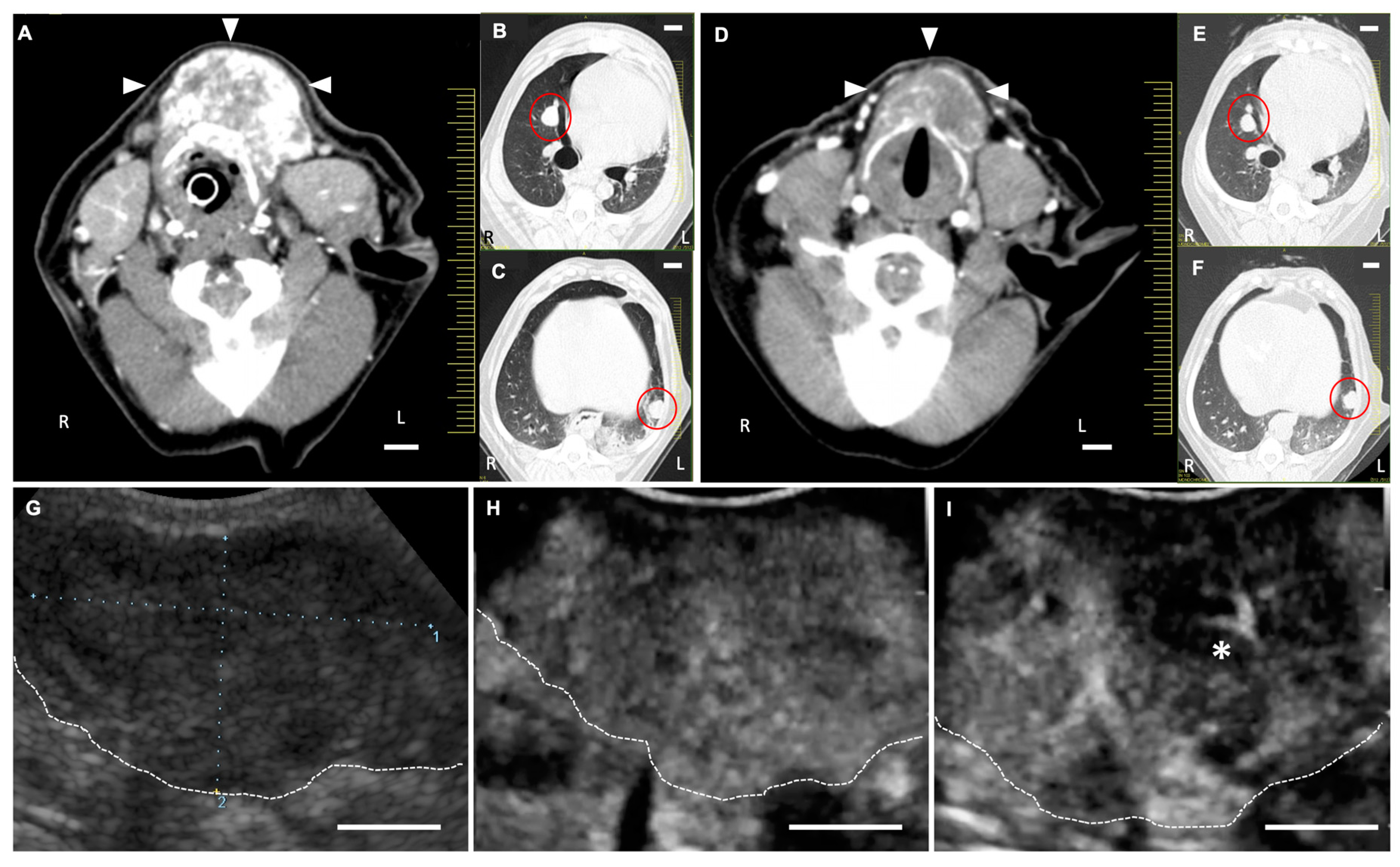
2.3. Anti-Tumor Effects of LBs in Combination with Therapeutic US and Liposomal Doxorubicin
3. Discussion
4. Materials and Methods
4.1. Preparation of LBs
4.1.1. Reagents
4.1.2. Preparation of Liposomes and LBs
4.2. US Contrast Effect and Safety of LBs in Healthy Dogs
4.2.1. Animals
4.2.2. CEUS for Liver and Laboratory Test
4.2.3. Imaging Analysis
4.3. CEUS in Dogs Bearing Focal Liver Lesions
4.3.1. Animals
4.3.2. Characterization of Focal Liver Lesions with Conventional Ultrasonography
4.3.3. CEUS Using LBs and Sonazoid
4.3.4. Evaluating the Diagnostic Accuracy of LB-CEUS
4.4. Anti-Tumor Effects of LBs Combined with US and Liposomal Doxorubicin
4.4.1. Animals
4.4.2. Treatment Protocol
4.4.3. CEUS for the Assessment of Tumor Vasculature
4.4.4. Measurement of Tumor Size
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.S. Introducing Theranostics Journal—From the Editor-in-Chief. Theranostics 2011, 1, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.H.; Dayton, P.A. Current status and prospects for microbubbles in ultrasound theranostics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound Microbubbles for Molecular Diagnosis, Therapy, and Theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef]
- Paefgen, V.; Doleschel, D.; Kiessling, F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol. 2015, 6, 197. [Google Scholar] [CrossRef]
- De Jong, N.; Frinking, P.J.A.; Bouakaz, A.; Goorden, M.; Schourmans, T.; Jingping, X.; Mastik, F. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound Med. Biol. 2000, 26, 487–492. [Google Scholar] [CrossRef]
- Wilson, S.R.; Burns, P.N. Microbubble-enhanced US in Body Imaging: What Role? Radiology 2010, 257, 24–39. [Google Scholar] [CrossRef]
- Kudo, N.; Okada, K.; Yamamoto, K. Sonoporation by single-shot pulsed ultrasound with microbubbles adjacent to cells. Biophys. J. 2009, 96, 4866–4876. [Google Scholar] [CrossRef]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the Liver—Update 2012. Ultrasound Med. Biol. 2013, 39, 187–210. [Google Scholar] [CrossRef]
- Wu, M.; Li, L.; Wang, J.; Zhang, Y.; Guo, Q.; Li, X.; Zhang, X. Contrast-enhanced US for characterization of focal liver lesions: A comprehensive meta-analysis. Eur. Radiol. 2018, 28, 2077–2088. [Google Scholar] [CrossRef]
- Liu, H.-L.; Fan, C.-H.; Ting, C.-Y.; Yeh, C.-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Foley, J.; Kassell, N.F.; Larrat, B.; Aubry, J.-F. Review of ultrasound mediated drug delivery for cancer treatment: Updates from pre-clinical studies. Transl. Cancer Res. 2014, 3, 494–511. [Google Scholar] [CrossRef]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Hagisawa, K.; Tanaka, K.; Sawamura, K.; Utoguchi, N.; Nishioka, T.; Maruyama, K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control. Release 2007, 117, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Unga, J.; Omata, D.; Kudo, N.; Ueno, S.; Munakata, L.; Shima, T.; Suzuki, R.; Maruyama, K. Development and evaluation of stability and ultrasound response of DSPC-DPSG-based freeze-dried microbubbles. J. Liposome Res. 2019, 29, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Unga, J.; Kageyama, S.; Suzuki, R.; Omata, D.; Maruyama, K. Scale-up production, characterization and toxicity of a freeze-dried lipid-stabilized microbubble formulation for ultrasound imaging and therapy. J. Liposome Res. 2019, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Omata, D.; Maruyama, T.; Unga, J.; Hagiwara, F.; Munakata, L.; Kageyama, S.; Shima, T.; Suzuki, Y.; Maruyama, K.; Suzuki, R. Effects of encapsulated gas on stability of lipid-based microbubbles and ultrasound-triggered drug delivery. J. Control. Release 2019, 311–312, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Sonoda, S.; Suzuki, R.; Yokouchi, M.; Kawasoe, Y.; Tachibana, K.; Maruyama, K.; Sakamoto, T.; Komiya, S. Combination of ultrasound and bubble liposome enhance the effect of doxorubicin and inhibit murine osteosarcoma growth. Cancer Biol. Ther. 2014, 12, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Nyman, H.T.; Kristensen, A.T.; Kjelgaard-Hansen, M.; McEvoy, F.J. Contrast-enhanced ultrasonography in normal canine liver. Evaluation of imaging and safety parameters. Vet. Radiol. Ultrasound 2005, 46, 243–250. [Google Scholar] [CrossRef]
- Kanemoto, H.; Ohno, K.; Nakashima, K.; Takahashi, M.; Fujino, Y.; Tsujimoto, H. Vascular and Kupffer imaging of canine liver and spleen using the new contrast agent sonazoid. J. Vet. Med. Sci. 2008, 70, 1265–1268. [Google Scholar] [CrossRef][Green Version]
- Watanabe, R.; Matsumura, M.; Chen, C.J.; Kaneda, Y.; Fujimaki, M. Characterization of tumor imaging with microbubble-based ultrasound contrast agent, sonazoid, in rabbit liver. Biol. Pharm. Bull. 2005, 28, 972–977. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Moriyasu, F.; Miyahara, T.; Yuki, M.; Iijima, H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med. Biol. 2007, 33, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Matsumura, M.; Chen, C.-J.; Kaneda, Y.; Ishihara, M.; Fujimaki, M. Gray-scale liver enhancement with Sonazoid (NC100100), a novel ultrasound contrast agent; detection of hepatic tumors in a rabbit model. Biol. Pharm. Bull. 2003, 26, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Iijima, H.; Moriyasu, F.; Waki, H. Definition of contrast enhancement phases of the liver using a perfluoro-based microbubble agent, perflubutane microbubbles. Ultrasound Med. Biol. 2009, 35, 1819–1827. [Google Scholar] [CrossRef]
- Nakamura, K.; Takagi, S.; Sasaki, N.; Bandula Kumara, W.R.; Murakami, M.; Ohta, H.; Yamasaki, M.; Takiguchi, M. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet. Radiol. Ultrasound 2010, 51, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, H.; Ohno, K.; Nakashima, K.; Takahashi, M.; Fujino, Y.; Nishimura, R.; Tsujimoto, H. Characterization of canine focal liver lesions with contrast-enhanced ultrasound using a novel contrast agent-Sonazoid. Vet. Radiol. Ultrasound 2009, 50, 188–194. [Google Scholar] [CrossRef]
- Samuelsson, E.; Shen, H.; Blanco, E.; Ferrari, M.; Wolfram, J. Contribution of Kupffer cells to liposome accumulation in the liver. Colloids Surf. B Biointerfaces 2017, 158, 356–362. [Google Scholar] [CrossRef]
- Hatanaka, K.; Kudo, M.; Minami, Y.; Ueda, T.; Tatsumi, C.; Kitai, S.; Takahashi, S.; Inoue, T.; Hagiwara, S.; Chung, H.; et al. Differential Diagnosis of Hepatic Tumors: Value of Contrast-Enhanced Harmonic Sonography Using the Newly Developed Contrast Agent, Sonazoid. Intervirology 2008, 51, 61–69. [Google Scholar] [CrossRef]
- Tamura, M.; Nakamura, K.; Osuga, T.; Shimbo, G.; Sasaki, N.; Morishita, K.; Ohta, H.; Takiguchi, M. Findings of contrast-enhanced ultrasonography with Sonazoid for cholangiocellular adenoma in three dogs. J. Vet. Med. Sci. 2019, 81, 1104–1108. [Google Scholar] [CrossRef]
- Trigo, F.J.; Thompson, H.; Breeze, R.G.; Nash, A.S. The pathology of liver tumours in the dog. J. Comp. Pathol. 1982, 92, 21–39. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Gabizon, A.A.; Barenholz, Y.; Bialer, M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: Pharmacokinetic studies in rodents and dogs. Pharm. Res. 1993, 10, 703–708. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Zabielska-Koczywąs, K.; Lechowski, R. The use of liposomes and nanoparticles as drug delivery systems to improve cancer treatment in dogs and cats. Molecules 2017, 22, 2167. [Google Scholar] [CrossRef]
- Vail, D.M.; Kravis, L.D.; Cooley, A.J.; Chun, R.; MacEwen, E.G. Preclinical trial of doxorubicin entrapped in sterically stabilized liposomes in dogs with spontaneously arising malignant tumors. Cancer Chemother. Pharmacol. 1997, 39, 410–416. [Google Scholar] [CrossRef]
- Sorenmo, K.; Samluk, M.; Clifford, C.; Baez, J.; Barrett, J.S.; Poppenga, R.; Overley, B.; Skorupski, K.; Oberthaler, K.; Van Winkle, T.; et al. Clinical and pharmacokinetic characteristics of intracavitary administration of pegylated liposomal encapsulated doxorubicin in dogs with splenic hemangiosarcoma. J. Vet. Intern. Med. 2007, 21, 1347–1354. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef]
- Skyba, D.M.; Price, R.J.; Linka, A.Z.; Skalak, T.C.; Kaul, S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 1998, 98, 290–293. [Google Scholar] [CrossRef]

| Parameters 1 | Pre-Injection of Lipid Bubbles | Post-Injection (24 h) of Lipid Bubbles | Unit |
|---|---|---|---|
| WBC | 106.8 ± 20.1 | 98.3 ± 17.8 | 102/μL |
| RBC | 705.0 ± 36.7 | 728.8 ± 70.5 | 104/μL |
| HGB | 16.0 ± 1.0 | 16.5 ± 2.0 | g/dL |
| HCT | 44.6 ± 2.9 | 46.1 ± 5.2 | % |
| PLT * | 41.0 ± 9.1 | 30.7 ± 9.2 | 104/μL |
| CRP | 0.5 ± 0.4 | 0.6 ± 0.2 | mg/dL |
| BUN * | 15.5 ± 1.8 | 11.5 ± 0.9 | mg/dL |
| CRE * | 0.72 ± 0.1 | 0.67 ± 0.1 | mg/dL |
| ALT | 45.7 ± 5.3 | 49.5 ± 4.6 | U/L |
| ALP | 153.3 ± 39.0 | 152.5 ± 32.3 | U/L |
| ALB | 3.3 ± 0.1 | 3.1 ± 0.3 | g/dL |
| Benign/ Malignant | No | Breed | Age (y) | Sex 1 | Diagnosis | Conventional B Mode | LB-CEUS | Sonazoid-CEUS 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesion Size 2 (cm) | Echo Pattern | Echo-Genicity 3 | Arterial 4 (0–30 s) | Portal 4 (30 s–2 min) | Kupffer 5 (10 min–) | Arterial 4 (0–30 s) | Portal 4 (30 s–2 min) | Kupffer 5 (10 min–) | ||||||
| Benign | 1 | Mix | 10 | M | Hepatocellular adenoma | 5.97 × 4.20 | solid | iso | hyper | iso | ND | hyper | iso | ND |
| 2 | Chihuahua | 12 | M | Cholangiocellular adenoma | 3.16 × 2.87 | mixed | hypo | hetero | hetero | CD | hetero | hetero | CD | |
| 3 | Mix | 12 | SF | Nodular hyperplasia | 2.89 × 2.01 | solid | iso | hyper | iso | ND | hyper | iso | ND | |
| 4 | Welsh Corgi | 14 | SF | Degeneration and necrosis of liver cells | 7.06 × 4.36 | solid | iso | iso | iso | ND | iso | iso | ND | |
| 5 | Boston Terrier | 13 | CM | Trichangiectasia | 1.04 × 0.92 | solid | hyper | iso | iso | ND | iso | iso | ND | |
| 6 | Chihuahua | 9 | SF | Vacuolar degeneration and fatty degeneration of liver cells | 1.55 × 1.54 | solid | iso | iso | iso | ND | n/a | n/a | n/a | |
| Malignant (primary) | 7 | Yorkshire Terrier | 9 | F | Hepatocellular carcinoma | 7.68 × 5.46 | solid | hypo | hetero | hetero | ID | hetero | hetero | ID |
| 8 | Miniature Dachshund | 12 | M | Hepatocellular carcinoma | 12.02 × 8.49 | solid | hypo | hetero | hetero | ID | hetero | hetero | ID | |
| 9 | French Bulldog | 11 | F | Hepatocellular carcinoma | 1.56 × 1.12 | solid | hypo | iso | hypo | CD | iso | hypo | CD | |
| 10 | Chihuahua | 11 | M | Hepatocellular carcinoma | 2.57 × 2.07 | solid | hypo | hypo | hypo | CD | hypo | hypo | CD | |
| 11 | Mix | 11 | SF | Hepatocellular carcinoma | 2.65 × 2.31 | solid | iso | hyper | hypo | CD | hyper | hypo | CD | |
| 12 | Miniature Dachshund | 14 | CM | Hepatocellular carcinoma | 4.52 × 4.11 | solid | iso | hyper | iso | CD | n/a | n/a | n/a | |
| 13 | Golden Retriever | 10 | CM | Hepatocellular carcinoma | 2.92 × 2.58 | solid | hypo | hyper | iso | CD | n/a | n/a | n/a | |
| 14 | Mix | 14 | SF | Hepatocellular carcinoma | 6.82 * | solid | hypo | hetero | hetero | ID | n/a | n/a | n/a | |
| 15 | Beagle | 13 | F | Hepatocellular carcinoma | 12.26 × 11.64 | solid | hypo | hetero | hetero | ID | n/a | n/a | n/a | |
| 16 | Miniature Schnauzer | 12 | CM | Cholangiocellular carcinoma | 6.05 × 3.76 | solid | hypo | hyper | iso | CD | n/a | n/a | n/a | |
| 17 | Beagle | 8 | SF | Epithelial malignant tumor | 6.29 × 5.93 | solid | hypo | hypo | hypo | CD | n/a | n/a | n/a | |
| Malignant (metastatic) | 18 | Miniature Dachshund | 11 | M | Hemangiosarcoma | 2.98 × 1.92 | solid | iso | hetero | hetero | CD | hetero | hetero | CD |
| 19 | Miniature Schnauzer | 9 | SF | Malignant melanoma | 1.52 × 0.96 | solid | hyper | hypo | hypo | CD | hypo | hypo | CD | |
| 20 | Jack Russell Terrier | 13 | CM | Epithelial malignant tumor | 9.31 × 5.72 | solid | iso | hetero | hetero | ID | hetero | hetero | ID | |
| 21 | Miniature Dachshund | 11 | CM | Nonepithelial malignant tumor | 2.14 × 1.40 | solid | hypo | hypo | hypo | CD | n/a | n/a | n/a | |
| No. | Breed | Age (y) | Sex 1 | Weight (kg) | Tumor Type | Tumor Site | Simultaneous Therapy |
|---|---|---|---|---|---|---|---|
| 1 | Papillon | 14 | SF | 2.5 | Hemangiopericytoma | Perianal | None |
| 2 | Toy Poodle | 7 | F | 3.8 | Ceruminous gland adenocarcinoma | Left ear | None |
| 3 | Welsh Corgi | 10 | F | 10.1 | Thyroid carcinoma (suspected) | Thyroid | Toceranib |
| No. | US Output Setting | Concentration of Doxil (mg/m2) | Number of Treatments | Tumor Volume | Survival Time 1 (days) | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (MHz) | Power Intensity (W/cm2) | Duty Cycle (%) | Irradiation Time (min) | A: At the First Measurement (cm3) | B: At the Last Measurement (cm3) | B/A (%) | |||||
| 1 | 1 | 2 | 50 | 20–30 | 16 | 2 | 178.0 | 51.4 | 28.9 | 52 | Died of a cause unrelated to the tumor |
| 2 | 1 | 2 | 50 or 100 | 30–40 | 8–16 | 6 | 4.4 | 1.3 | 28.2 | 127 | Died of a cause unrelated to the tumor |
| 3 | 1 | 2 | 50 | 15 | 15 | 4 | 41.0 | 18.1 | 44.3 | n/a | Lost to follow-up |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoe, I.; Murahata, Y.; Harada, K.; Sunden, Y.; Omata, D.; Unga, J.; Suzuki, R.; Maruyama, K.; Okamoto, Y.; Osaki, T. A Pilot Study on Efficacy of Lipid Bubbles for Theranostics in Dogs with Tumors. Cancers 2020, 12, 2423. https://doi.org/10.3390/cancers12092423
Yokoe I, Murahata Y, Harada K, Sunden Y, Omata D, Unga J, Suzuki R, Maruyama K, Okamoto Y, Osaki T. A Pilot Study on Efficacy of Lipid Bubbles for Theranostics in Dogs with Tumors. Cancers. 2020; 12(9):2423. https://doi.org/10.3390/cancers12092423
Chicago/Turabian StyleYokoe, Inoru, Yusuke Murahata, Kazuki Harada, Yuji Sunden, Daiki Omata, Johan Unga, Ryo Suzuki, Kazuo Maruyama, Yoshiharu Okamoto, and Tomohiro Osaki. 2020. "A Pilot Study on Efficacy of Lipid Bubbles for Theranostics in Dogs with Tumors" Cancers 12, no. 9: 2423. https://doi.org/10.3390/cancers12092423
APA StyleYokoe, I., Murahata, Y., Harada, K., Sunden, Y., Omata, D., Unga, J., Suzuki, R., Maruyama, K., Okamoto, Y., & Osaki, T. (2020). A Pilot Study on Efficacy of Lipid Bubbles for Theranostics in Dogs with Tumors. Cancers, 12(9), 2423. https://doi.org/10.3390/cancers12092423





