Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
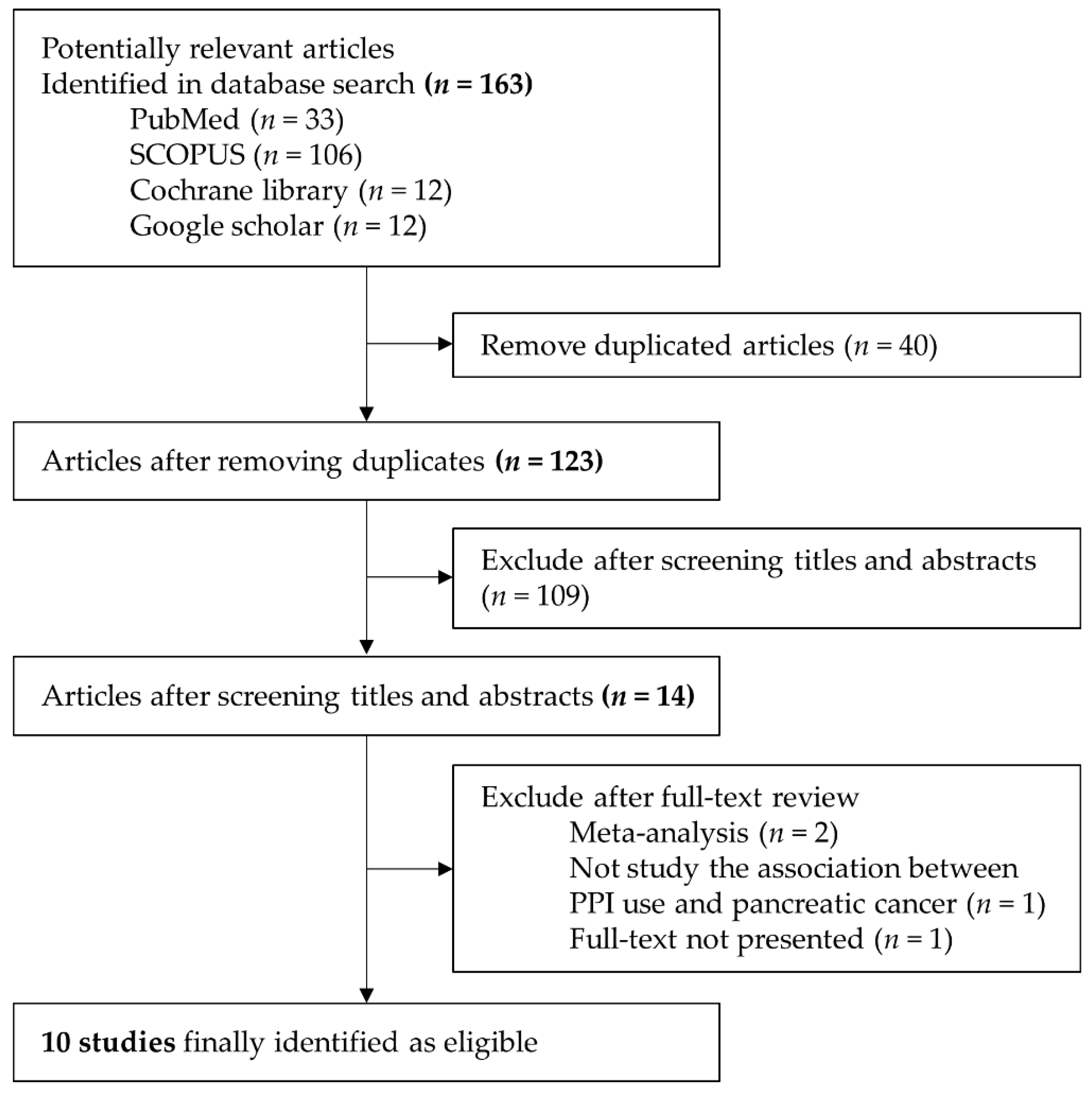
3.1. Selected Studies
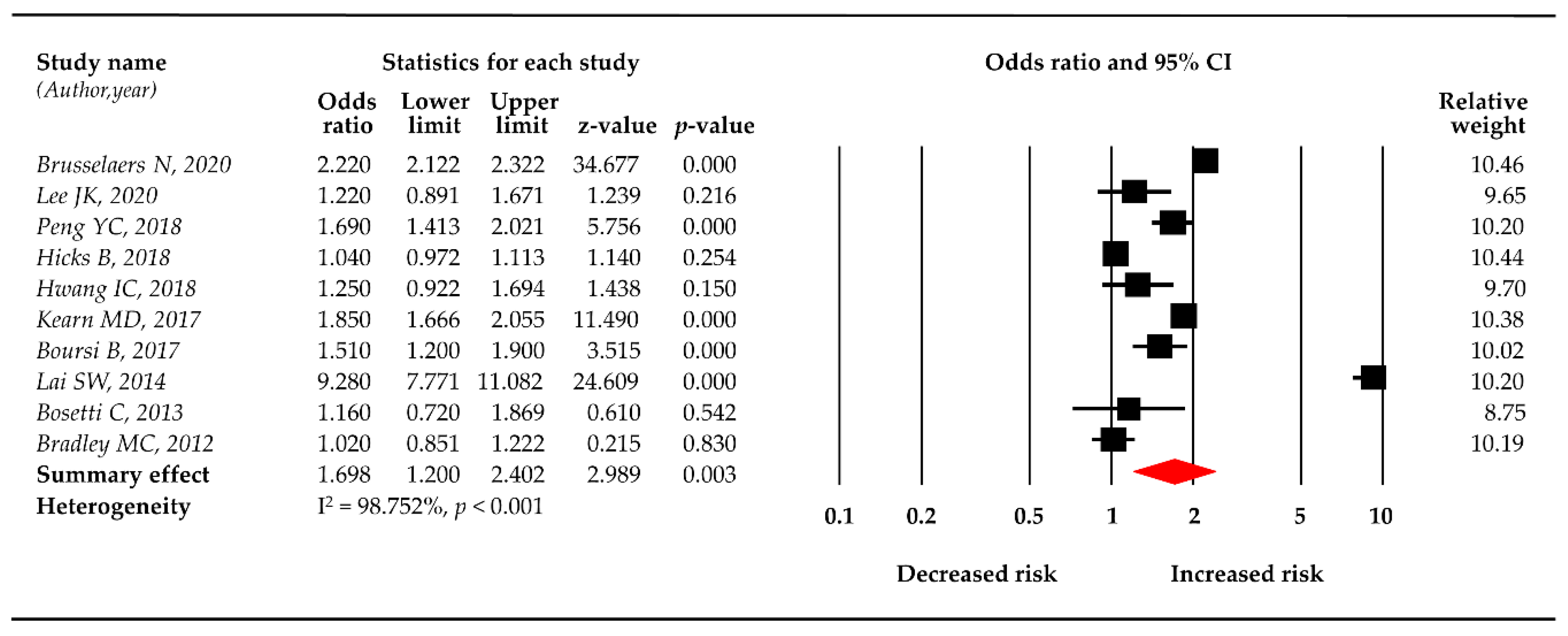
3.2. PPI Use and the Risk of Pancreatic Cancer
3.3. Sensitivity Analysis
3.4. Subgroup Analysis
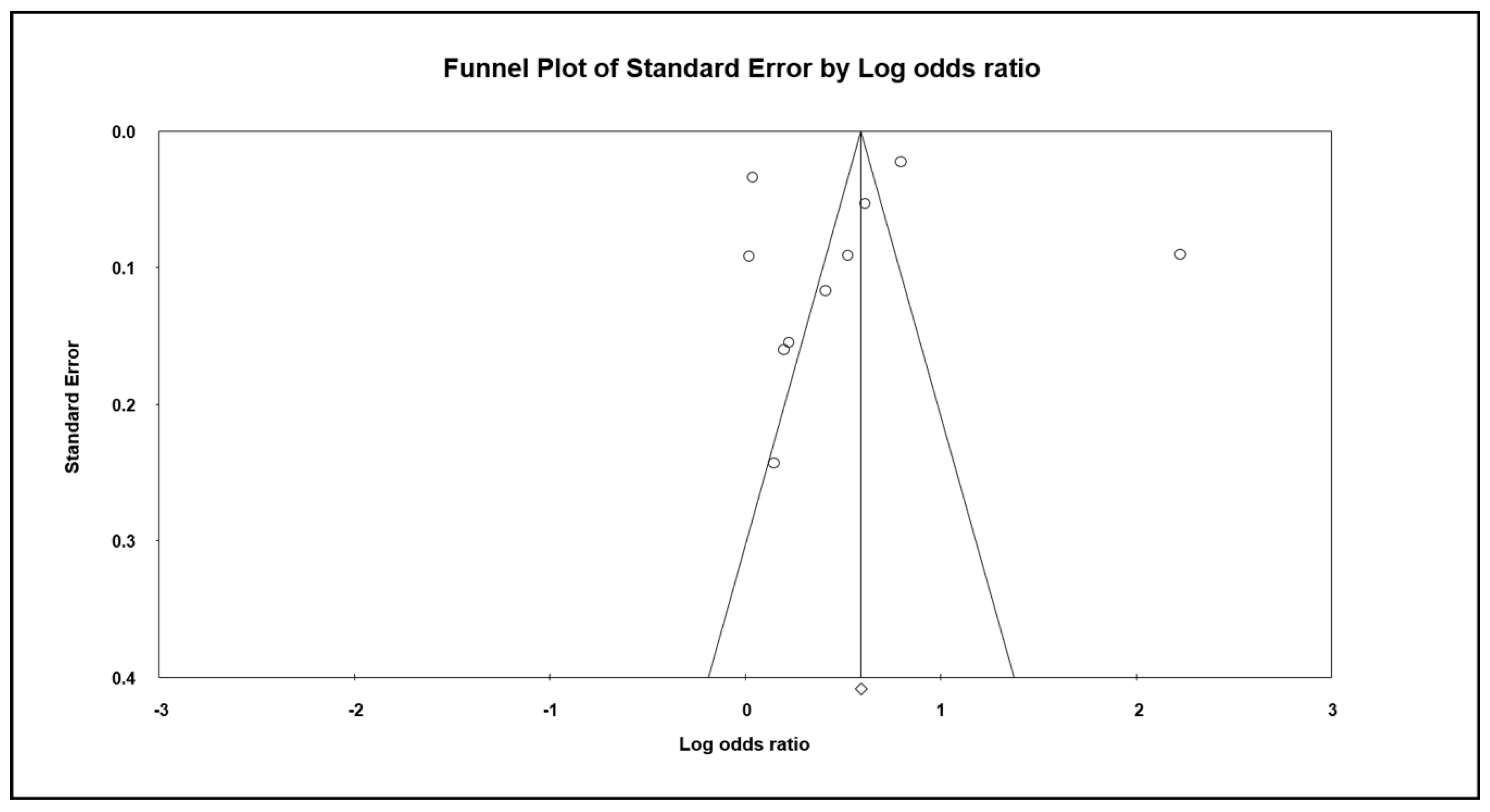
3.5. Publication Bias
4. Discussion
4.1. Increased Production of Gastrin
4.2. Bacterial Overgrowth and Nitrosamine
4.3. The Biological Link Between PPI Use and Other Cancers
4.4. Data Interpretation
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer Statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, P.; Hoorn, S.V.; Christophi, C.; Nikfarjam, M. Association of Diabetes Mellitus and Pancreatic Adenocarcinoma: A Meta-Analysis of 88 Studies. Ann. Surg. Oncol. 2014, 21, 2453–2462. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Pergolini, I.; Sahora, K.; Ferrone, C.R.; Morales-Oyarvide, V.; Wolpin, B.M.; Mucci, L.A.; Brugge, W.R.; Mino-Kenudson, M.; Patino, M.; Sahani, D.V.; et al. Long-Term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017, 153, 1284–1294.e1. [Google Scholar] [CrossRef]
- Iodice, S.; Gandini, S.; Maisonneuve, P.; Lowenfels, A.B. Tobacco and the Risk of Pancreatic Cancer: A Review and Meta-Analysis. Langenbeck’s Arch. Surg. 2008, 393, 535–545. [Google Scholar] [CrossRef]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Smith, G.D.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Fuchs, C.S. Physical Activity, Obesity, Height, and the Risk of Pancreatic Cancer. JAMA 2001, 286, 921–929. [Google Scholar] [CrossRef]
- Nöthlings, U.; Wilkens, L.R.; Murphy, S.P.; Hankin, J.H.; Henderson, B.E.; Kolonel, L.N. Meat and Fat Intake as Risk Factors for Pancreatic Cancer: The Multiethnic Cohort Study. J. Natl. Cancer Inst. 2005, 97, 1458–1465. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Red and Processed Meat Consumption and Risk of Pancreatic Cancer: Meta-Analysis of Prospective Studies. Br. J. Cancer 2012, 106, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, Y.; Liu, W. Helicobacter Pylori Infection and Pancreatic Cancer Risk: A Meta-Analysis. J. Cancer Res. Ther. 2016, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Hao, N.-B.; Hu, C.-J.; Yong, X.; Lü, M.-H.; Cheng, B.-J.; Zhang, Y.; Yang, S.-M. HBV Infection Increases the Risk of Pancreatic Cancer: A Meta-Analysis. Cancer Causes Control. 2013, 24, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-H.; Fu, J.-J.; Wang, X.-L.; Zhu, J.-Y.; Ye, X.-H.; Chen, S.-D. Hepatitis B or C Viral Infection and Risk of Pancreatic Cancer: A Meta-Analysis of Observational Studies. World J. Gastroenterol. 2013, 19, 4234–4241. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Proton Pump Inhibitors: An Update of Their Clinical Use and Pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef]
- Sheen, E.; Triadafilopoulos, G. Adverse Effects of Long-Term Proton Pump Inhibitor Therapy. Dig. Dis. Sci. 2011, 56, 931–950. [Google Scholar] [CrossRef]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiriac, S.A.; Ciobica, A.; Boiculese, L. Proton Pump Inhibitors Therapy and Risk of Clostridium Difficile Infection: Systematic Review and Meta-Analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef]
- Nassar, Y.; Richter, S. Proton-pump Inhibitor Use and Fracture Risk: An Updated Systematic Review and Meta-analysis. J. Bone Metab. 2018, 25, 141–151. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Association of Long-Term Proton Pump Inhibitor Therapy with Bone Fractures and Effects on Absorption of Calcium, Vitamin B12, Iron, and Magnesium. Curr. Gastroenterol. Rep. 2010, 12, 448–457. [Google Scholar] [CrossRef]
- Danziger, J.; William, J.H.; Scott, D.J.; Lee, J.; Lehman, L.-W.; Mark, R.G.; Howell, M.D.; Celi, L.A.; Mukamal, K.J. Proton-Pump Inhibitor Use Is Associated with Low Serum Magnesium Concentrations. Kidney Int. 2013, 83, 692–699. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Quesenberry, C.P.; A Corley, D. Proton Pump Inhibitor and Histamine-2 Receptor Antagonist Use and Iron Deficiency. Gastroenterology 2017, 152, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; A Corley, D. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B 12 Deficiency. JAMA 2013, 310, 2435. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.L.; Fixen, D.R.; Linnebur, S.A. Adverse Effects of Proton-Pump Inhibitor Use in Older Adults: A Review of the Evidence. Ther. Adv. Drug Saf. 2017, 8, 273–297. [Google Scholar] [CrossRef]
- Ahn, J.S.; Eom, C.-S.; Jeon, C.Y.; Park, S.M. Acid Suppressive Drugs and Gastric Cancer: A Meta-Analysis of Observational Studies. World J. Gastroenterol. 2013, 19, 2560–2568. [Google Scholar] [CrossRef]
- Shao, Y.-H.J.; Chan, T.-S.; Tsai, K.; Wu, S.-Y. Association between Proton Pump Inhibitors and the Risk of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 460–468. [Google Scholar] [CrossRef]
- Cheung, K.-S.; Leung, W.K. Long-Term Use of Proton-Pump Inhibitors and Risk of Gastric Cancer: A Review of the Current Evidence. Ther. Adv. Gastroenterol. 2019, 12, 1756284819834511. [Google Scholar] [CrossRef]
- Song, H.J.; Jiang, X.; Henry, L.; Nguyen, M.H.; Park, H. Proton Pump Inhibitors and Risk of Liver Cancer and Mortality in Patients with Chronic Liver Disease: A Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2020, 76, 851–866. [Google Scholar] [CrossRef]
- Ahn, J.S.; Park, S.M.; Eom, C.S.; Kim, S.; Myung, S.-K. Use of Proton Pump Inhibitor and Risk of Colorectal Cancer: A Meta-analysis of Observational Studies. Korean J. Fam. Med. 2012, 33, 272–279. [Google Scholar] [CrossRef]
- Lai, S.-W.; Sung, F.-C.; Lin, C.-L.; Liao, K.-F. Use of Proton Pump Inhibitors Correlates with Increased Risk of Pancreatic Cancer: A Case-Control Study in Taiwan. Kuwait Med. J. 2014, 46, 44–48. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.G.; Stewart, L. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Merchant, S.A.; Schneider, J.L.; Jensen, C.D.; Fireman, B.H.; Quesenberry, C.P.; Corley, D.A. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am. J. Gastroenterol. 2020, 115, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-C.; Lin, C.-L.; Hsu, W.-Y.; Lu, I.-T.; Yeh, H.-Z.; Chang, C.-S.; Kao, C.-H. Proton Pump Inhibitor Use Is Associated with Risk of Pancreatic Cancer: A Nested Case-Control Study. Dose-Response 2018, 16, 1559325818803283. [Google Scholar] [CrossRef] [PubMed]
- Hicks, B.M.; Friis, S.; Pottegård, A. Use of Proton Pump Inhibitors and Risk of Pancreatic Cancer. Pharmacoepidemiol. Drug Saf. 2018, 27, 926–930. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Park, S. Association between Proton Pump Inhibitor Use and the Risk of Pancreatic Cancer: A Korean Nationwide Cohort Study. PLoS ONE 2018, 13, e0203918. [Google Scholar] [CrossRef]
- Bradley, M.C.; Murray, L.J.; Cantwell, M.M.; Hughes, C.M. Proton Pump Inhibitors and Histamine-2-Receptor Antagonists and Pancreatic Cancer Risk: A Nested Case–Control Study. Br. J. Cancer 2011, 106, 233–239. [Google Scholar] [CrossRef]
- Kearns, M.D.; Boursi, B.; Yang, Y.-X. Proton Pump Inhibitors on Pancreatic Cancer Risk and Survival. Cancer Epidemiol. 2017, 46, 80–84. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by A Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Boursi, B.; Finkelman, B.S.; Giantonio, B.J.; Haynes, K.; Rustgi, A.K.; Rhim, A.D.; Mamtani, R.; Yang, Y.-X. A Clinical Prediction Model to Assess Risk for Pancreatic Cancer among Patients with New-Onset Diabetes. Gastroenterology 2016, 152, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Lucenteforte, E.; Bracci, P.M.; Negri, E.; Neale, R.; Risch, H.A.; Olson, S.; Gallinger, S.; Miller, A.B.; Bueno-De-Mesquita, H.B.; et al. Ulcer, Gastric Surgery and Pancreatic Cancer Risk: An Analysis from the International Pancreatic Cancer Case–Control Consortium (PanC4). Ann. Oncol. 2013, 24, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Sadr-Azodi, O.; Engstrand, L. Long-Term Proton Pump Inhibitor Usage and the Association with Pancreatic Cancer in Sweden. J. Gastroenterol. 2019, 55, 453–461. [Google Scholar] [CrossRef] [PubMed]
- A Risch, H. Etiology of Pancreatic Cancer, With a Hypothesis Concerning the Role of N-Nitroso Compounds and Excess Gastric Acidity. J. Natl. Cancer Inst. 2003, 95, 948–960. [Google Scholar] [CrossRef]
- Waldum, H.L.; Sordal, O.; Mjones, P.G. The Enterochromaffin-Like [ECL] Cell-Central in Gastric Physiology and Pathology. Int. J. Mol. Sci. 2019, 20, 2444. [Google Scholar] [CrossRef]
- Noble, P.J.M.; Wilde, G.; White, M.R.H.; Pennington, S.; Dockray, G.J.; Varro, A. Stimulation of Gastrin-CCKB Receptor Promotes Migration of Gastric AGS Cells via Multiple Paracrine Pathways. Am. J. Physiol. Liver Physiol. 2003, 284, G75–G84. [Google Scholar] [CrossRef][Green Version]
- Thorburn, C.M.; Friedman, G.D.; Dickinson, C.J.; Vogelman, J.H.; Orentreich, N.; Parsonnet, J. Gastrin and Colorectal Cancer: A Prospective Study. Gastroenterology 1998, 115, 275–280. [Google Scholar] [CrossRef]
- Chao, C.; Hellmich, M.R. Gastrin, Inflammation, and Carcinogenesis. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 33–39. [Google Scholar] [CrossRef]
- Klinkenberg-Knol, E.C.; Festen, H.P.M.; Jansen, J.B.M.J.; Lamers, C.B.H.W.; Nelis, F.; Snel, P.; Luckers, A.; Dekkers, C.P.M.; Havu, N.; Meuwissen, S.G.M. Long-Term Treatment with Omeprazole for Refractory Reflux Esophagitis: Efficacy and Safety. Ann. Intern. Med. 1994, 121, 161–167. [Google Scholar] [CrossRef]
- Smith, J.P.; Liu, G.; Soundararajan, V.; McLaughlin, P.J.; Zagon, I.S. Identification and Characterization of CCK-B/Gastrin Receptors in Human Pancreatic Cancer Cell Lines. Am. J. Physiol. Integr. Comp. Physiol. 1994, 266, R277–R283. [Google Scholar] [CrossRef]
- Smith, J.P.; Fantaskey, A.P.; Liu, G.; Zagon, I.S. Identification of Gastrin as a Growth Peptide in Human Pancreatic Cancer. Am. J. Physiol. Integr. Comp. Physiol. 1995, 268, R135–R141. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, A.; Wang, T.C. Gastrin and Cancer: A Review. Cancer Lett. 2006, 238, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Fonkoua, L.K.; Moody, T.W. The Role of Gastrin and CCK Receptors in Pancreatic Cancer and other Malignancies. Int. J. Boil. Sci. 2016, 12, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Cunningham, D.; Russell, C.; Norman, A.R.; Kurzawinski, T.; Harper, P.; Harrison, P.; Middleton, G.; Daniels, F.; Hickish, T.; et al. Gastrazole (JB95008), a Novel CCK2/Gastrin Receptor Antagonist, in the Treatment of Advanced Pancreatic Cancer: Results from Two Randomised Controlled Trials. Br. J. Cancer 2006, 94, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.T.; Smith, S.C.; Bouvier, C.V.; Michaeli, D.; Hochhauser, D.; Davidson, B.R.; Kurzawinski, T.R.; Watkinson, A.F.; Van Someren, N.; Pounder, R.E.; et al. Phase II Study of Anti–Gastrin-17 Antibodies, Raised to G17DT, in Advanced Pancreatic Cancer. J. Clin. Oncol. 2002, 20, 4225–4231. [Google Scholar] [CrossRef]
- Gilliam, A.D.; Broome, P.; Topuzov, E.G.; Garin, A.M.; Pulay, I.; Humphreys, J.; Whitehead, A.; Takhar, A.; Rowlands, B.J.; Beckingham, I.J. An International Multicenter Randomized Controlled Trial of G17DT in Patients With Pancreatic Cancer. Pancreas 2012, 41, 374–379. [Google Scholar] [CrossRef]
- Todisco, A. Molecular Mechanisms for the Growth Factor Action of Gastrin. J. Gastroenterol. 2000, 35 (Suppl. S12), 57–64. [Google Scholar] [CrossRef]
- Ferrand, A.; Kowalski-Chauvel, A.; Bertrand, C.; Escrieut, C.; Mathieu, A.; Portolan, G.; Pradayrol, L.; Fourmy, D.; Dufresne, M.; Seva, C. A Novel Mechanism for JAK2 Activation by a G Protein-coupled Receptor, the CCK2R. J. Boil. Chem. 2005, 280, 10710–10715. [Google Scholar] [CrossRef]
- Daulhac, L.; Kowalski-Chauvel, A.; Pradayrol, L.; Vaysse, N.; Seva, C. Gastrin Stimulates the Formation of a p60Src/p125FAK Complex Upstream of the Phosphatidylinositol 3-Kinase Signaling Pathway. FEBS Lett. 1999, 445, 251–255. [Google Scholar] [CrossRef]
- Cayrol, C.; Bertrand, C.; Kowalski-Chauvel, A.; Daulhac, L.; Cohen-Jonathan-Moyal, E.; Ferrand, A.; Seva, C. αV Integrin: A New Gastrin Target in Human Pancreatic Cancer Cells. World J. Gastroenterol. 2011, 17, 4488–4495. [Google Scholar] [CrossRef]
- Mirvish, S.S. Role of N-Nitroso Compounds (NOC) and N-Nitrosation in Etiology of Gastric, Esophageal, Nasopharyngeal and Bladder Cancer and Contribution to Cancer of Known Exposures to NOC. Cancer Lett. 1995, 93, 17–48. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Bingham, S. Dietary Meat, Endogenous Nitrosation and Colorectal Cancer. Biochem. Soc. Trans. 2007, 35, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Dallinga, J.W.; Pachen, D.; Lousberg, A.; Van Geel, J.; Houben, G.; Stockbrügger, R.W.; Van Maanen, J.; Kleinjans, J. Volatile N-Nitrosamines in Gastric Juice of Patients with Various Conditions of the Gastrointestinal Tract Determined by Gas Chromatography–Mass Spectrometry and Related to Intragastric pH and Nitrate and Nitrite Levels. Cancer Lett. 1998, 124, 119–125. [Google Scholar] [CrossRef]
- Xu, G.; Reed, P. N-Nitroso Compounds in Fresh Gastric Juice and Their Relation to Intragastric pH and Nitrite Employing An Improved Analytical Method. Carcinogenesis 1993, 14, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A. Pancreatic Cancer: Helicobacter Pylori Colonization, N-Nitrosamine Exposures, and ABO Blood Group. Mol. Carcinog. 2011, 51, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, N.; Brimblecombe, R.W.; Elder, J.; Heatley, R.V.; Misiewicz, J.J.; Northfield, T.C.; Pottage, A. Effects of Acid Suppression on Microbial Flora of Upper Gut. Dig. Dis. Sci. 1995, 40, 81S–95S. [Google Scholar] [CrossRef] [PubMed]
- Parsa, I.; Marsh, W.H.; Sutton, A.L. An In Vitro Model of Human Pancreas Carcinogenesis: Effects of Nitroso Compounds. Cancer 1981, 47, 1543–1551. [Google Scholar] [CrossRef]
- Kokkinakis, D.M.; Scarpelli, D.G. DNA Alkylation in the Hamster Induced by Two Pancreatic Carcinogens. Cancer Res. 1989, 49, 3184–3189. [Google Scholar]
- Kokkinakis, D.M.; Subbarao, V. The Significance of DNA Damage, Its Repair and Cell Proliferation during Carcinogen Treatment in the Initiation of Pancreatic Cancer in the Hamster Model. Cancer Res. 1993, 53, 2790–2795. [Google Scholar]
- Howatson, A.G.; Carter, D.C. Pancreatic Carcinogenesis: Effect of Secretin in the Hamster-Nitrosamine Model2. J. Natl. Cancer Inst. 1987, 78, 101–105. [Google Scholar] [CrossRef]
- Laine, L.; Ahnen, D.; McClain, C.; Solcia, E.; Walsh, J.H. Review Article: Potential Gastrointestinal Effects of Long-Term Acid Suppression with Proton Pump Inhibitors. Aliment. Pharmacol. Ther. 2000, 14, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Chang, W.; Jin, G.; Wang, T.C. Gastrin and Upper GI Cancers. Curr. Opin. Pharmacol. 2016, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Nadella, S.; Osborne, N. Gastrin and Gastric Cancer. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.; Khan, K.; Savage, K.; Rode, J.; Varro, A.; Michaeli, D.; Grimes, S.; Brett, B.; Pounder, R.; Dhillon, A. Expression and Processing of Gastrin in Hepatocellular Carcinoma, Fibrolamellar Carcinoma and Cholangiocarcinoma. J. Hepatol. 1999, 30, 519–526. [Google Scholar] [CrossRef]
- Savage, K.; Waller, H.A.; Stubbs, M.; Khan, K.; Watson, S.A.; Clarke, P.A.; Grimes, S.; Michaeli, D.; Dhillon, A.P.; Caplin, M.E. Targeting of Cholecystokinin B/Gastrin Receptor in Colonic, Pancreatic and Hepatocellular Carcinoma Cell Lines. Int. J. Oncol. 2006, 29, 1429–1435. [Google Scholar] [CrossRef]
- Pobel, D.; Riboli, E.; Cornée, J.; Hémon, B.; Guyader, M. Nitrosamine, Nitrate and Nitrite in Relation to Gastric Cancer: A Case-Control Study in Marseille, France. Eur. J. Epidemiol. 1995, 11, 67–73. [Google Scholar] [CrossRef]
- Kromer, W.; Krüger, U.; Huber, R.; Hartmann, M.; Steinijans, V. Differences in pH-Dependent Activation Rates of Substituted Benzimidazoles and Biological In Vitro Correlates. Pharmacology 1998, 56, 57–70. [Google Scholar] [CrossRef]
- Horn, J. Review Article: Understanding the Pharmacodynamic and Pharmacokinetic Differences between Proton Pump Inhibitors—Focus on Pka and Metabolism. Aliment. Pharmacol. Ther. Symp. Ser. 2006, 2, 340–350. [Google Scholar] [CrossRef]
| Study (Author, Year) | Study Design | Country | Period of Recruitment | No. of Study Population (Case/Control) | Mean Age (Years) (Case/Control) | Percentage of Males (Case/Control) | Confounder Adjusted in the Multivariate Analysis | Quality Assessment (NOS) |
|---|---|---|---|---|---|---|---|---|
| Brusselaers N, 2020 | Cohort | Sweden | 2005–2012 | 796492 | NA | 41.5 | Age, indications for gastric acid suppressive therapy, diabetes | 8 |
| Lee JK, 2020 | Case–control | USA | 1996–2016 | 567/4870 | 67.8/67.3 | 50.6/ 51.5 | Chronic alcohol consumption, smoking, BMI, family history of each cancer, cystic fibrosis, chronic pancreatitis, diabetes mellitus, pancreatic cysts | 8 |
| Peng YC, 2018 | Case–control | Taiwan | 2006–2011 | 1087/1087 | 68.3/67.4 | 60.9/59.8 | Age, chronic pancreatitis, biliary tract disease | 6 |
| Hicks B, 2018 | Case–control | Denmark | 2000–2015 | 6921/34605 | NA | NA | Diabetes, alcohol-related disease, COPD, chronic pancreatitis, gallstones, peptic ulcer, Helicobacter pylori infection, hepatitis B and C infection, use of low-dose aspirin, NSAIDs, statins, HRT, CCI, highest achieved education | 7 |
| Hwang IC, 2018 | Cohort | Korea | 2002–2013 | 453655 | NA | 53.5 | Age, BMI, smoking, alcohol, drinking, physical activity, diabetes, chronic pancreatitis, CCI, SES | 9 |
| Kearn MD, 2017 | Nested case–control, Cohort | UK | 1995–2013 | 4113/16072 | 70.9/71.1 | 51.4/51.1 | Diabetes, smoking, alcohol, obesity | 6 |
| Boursi B, 2017 | Cohort | UK | 1995–2013 | 19146 | 62.7 | 53.6 | NA | 9 |
| Lai SW, 2014 | Case–control | Taiwan | 2000–2010 | 977/3908 | 68.38/68.11 | 60.59/60.59 | Acute pancreatitis, chronic pancreatitis, diabetes, obesity, H2RA, statin, non-statin lipid lowering, both ASA and COX2i | 6 |
| Bosetti C, 2013 | Case–control | USA, Canada, Australia | 56/51 | NA | 56.5/56.6 | NA | 5 | |
| Bradley MC, 2012 | Case–control | UK | 1995–2006 | 1141/7954 | 57.3 | 533.7 | Smoking, BMI, alcohol, history of chronic pancreatitis, use of other drugs (NSAIDs, steroids, HRT), diabetes, prior cancer | 7 |
| Excluded Study (Author,year) | Observed OR | Effect Size and 95% Confidence Interval | Test of Null (Two-Tailed) | |||
|---|---|---|---|---|---|---|
| Mean OR without This Study | Lower Limit | Upper Limit | z-Value | p-Value | ||
| Brusselaers N, 2020 | 2.220 | 1.641 | 1.049 | 2.567 | 2.171 | 0.030 |
| Lee JK, 2020 | 1.220 | 1.759 | 1.217 | 2.540 | 3.008 | 0.003 |
| Peng YC, 2018 | 1.690 | 1.698 | 1.163 | 2.479 | 2.740 | 0.006 |
| Hicks B, 2018 | 1.040 | 1.754 | 1.214 | 2.535 | 2.992 | 0.003 |
| Hwang IC, 2018 | 1.250 | 1.800 | 1.273 | 2.545 | 3.328 | 0.001 |
| Kearn MD, 2017 | 1.850 | 1.678 | 1.120 | 2.516 | 2.507 | 0.012 |
| Boursi B, 2017 | 1.510 | 1.720 | 1.184 | 2.497 | 2.849 | 0.004 |
| Lai SW, 2014 | 9.280 | 1.405 | 1.062 | 1.859 | 2.379 | 0.017 |
| Bosetti C, 2013 | 1.160 | 1.761 | 2.223 | 2.535 | 3.043 | 0.002 |
| Bradley MC, 2012 | 1.020 | 1.799 | 1.245 | 2.600 | 3.125 | 0.002 |
| Subgroup | No. of Studies | Effect Size and 95% Confidence Interval | Test of Null (Two-Tailed) | |||
|---|---|---|---|---|---|---|
| OR | Lower Limit | Upper Limit | z-Value | p-Value | ||
| Study design | ||||||
| Case–control | 7 | 1.725 | 1.005 | 2.959 | 1.978 | 0.048 |
| Cohort | 3 | 1.647 | 1.134 | 2.392 | 2.620 | 0.009 |
| Quality of study | ||||||
| High (NOS > 7) | 4 | 1.534 | 1.081 | 2.176 | 2.394 | 0.017 |
| Low (NOS ≤ 7) | 6 | 1.824 | 1.005 | 3.312 | 1.975 | 0.048 |
| Countries | ||||||
| Asia | 3 | 2.705 | 0.751 | 9.746 | 1.522 | 0.128 |
| Western | 7 | 1.388 | 0.996 | 1.934 | 1.934 | 0.053 |
| Type of drugs | ||||||
| Omeprazole | 3 | 2.113 | 0.697 | 6.411 | 1.322 | 0.186 |
| Pantoprazole | 3 | 2.524 | 0.484 | 13.156 | 1.099 | 0.272 |
| Lansoprazole | 3 | 2.985 | 0.771 | 11.556 | 1.584 | 0.113 |
| Rabeprazole | 2 | 5.401 | 1.984 | 14.703 | 3.301 | 0.001 |
| Esomeprazole | 3 | 2.583 | 0.475 | 14.056 | 1.098 | 0.272 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.-E.; Kim, A.-S.; Kim, M.-R.; Ko, H.-J.; Jung, M.K. Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies. Cancers 2020, 12, 2220. https://doi.org/10.3390/cancers12082220
Hong H-E, Kim A-S, Kim M-R, Ko H-J, Jung MK. Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies. Cancers. 2020; 12(8):2220. https://doi.org/10.3390/cancers12082220
Chicago/Turabian StyleHong, Hee-Eun, A-Sol Kim, Mi-Rae Kim, Hae-Jin Ko, and Min Kyu Jung. 2020. "Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies" Cancers 12, no. 8: 2220. https://doi.org/10.3390/cancers12082220
APA StyleHong, H.-E., Kim, A.-S., Kim, M.-R., Ko, H.-J., & Jung, M. K. (2020). Does the Use of Proton Pump Inhibitors Increase the Risk of Pancreatic Cancer? A Systematic Review and Meta-Analysis of Epidemiologic Studies. Cancers, 12(8), 2220. https://doi.org/10.3390/cancers12082220





