Oncological Outcomes of Metastasis-Directed Therapy in Oligorecurrent Prostate Cancer Patients Following Radical Prostatectomy
Abstract
1. Introduction
2. Results
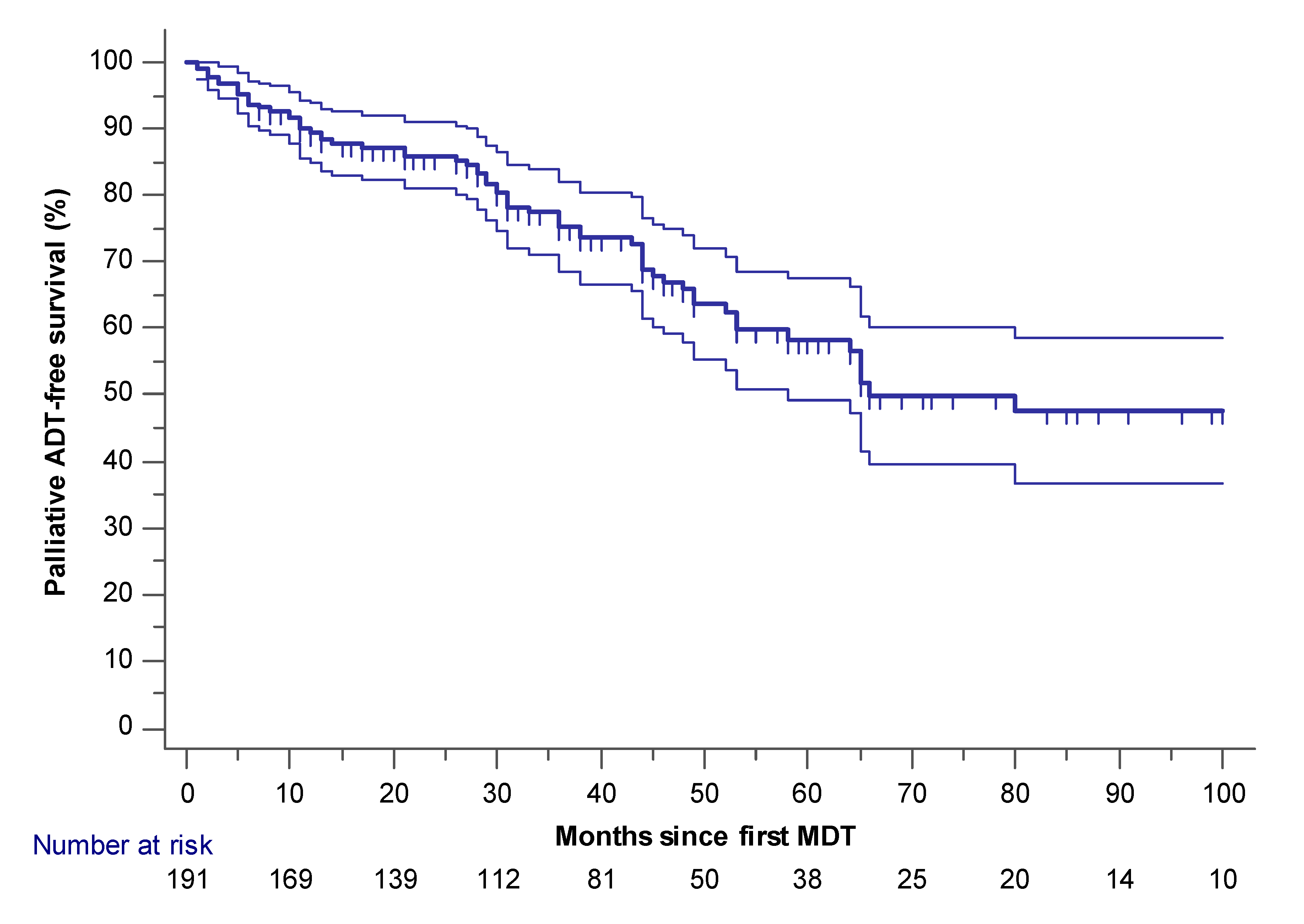
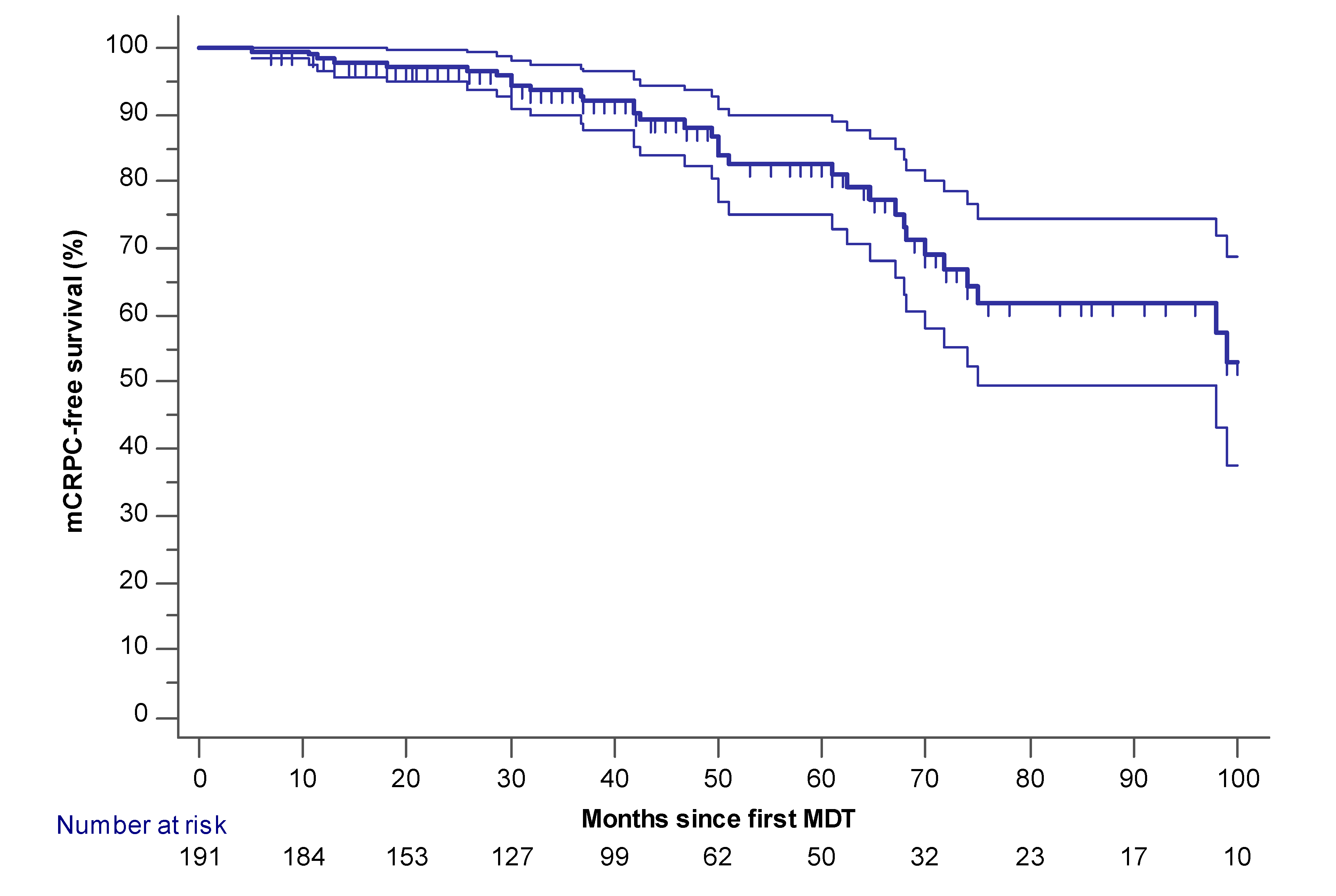
2.1. Palliative ADT-Free and mCRPC Free Survival
2.2. Multivariate Cox Proportional Hazard Regression Model Predicting Palliative ADT
2.3. Secondary Endpoints
3. Discussion
4. Methods
4.1. Radiotherapy
4.1.1. Stereotactic Body Radiation Therapy
4.1.2. Pelvic and/or Para-Aortic Lymph Node (PALN) Irradiation
- Primary pelvic and/or PALN with a simultaneous integrated boost (SIB) on the visible LN.
- Postoperative pelvic and/or PALN (adjuvant/salvage) after pelvic and/or peri-aortic LN dissection.
4.2. Salvage Lymphadenectomy
4.3. Metastasectomy
4.4. Follow-Up
4.5. Endpoints
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen Deprivation Therapy |
| BCR | Biochemical Recurrence |
| CI | Confidence Interval |
| CRPC | Castration-Resistant Prostate Cancer |
| CT | Computed Tomography |
| EAU | European Association of Urology |
| GTV | Gross Tumor Volume |
| HR | Hazard Ratio |
| ICECAP | Intermediate Clinical Endpoints of Cancer of the Prostate |
| IQR | Interquartile Range |
| ISUP | International Society of Urological Pathology |
| LN | Lymph Node |
| mCRPC | Metastatic Castration-Resistant Prostate Cancer |
| MDT | Metastasis-Directed Therapy |
| NA | Not Available |
| PCa | Prostate Cancer |
| PALN | Para-Aortic Lymph Node |
| PSA | Prostate-Specific Antigen |
| PSMA | Prostate Specific Membrane Antigen |
| PTV | Planning Target Volume |
| RP | Radical Prostatectomy |
| RT | Radiotherapy |
| SBRT | Stereotactic Body Radiation Therapy |
| SIB | Simultaneous Integrated Boost |
| sLND | Salvage Lymph Node Dissection/Salvage Lymphadenectomy |
References
- Battaglia, A.; De Meerleer, G.; Tosco, L.; Moris, L.; Van den Broeck, T.; Devos, G.; Everaerts, W.; Joniau, S. Novel Insights into the Management of Oligometastatic Prostate Cancer: A Comprehensive Review. Eur. Urol. Oncol. 2019, 2, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Bossi, A.; Decaestecker, K.; De Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach Iii, M.; Briganti, A. Platinum Priority-Review-Prostate Cancer Metastasis-directed Therapy of Regional and Distant Recurrences After Curative Treatment of Prostate Cancer: A Systematic Review of the Literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Zilli, T.; Ost, P. Metastasis-directed therapy: A new standard for oligorecurrent prostate cancer? Oncotarget 2018, 9, 34196–34197. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Mehra, M.; Nair, S.; Lawson, J.; Small, E.J. Association of metastasis-free survival (MFS) and overall survival (OS) in nonmetastatic castration-resistant prostate cancer (nmCRPC). J. Clin. Oncol. 2018, 36, 5032. [Google Scholar] [CrossRef]
- Xie, W.; Regan, M.M.; Buyse, M.; Halabi, S.; Kantoff, P.; Sartor, O.; Soule, H.; Clarke, N.W.; Collette, L.; Dignam, J.J.; et al. Metastasis-free survival is a strong Surrogate of overall survival in localized prostate cancer. J. Clin. Oncol. 2017, 35, 3097–3104. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Tilki, D.; Mandel, P.; Seeliger, F.; Kretschmer, A.; Karl, A.; Ergün, S.; Seitz, M.; Stief, C.G. Salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy. J. Urol. 2015, 193, 90–484. [Google Scholar] [CrossRef]
- Osmonov, D.K.; Aksenov, A.V.; Trick, D.; Naumann, C.M.; Hamann, M.F.; Faddan, A.A.; Jünemann, K.P. Cancer-specific and overall survival in patients with recurrent prostate cancer who underwent salvage extended pelvic lymph node dissection. BMC Urol. 2016, 16. [Google Scholar] [CrossRef]
- Suardi, N.; Gandaglia, G.; Gallina, A.; Di Trapani, E.; Scattoni, V.; Vizziello, D.; Cucchiara, V.; Bertini, R.; Colombo, R.; Picchio, M.; et al. Long-term Outcomes of Salvage Lymph Node Dissection for Clinically Recurrent Prostate Cancer: Results of a Single-institution Series with a Minimum Follow-up of 5 Years. Eur. Urol. 2015, 67, 299–309. [Google Scholar] [CrossRef]
- Porres, D.; Pfister, D.; Thissen, A.; Kuru, T.H.; Zugor, V.; Buettner, R.; Knuechel, R.; Verburg, F.A.; Heidenreich, A. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis. 2016, 20, 85–92. [Google Scholar] [CrossRef]
- Christian Rischke, H.; Schultze-Seemann, W.; Wieser, G.; Krönig, M.; Drendel, V.; Stegmaier, P.; Krauss, T.; Henne, K.; Volegova-Neher, N.; Schlager, D.; et al. Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther Onkol. 2015, 191, 310–320. [Google Scholar] [CrossRef]
- Berkovic, P.; De Meerleer, G.; Delrue, L.; Lambert, B.; Fonteyne, V.; Lumen, N.; Decaestecker, K.; Villeirs, G.; Vuye, P.; Ost, P. Salvage Stereotactic Body Radiotherapy for Patients With Limited Prostate Cancer Metastases: Deferring Androgen Deprivation Therapy. Clin. Genitourin. Cancer 2013, 11, 27–32. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Spratt, D.E.; Evans, M.J.; Davis, B.J.; Doran, M.G.; Lee, M.X.; Shah, N.; Wongvipat, J.; Carnazza, K.E.; Klee, G.G.; Polkinghorn, W.; et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015, 75, 4688–4696. [Google Scholar] [CrossRef]
- Bartek, J.; Mistrik, M.; Bartkova, J. Androgen receptor signaling fuels DNA repair and radioresistance in prostate cancer. Cancer Discov. 2013, 3, 1222–1224. [Google Scholar] [CrossRef]
- Jackson, W.C.; Suresh, K.; Tumati, V.; Allen, S.G.; Dess, R.T.; Salami, S.S.; George, A.; Kaffenberger, S.D.; Miller, D.C.; Hearn, J.W.D.; et al. Intermediate Endpoints After Postprostatectomy Radiotherapy: 5-Year Distant Metastasis to Predict Overall Survival. Eur. Urol. 2018, 74, 413–419. [Google Scholar] [CrossRef]
- Grochtdreis, T.; König, H.H.; Dobruschkin, A.; von Amsberg, G.; Dams, J. Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: A systematic review. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Parikh, N.R.; Chang, E.M.; Nickols, N.G.; Rettig, M.; Raldow, A.; King, C.R.; Steinberg, M.L.; Feng, F.Y.; Spratt, D.E.; Tran, P.T.; et al. Cost-Effectiveness of Metastasis-Directed Therapy in Oligorecurrent Hormone-Sensitive Prostate Cancer. Int. J. Radiat. Oncol. 2019, 105, S100. [Google Scholar] [CrossRef]
- Fossati, N.; Suardi, N.; Gandaglia, G.; Bravi, C.A.; Soligo, M.; Karnes, R.J.; Shariat, S.; Battaglia, A.; Everaerts, W.; Joniau, S.; et al. Identifying the Optimal Candidate for Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer: Results from a Large, Multi-institutional Analysis. Eur. Urol. 2019, 75, 176–183. [Google Scholar] [CrossRef]
- Decaestecker, K.; De Meerleer, G.; Lambert, B.; Delrue, L.; Fonteyne, V.; Claeys, T.; De Vos, F.; Huysse, W.; Hautekiet, A.; Maes, G.; et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat. Oncol. 2014, 9. [Google Scholar] [CrossRef]
- De Bleser, E.; Jereczek-Fossa, B.A.; Pasquier, D.; Zilli, T.; Van As, N.; Siva, S.; Fodor, A.; Dirix, P.; Gomez-Iturriaga, A.; Trippa, F.; et al. Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur. Urol. 2019, 76, 732–739. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Goetghebeur, E.; et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J. Clin. Oncol. 2020, 38, 10–100. [Google Scholar] [CrossRef]
- Bravi, C.A.; Fossati, N.; Gandaglia, G.; Suardi, N.; Mazzone, E.; Robesti, D.; Osmonov, D.; Juenemann, K.-P.; Boeri, L.; Jeffrey Karnes, R.; et al. Long-term Outcomes of Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Not as Good as Previously Thought. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Fonteyne, V.; De Gersem, W.; De Neve, W.; Jacobs, F.; Lumen, N.; Vandecasteele, K.; Villeirs, G.; De Meerleer, G. Hypofractionated Intensity-Modulated Arc Therapy for Lymph Node Metastasized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1013–1020. [Google Scholar] [CrossRef]
- Draulans, C.; Joniau, S.; Fonteyne, V.; Delrue, L.; Decaestecker, K.; Everaerts, W.; Dirix, P.; Van den Bergh, L.; Crijns, W.; Vandendriessche, H.; et al. Benefits of Elective Para-Aortic Radiotherapy for pN1 Prostate Cancer Using Arc Therapy (Intensity-Modulated or Volumetric Modulated Arc Therapy): Protocol for a Nonrandomized Phase II Trial. JMIR Res. Protoc. 2018, 7, e11256. [Google Scholar] [CrossRef]
- Ost, P.; De Meerleer, G.; De Gersem, W.; Impens, A.; De Neve, W. Analysis of prostate bed motion using daily cone-beam computed tomography during postprostatectomy radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 188–194. [Google Scholar] [CrossRef]
- Devos, G.; Muilwijk, T.; Raskin, Y.; Calderon, V.; Moris, L.; Van den Broeck, T.; Berghen, C.; De Meerleer, G.; Albersen, M.; Van Poppel, H.; et al. Comparison of Peri-operative and Early Oncological Outcomes of Robot-Assisted vs. Open Salvage Lymph Node Dissection in Recurrent Prostate Cancer. Front. Oncol. 2019, 9, 781. [Google Scholar] [CrossRef]
- Battaglia, A.; Devos, G.; Decaestecker, K.; Witters, M.; Moris, L.; Van den Broeck, T.; Berghen, C.; Everaerts, W.; Albersen, M.; Tsaturyan, A.; et al. Metastasectomy for visceral and skeletal oligorecurrent prostate cancer. World J. Urol. 2019, 37, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]

| Median Age at Primary Treatment in Years (IQR; Mean) | 61.3 (56–66; 60.9) |
| Median PSA at time of RP (IQR, ng/mL) | 9.2 (6.4–14.7) |
| <20 ng/mL | 158 (82.7%) |
| ≥20 ng/mL | 25 (13.1%) |
| NA | 8 (4.2%) |
| Technique | |
| Open | 132 (69.1%) |
| Laparoscopy | 13 (24.1%) |
| Robot | 46 (6.8%) |
| Concomitant pelvic LN dissection during RP | |
| Yes | 128 (67%) |
| No | 63 (33%) |
| Median number of LN removed during pelvic LN dissection (IQR) | 11 (6–20) |
| Pathological N-stage | |
| pN1 | 37 (19.3%) |
| Pathological ISUP (%) | |
| 1 | 8 (4.2%) |
| 2 | 42 (22%) |
| 3 | 54 (28.3%) |
| 4 | 43 (22.5%) |
| 5 | 37 (19.4%) |
| NA | 7 (3.6%) |
| Pathological T-stage | |
| pT2 | 68 (35.6%) |
| pT3a | 57 (29.8%) |
| pT3b | 59 (30.9%) |
| pT4 | 2 (1%) |
| NA | 5 (2.6%) |
| Positive section margins | |
| R0 | 118 (61.8%) |
| R1 | 65 (34%) |
| NA | 8 (4.2%) |
| Adjuvant/salvage RT following RP | |
| No adjuvant/salvage RT | 66 (34.6%) |
| Adjuvant RT | 15 (7.9%) |
| Salvage RT | 110 (57.6%) |
| Template adjuvant/salvage RT | |
| Prostate bed | 90 (47.1%) |
| Prostate bed + pelvic LN region | 35 (18.3%) |
| Concomitant ADT during adjuvant/salvage RT | 24 (12.6%) |
| Median Age at MDT in Years (IQR; Mean) | 66.9 (63–71.8; 67) |
| Median time from positive imaging to MDT (months, IQR) | 2.6 (1.7–4.7) |
| Number of lesions on imaging (%) | |
| 1–3 | 171 (89.6%) |
| 4–5 | 20 (10.4%) |
| Type of imaging used (%) | |
| 11C-choline PET/CT | 60 (31.4%) |
| 68Ga- or 18F-PSMA PET/CT | 113 (59.2%) |
| Bone scan and/or CT-scan | 18 (9.4%) |
| Type of recurrence (patient-based): | |
| N1 * | 101 (52.9%) |
| M1a | 34 (17.8%) |
| M1b | 45 (23.6%) |
| M1c | 11 (5.8%) |
| Location of lesions on imaging (lesion-based analysis) | n = 350 |
| Lymph nodes | 270 |
| Internal iliac | 29 |
| Obturator | 35 |
| External iliac | 65 |
| Common iliac | 49 |
| Presacral | 24 |
| Perirectal | 11 |
| Retroperitoneal | 46 |
| inguinal | 7 |
| Mediastinal | 4 |
| Supraclavicular | 0 |
| Skeletal | 69 |
| Appendicular | 5 |
| Axial | 64 |
| Visceral | 11 |
| Any ADT prior to first MDT (e.g., during adjuvant/salvage RT)(%) | |
| Yes | 60 (31.4%) |
| Bicalutamide | 22 |
| LHRH agonist/antagonist | 35 |
| NA | 3 |
| No | 131 (68.6%) |
| Type of first MDT (%) | |
| Radiotherapy-based | 81 (42.4%) |
| Elective field radiation | 31 |
| SBRT | 50 |
| Metastasectomy | 10 (5.2%) |
| sLND | 99 (51.8%) |
| Adjuvant RT | 11 |
| Salvage RT | 21 |
| Combination: SBRT + sLND | 1 (0.5%) |
| Median PSA at time of first MDT (IQR, ng/mL) | 1.4 (0.6–3.4) |
| Salvage Lymphadenectomy (n = 100) | |
| Median PSA at time of sLND (IQR, ng/mL) | 1.4 (0.6–3) |
| ASA classification at time of sLND | |
| 1–2 | 69 (69%) |
| 3–4 | 16 (16%) |
| NA | 15 (15%) |
| Median BMI preoperative (IQR) | 26 (24–30) |
| Median operation time (min, IQR) | 150 (120–190) |
| Median blood loss (mL, IQR) | 200 (100–450) |
| Hospital stay (IQR, days) | 5 (2–7) |
| Technique | |
| Open | 65 (65%) |
| Robot | 33 (33%) |
| Laparoscopy | 2 (2%) |
| Template | |
| Pelvic sLND | 63 (63%) |
| Retroperitoneal sLND | 8 (8%) |
| Pelvic + retroperitoneal sLND | 29 (29%) |
| Median number of LN removed (IQR) | 19 (11–28) |
| Median of positive LN at final pathology (IQR) | 2 (1–5) |
| Adjuvant/salvage RT following sLND | |
| Yes | 32 (32%) |
| Concomitant ADT | 31 |
| Template pelvis | 17 |
| Template Pelvis + retroperitoneal LN | 15 |
| No | 68 (68%) |
| Radiotherapy (n = 82) | |
| Median PSA at time of RT (IQR, ng/mL) | 1.3 (0.45–3.95) |
| Type of RT | |
| SBRT | 51 (62.2%) |
| Concomitant ADT | 41 |
| Median months concomitant ADT (IQR) | 1 (1–1) |
| Elective field radiation | 31 (37.8%) |
| Concomitant ADT | 24 |
| Median months concomitant ADT (IQR) | 18 (6–24) |
| Metastasectomy (n = 10) | |
| Median PSA at time of metastasectomy (IQR, ng/mL) | 3.4 (1.3–4.9) |
| ASA-classification at time of metastasectomy | |
| 1 | 2 (20%) |
| 2 | 7 (70%) |
| NA | 1 (10%) |
| Median BMI preoperative | 28 (25–29) |
| Type of metastasectomy | |
| Lung | 6 |
| Rectum | 1 |
| Testis | 1 |
| Liver | 1 |
| Adrenal gland | 1 |
| Median hospital stay (IQR, days) | 4 (2–7) |
| Variable | Univariate HR (95% CI); p-Value | Multivariate HR (95% CI); p-Value |
|---|---|---|
| Number of lesions | ||
| 4–5 lesions vs. 1–3 lesions | 1.89 (0,89–4); 0.09 | / |
| Type of recurrence | ||
| M1a-b-c vs. N1 | 1.81 (1.09–2.98); 0.019 | 1.88 (0.94–3.8); 0.07 |
| PSA at time of sLND | 1.08 (1.02–1.14); 0.006 | 1.04 (0.97–1.12); 0.21 |
| pT-stage | ||
| pT3b-T4 vs. T2-T3a | 1.5 (0.9–2.5); 0.11 | / |
| pN1 at RP | 2.26 (1.2–4.3); 0.01 | 1.44 (0.7–2.9); 0.31 |
| Pathological ISUP grade group | ||
| ISUP 5 vs. ISUP ≤4 | 2.26 (1.32–3.8); 0.0028 | 2.6 (1.4–5.1); 0.0029 |
| Imaging technique prior to MDT | ||
| No PSMA PET/CT vs. PSMA PET/CT | 1.32 (1.76–2.3); 0.31 | / |
| Time from primary treatment until first MDT | 1 (0.99–1.01); 0.2 | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devos, G.; Berghen, C.; Van Eecke, H.; Stichele, A.V.; Van Poppel, H.; Goffin, K.; Mai, C.; De Wever, L.; Albersen, M.; Everaerts, W.; et al. Oncological Outcomes of Metastasis-Directed Therapy in Oligorecurrent Prostate Cancer Patients Following Radical Prostatectomy. Cancers 2020, 12, 2271. https://doi.org/10.3390/cancers12082271
Devos G, Berghen C, Van Eecke H, Stichele AV, Van Poppel H, Goffin K, Mai C, De Wever L, Albersen M, Everaerts W, et al. Oncological Outcomes of Metastasis-Directed Therapy in Oligorecurrent Prostate Cancer Patients Following Radical Prostatectomy. Cancers. 2020; 12(8):2271. https://doi.org/10.3390/cancers12082271
Chicago/Turabian StyleDevos, Gaëtan, Charlien Berghen, Henri Van Eecke, Arthur Vander Stichele, Hendrik Van Poppel, Karolien Goffin, Cindy Mai, Liesbeth De Wever, Maarten Albersen, Wouter Everaerts, and et al. 2020. "Oncological Outcomes of Metastasis-Directed Therapy in Oligorecurrent Prostate Cancer Patients Following Radical Prostatectomy" Cancers 12, no. 8: 2271. https://doi.org/10.3390/cancers12082271
APA StyleDevos, G., Berghen, C., Van Eecke, H., Stichele, A. V., Van Poppel, H., Goffin, K., Mai, C., De Wever, L., Albersen, M., Everaerts, W., De Meerleer, G., & Joniau, S. (2020). Oncological Outcomes of Metastasis-Directed Therapy in Oligorecurrent Prostate Cancer Patients Following Radical Prostatectomy. Cancers, 12(8), 2271. https://doi.org/10.3390/cancers12082271







