Immunoradiotherapy as an Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent
Abstract
1. Introduction
2. The Rationale for the Use of RT
3. Immunotherapy in Lung Cancer
4. The Role of Radiation in the Immune Response to Cancer
5. Immunoradiotherapy in Metastatic Lung Cancer
5.1. How did the Preclinical Evidence for the Combination of Ici and Rt Translate into the Clinical Setting?
5.2. Can This Retrospective Data Be Replicated in Clinical Trials?
| Author/Trial | Phase | N | Treatment Arms | ORR (%) | Median PFS (months) | Median OS (months) | irAEs ≥ G 3 (%) |
|---|---|---|---|---|---|---|---|
| Theelen et al. [57]/PEMBRO-RT | II randomized | 76 | SABR + pembrolizumab Pembrolizumab | 36 vs. 18 | 6.6 vs. 1.9 | 15.6 vs. 7.6 | 11 |
| Welsh et al. [55] | II randomized | 72 | SABR/Conventional RT + pembrolizumab Pembrolizumab | 22 vs. 25 * | 10.9 vs. 8.4 * | NR | 15 |
| Patel JD et al. [59] COSINR | I randomized | 35 | SABR + concurrent ipilimumab/nivolumab SABR + sequential ipilimumab/nivolumab | 68 (total) | 6.2 vs. 5.9 | NR | 11 |
| Bauml et al. [60] | II | 45 | Locally ablative therapy (surgery/SABR) + pembrolizumab | NR | 19.1 | 41.6 | 10 |
| Formenti et al. [54] | I/II | 39 | SABR + ipilimumab | 31 | 7.1 | 13.0 | 10.3 |
5.3. Could the Safety Profile of the Combination Be an Issue?
6. Immunoradiotherapy in Locally Advanced NSCLC
6.1. What Is the Evidence for Administering ICI Consolidation Therapy?
6.2. Does Immunotherapy Have a Role as Part of Definitive Therapy?
| Trial | Phase | N | Stage | RT Dose (Gy) | ICI Agent | IT Sequence | ORR (%) | OS (%) | PFS | Toxicity ≥ G3 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PACIFIC [8] (Randomized) | 3 | 714 | Unresectable III | 54–66 | Durvalumab | Consolidation | 28.4% | 1-yr: 83.1 2-yr: 66.3 | Median 18.8 months | 30.5 |
| LUN 14–179 [77] | 2 | 92 | Unresectable IIIA/B | 59.4–66 | Pembrolizumab | Consolidation | NR | 1-yr: 80.5 2-yr: 68.7 | Median 15.4 months | 6.5 |
| ETOP NICOLAS [82] | 2 | 80 | Unresectable IIIA/B | 66 | Nivolumab | Concurrent + Consolidation | NR | 1-yr: 79 | 1-yr: 54% | 10.9 |
| DETERRED [83] | 2 | 40 | Unresectable III | 60–66 | Atezolizumab | Concurrent + Consolidation | NR | 1-yr: 79 | 1-yr: 57% | 27.5 |
6.3. Can immunotherapy Take the Place of Chemotherapy in Definitive Therapy?
6.4. Is the Neoadjuvant Setting a Good Fit for Immunoradiotherapy?
7. Early Stage NSCLC and Small-Cell Lung Cancer: Future Directions for Immunoradiotherapy
8. Optimizing the Efficacy of Immunoradiotherapy in Lung Cancer
8.1. RT Fractionation and ICI Agent
8.2. RT and ICI Sequence
8.3. Number of Irradiated Lesions and Tumor Location
8.4. Biomarkers
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Stadistics 2017. Ca Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Choi, J.L.; Simone, C.B., 2nd. Stereotactic body radiation therapy versus surgery for early stage non-small cell lung cancer: Clearing a path through an evolving treatment landscape. J. Thorac. Dis. 2019, 11 (Suppl. 9), S1360–S1365. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, J.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomized, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Juan, O.; Popat, S. Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Schonewolf, C.A.; Verma, V.; Post, C.M.; Berman, A.T.; Frick, M.A.; Vachani, A.; Lin, C.; Simone, C.B., 2nd. Outcomes of invasive mediastinal nodal staging versus positron emission tomography staging alone for early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Lung Cancer 2018, 117, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.; Ciunci, C.; Hertan, L.; Gomez, D. Special topics in immunotherapy and radiation therapy: Reirradiation and palliation. Transl. Lung Cancer Res. 2017, 6, 119–130. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csöszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1- Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Morales-Orue, I.; Castilla-Martinez, J.; Zafra-Martin, J.; Kannemann, A.; Blanco, J.; Lloret, M.; Lara, P.C. Stereotactic ablative radiotherapy combined with immune checkpoint inhibitors reboots the immune response assisted by immunotherapy in metastatic lung cancer: A systematic review. Int. J. Mol. Sci. 2019, 20, 2173. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer Version 6. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 20 June 2020).
- Ferlay, J.; Steliarova-Foucher, E.; Lorlet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Dandekar, V.K.; Young, J.; Kiel, K.; Bonomi, P.; Fidler, M.J.; Batus, M.; Sher, D. Efficacy and Tolerability of Palliative Split-Course Thoracic Chemoradiotherapy for Symptomatic Non-Small Cell Lung Cancer. Am. J. Clin. Oncol. 2015, 38, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Couñago, F.; Luna, J.; Guerrero, L.L.; Vaquero, B.; Guillen-Sacoto, M.C.; Gonzalez-Merino, T.; Taboada, B.; Diaz, V.; Rubio-Viqueira, B.; Diaz-Gavela, T.; et al. Management of oligomtastatic non-small cell lung cancer patients: Current controversies and future directions. World J. Clin. Oncol. 2019, 10, 318–339. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodriguez, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 2020, JCO2000828. [Google Scholar] [CrossRef]
- Aupérin, A.; Péchoux, C.L.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Spigel, D.R.; Reckamp, K.L.; Rizvi, N.A.; Poddubskaya, E.; West, H.J.; Eberhardt, W.E.E.; Baas, P.; Antonia, S.J.; Pluzanski, A.; Vokes, E.E.; et al. A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs. docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2015, 33 (Suppl. 15), 8009. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Horn, L.; Borghaei, H.; Spigel, D.R.; Steins, M.; Ready, N.; Quan, L.; Chow, M.; Vokes, E.E.; Felip, E.; et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2015, 33 (Suppl. 18). [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Cobo Dols, M.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Senler, F.C.; Csöszi, T.; Fülöp, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2451. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Langer, C.J.; Gadgeel, S.M.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Pemetrexed-Carboplatin Plus Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC: KEYNOTE-021 Cohort G Update. In Proceedings of the IASLC 18th World Conference on Lung Cancer, Yokohama, Japan, 15–18 October 2017. [Google Scholar]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczȩsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; van Thienen, H.; Blank, C.U. Toxicity patterns with immunomodulating antibodies and their combinations. Semin. Oncol. 2015, 42, 423–428. [Google Scholar] [CrossRef]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Berking, C.; Bergmann, T.; Bockmeyer, C.L.; Eigentler, T.; Fluck, M.; et al. The price of tumor control: An analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS ONE 2013, 8, e53745. [Google Scholar] [CrossRef]
- Connell, P.P.; Hellman, S. Advances in radiotherapy and implications for the next century: A historical perspective. Cancer Res. 2009, 69, 383–392. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Yazan, A.; Puja, V.; Sungjune, K. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Mole, R.H. Whole Body Irradiation—Radiobiology or Medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Demaria, S.; Golden, E.B.; Formenti, S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015, 1, 1325–1332. [Google Scholar] [CrossRef]
- Grass, G.D.; Krishna, N.; Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 2016, 40, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, G.D. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Probst, H.C.; Vuong, V.; Landshammer, A.; Muth, S.; Yagita, H.; Schwendener, R.; Pruschy, M.; Knuth, A.; van den Broek, M. Radiotherapy Promotes Tumor-Specific Effector CD8 + T Cells via Dendritic Cell Activation. J. Immunol. 2012, 189, 558–566. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Liang, H.; Deng, L.; Hou, Y.; Meng, X.; Huang, X.; Rao, E.; Zheng, W.; Maureci, H.; Mack, M.; Xu, M.; et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Vatner, R.E.; Cooper, B.T.; Vanpouille-Box, C.; Demaria, S.; Formenti, S.C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 2014, 4, 325. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. Mechanisms of Tumor Cell–Intrinsic Immune Evasion. Annu. Rev. Cancer Biol. 2018, 4, 213–228. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. Cancer Clin. Trials 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wao, H.; Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Survival of patients with non-small cell lung cancer without treatment: A systematic review and meta-analysis. Syst. Rev. 2013, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, H.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicenter, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Morales-Orue, I.; Rodriguez-Abreu, D.; Lara-Jimenez, P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin. Transl. Radiat. Oncol. 2018, 9, 5–11. [Google Scholar] [CrossRef]
- Torok, J.A.; Salama, J.K. Combining immunotherapy and radiotherapy for the STR treatment. Nat. Rev. Clin. Oncol. 2019, 16, 666–667. [Google Scholar] [CrossRef]
- Sharvedian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Desideri, I.; Francolini, G.; Scotti, V.; Pezzulla, D.; Becherini, C.; Terziani, F.; Paoli, C.D.; Olmetto, E.; Visani, L.; Meattini, I.; et al. Benefit of ablative versus palliative-only radiotherapy in combination with nivolumab in patients affected by metastatic kidney and lung cancer. Clin. Transl. Oncol. 2019, 21, 933–938. [Google Scholar] [CrossRef]
- Ratnayake, G.; Shanker, M.; Roberts, K.; Mason, R.; Hughes, B.G.M.; Lwin, Z.; Jain, V.; O’Byrne, K.; Lehman, M.; Chua, B. Prior or concurrent radiotherapy and nivolumab immunotherapy in non-small lung cancer. Asia Pac. J. Clin. Oncol. 2020, 16, 56–62. [Google Scholar] [CrossRef]
- Samstein, R.; Rimner, A.; Barker, C.A.; Yamada, Y. Combined immune checkpoint blockade and radiation therapy: Timing and dose fractionation associated with greatest survival duration among over 750 treated patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99 (Suppl. 2), S129–S130. [Google Scholar] [CrossRef]
- Tang, C.; Welsh, J.W.; de Groot, P.; Massarelli, E.; Chang, J.Y.; Hess, K.R.; Basu, S.; Curran, M.A.; Cabanillas, M.E.; Subbiah, V.; et al. Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T cells. Clin. Cancer Res. 2017, 23, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.-P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; De Andrade, L.F.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Tang, C.; de Groot, P.; Naing, A.; Raju, U.; Shaaban, S.; Chang, J.Y.; Cushman, T.; Heymach, J.; Dadu, R.; et al. Phase II 5-arm trial of ipilimumab plus lung or liver stereotactic radiation for patients with advanced malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1315. [Google Scholar] [CrossRef]
- Chicas-Sett, R.; Morales-Orue, I.; Castilla-Martinez, J.F.; Blanco, J.; Kannemann, A.; Zafra, J.; Zajac, M.; Lloret, M.; Lara, P.C. I-SABR induces local and abscopal responses in metastatic patients after failure to ICI treatment. Radiother. Oncol. 2019, 133, S24. [Google Scholar] [CrossRef]
- Theelen, W.; Peulen, H.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs. Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Welsh, J.W.; Menon, H.; Tang, C.; Verma, V.; Altan, M.; Hess, K.R.; de Groot, P.; Nguyen, Q.; Simon, G.R.; Ferdinandos Skoulidis, F.; et al. Randomized phase I/II trial of pembrolizumab with and without radiotherapy for metastatic non-small cell lung cancer. J. Clin. Oncol. 2019, 37 (Suppl. 15), 9104. [Google Scholar] [CrossRef]
- Patel, J.D.; Bestvina, C.M.; Karrison, T.; Jelinek, M.J.; Juloori, A.; Pointer, K.; Hoffman, P.C.; Pitroda, S.P.; Vokes, E.E.; Chmura, S.J. Randomized phase I trial to evaluate Concurrent or Sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV non-small cell lung cancer (COSINR study). J. Clin. Oncol. 2020, 38, 9616. [Google Scholar] [CrossRef]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Deshpande, C.; Miller, L.; Patel, P.; Alley, E.; et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1283–1290. [Google Scholar] [CrossRef]
- Cheema, P.K.; Rothenstein, J.; Melosky, B.; Brade, A.; Hirsh, V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr. Oncol. 2019, 26, 37–42. [Google Scholar] [CrossRef]
- Kumar, S.S.; Higgins, K.A.; McGarry, R.C. Emerging Therapies for Stage III Non-Small Cell Lung Cancer: Stereotactic Body Radiation Therapy and Immunotherapy. Front. Oncol. 2017, 7, 197. [Google Scholar] [CrossRef]
- Albain, K.S.; Swann, R.S.; Rusch, V.W.; Turrisi, A.T., 3rd; Shepherd, F.A.; Smith, C.; Chen, Y.; Livingston, R.B.; Feins, R.H.; Gandara, D.R.; et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small cell lung cancer: A phase III randomised controlled trial. Lancet 2009, 374, 379–386. [Google Scholar] [CrossRef]
- Curran, W.J., Jr.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef]
- Vokes, E.E.; Herndon, J.E., 2nd; Kelley, M.J.; Cicchetti, M.G.; Ramnath, N.; Neill, H.; Atkins, J.N.; Watson, D.M.; Akerley, W.; Green, M.R.; et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and leukemia group B. J. Clin. Oncol. 2007, 25, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Neubauer, M.; Yiannoutsos, C.; McGarry, R.; Arseneau, J.; Ansari, R.; Reynolds, C.; Govindan, R.; Melnyk, A.; Fisher, W.; et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncol. J. Clin. Oncol. 2008, 26, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; Flentje, M.; Schmidt, M.; Pöllinger, B.; Gosse, H.; Willner, J.; Ulm, K.; Bronchial Carcinoma Therapy Group. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non-small-cell lung cancer: Study CTRT99/97 by the Bronchial Carcinoma Therapy Group. J. Clin. Oncol. 2006, 24, 4397–4404. [Google Scholar] [CrossRef] [PubMed]
- Flentje, M.; Huber, R.M.; Engel-Riedel, W.; Andreas, S.; Kollmeier, J.; Staar, S.; Dickgreber, N.; Vaissiere, N.; De Almeida, C.; Edlich, B.; et al. GILT--A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non–small cell lung cancer. Strahlenther. Onkol. 2016, 192, 216–222. [Google Scholar] [CrossRef]
- Ahn, J.S.; Ahn, Y.C.; Kim, J.H.; Lee, C.G.; Cho, E.K.; Lee, K.C.; Chen, M.; Kim, D.W.; Kim, H.K.; Min, Y.J.; et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non–small-cell lung cancer: KCSG-LU05-04. J. Clin. Oncol. 2015, 33, 2660–2666. [Google Scholar] [CrossRef]
- Kelly, K.; Chansky, K.; Gaspar, L.E.; Albain, K.S.; Jett, J.; Ung, Y.C.; Lau, D.H.; Crowley, J.J.; Gandara, D.R. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-smallcell lung cancer: SWOG S0023. J. Clin. Oncol. 2008, 26, 450–456. [Google Scholar] [CrossRef]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.E.; Bosquée, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Giaccone, G.; Bazhenova, L.A.; Nemunaitis, J.; Tan, M.; Juhász, E.; Ramlau, R.; van den Heuvel, M.M.; Lal, R.; Kloecker, G.H.; Eaton, K.D.; et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non–small cell lung cancer. Eur. J. Cancer 2015, 51, 2321–2329. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez Aguillo, M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized phase III trial of pemetrexed–cisplatin or etoposide–cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Botticella, A.; Mezquita, L.; Le Pechoux, C.; Planchard, D. Durvalumab for stage III non-small-cell lung cancer patients: Clinical evidence and real-world experience. Ther. Adv. Respir. Dis. 2019, 13, 1753466619885530. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-year overall survival update from the PACIFIC trial. J. Clin. Oncol. 2019, 37 (Suppl. 15). [Google Scholar] [CrossRef]
- Durm, G.A.; Althouse, S.K.; Sadiq, A.A.; Jalal, S.I.; Jabbour, S.; Zon, R.; Kloecker, G.H.; Fisher, W.B.; Reckamp, K.L.; Kio, E.A.; et al. Phase II trial of concurrent chemoradiation with consolidation pembrolizumab in patients with unresectable stage III non–small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Durm, G.A.; Althouse, S.K.; Sadiq, A.A.; Jalal, S.I.; Jabbour, S.; Zon, R.; Kloecker, G.H.; Fisher, W.B.; Reckamp, K.L.; Kio, E.A.; et al. Updated Results of a Phase II Trial of Concurrent Chemoradiation with Consolidation Pembrolizumab in Patients with Unresectable Stage III NSCLC. J. Thorac. Oncol. 2018, 13, S321. [Google Scholar] [CrossRef]
- Gerber, D.E.; Urbanic, J.J.; Langer, C.; Hu, C.; Chang, I.F.; Lu, B.; Movsas, B.; Jeraj, R.; Curran, W.J.; Bradley, J.D. Treatment design and rationale for a randomized trial of cisplatin and etoposide plus thoracic radiotherapy followed by nivolumab or placebo for locally advanced non–small-cell lung cancer (RTOG 3505). Clin. Lung Cancer 2017, 18, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Felip, E.; Dafni, U.; Belka, C.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial. Lung Cancer 2019, 133, 83–87. [Google Scholar] [CrossRef]
- Fitzgerald, K.; Simone, C.B., 2nd. Combining Immunotherapy with Radiation Therapy in Non-Small Cell Lung Cancer. Thorac. Surg. Clin. 2020, 30, 221–239. [Google Scholar] [CrossRef]
- Peters, S.; Felip, E.; Dafni, U.; Tufman, A.; Guckenberger, M.; Irigoyen, A.; Nadal, E.; Becker, A.; Vees, H.; Pless, M.; et al. Efficacy evaluation of concurrent nivolumab addition to a first-line, concurrent chemo-radiotherapy regimen in unresectable locally advanced NSCLC: Results from the European Thoracic Oncology Platform (ETOP 6–14) NICOLAS phase II trial. Ann. Oncol. 2019, 30 (Suppl. 5), mdz259. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.; Mok, I.; Young, J.A.; Phan, S.; Sandler, A.; Papadimitrakopoulou, V.; Heymach, J.; Tsao, A.S. DETERRED: Phase II trial combining atezolizumab concurrently with chemoradiation therapy in locally advanced non-small cell lung cancer. J. Thorac. Oncol. 2018, 3, S320–S321. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Nadal, E.; Insa, A.; Campelo, R.G.; Casal, J.; Domine, M.; Majem, M.; Rodriguez-Abreu, D.; Martinez-Marti, A.; De Castro, J.; et al. OA13.05 NADIM Study: Updated Clinical Research and Outcomes. J. Thorac. Oncol. 2019, 14, S241. [Google Scholar] [CrossRef]
- Senthi, S.; Lagerwaard, F.J.; Haasbeek, C.J.; Slotman, B.J.; Senan, S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol. 2012, 13, 802–809. [Google Scholar] [CrossRef]
- Nesbit, E.G.; Leal, T.A.; Kruser, T.J. What is the role of radiotherapy for extensive-stage small cell lung cancer in the immunotherapy era? Transl. Lung Cancer Res. 2019, 8, S153–S162. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.W.; Heymach, J.V.; Chen, D.; Verma, V.; Cushman, T.R.; Hess, K.R.; Shroff, G.; Tang, C.; Skoulidis, F.; Jeter, M.; et al. Phase I Trial of Pembrolizumab and Radiation Therapy after Induction Chemotherapy for Extensive-Stage Small Cell Lung Cancer. J. Thorac. Oncol. 2020, 15, 266–273. [Google Scholar] [CrossRef]
- Garelli, E.; Rittmeyer, A.; Putora, P.M.; Glatzer, M.; Dressel, R.; Andreas, S. Abscopal effect in lung cancer: Three case reports and a concise review. Immunotherapy 2019, 11, 1445–1461. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.P.; Martin, R.F.; Martin, O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015, 356, 82–90. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 25379–25388. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Chen, D.; Menon, H.; Verma, V.; Guo, C.; Ramapriyan, R.; Barsoumian, H.; Younes, A.; Hu, Y.; Wasley, M.; Cortez, M.A.; et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for metastatic non-small cell lung cancer: Retrospective analysis of two single-institution prospective trials. J. Immunother. Cancer 2020, 8, e000492. [Google Scholar] [CrossRef]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.J.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [PubMed]
- MPDL3280A and Stereotactic Ablative Radiotherapy in Patients with Non-Small Cell Lung Cancer. Available online: https://ClinicalTrials.gov/show/NCT02400814 (accessed on 10 May 2020).
- Tubin, S.; Popper, H.H.; Brcic, L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): Improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat. Oncol. 2019, 14, 21. [Google Scholar] [CrossRef]
- Farach, A.; Farach-Carson, M.C.; Butler, E.B.; Chang, J.C.; Teh, B.S. The role of combined radiation and immunotherapy in breast cancer treatment. J. Radiat. Oncol. 2015, 4, 347–354. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Ghasemzadeh, A.; Nirschl, T.R.; Theodros, D.; Kochel, C.M.; Francica, B.J.; Muroyama, Y.; Anders, R.A.; Sharabi, A.B.; Velarde, E.; et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin. Cancer Res. 2018, 24, 5058–5071. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.E.; Rodríguez, I.; Mayorga, L.; Labiano, T.; Barbes, B.; Etxeberria, I.; Ponz-Sarvise, M.; Azpilikueta, A.; Bolaños, E.; Sanmamed, M.F.; et al. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol. Cancer Ther. 2019, 18, 621–631. [Google Scholar] [CrossRef]
- Gerber, S.A.; Sedlacek, A.L.; Cron, K.R.; Murphy, S.P.; Frelinger, J.G.; Lord, E.M. IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am. J. Pathol. 2013, 182, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Adams, D.K.; He, J.; Kalhor, N.; Zhang, M.; Xu, T.; Gao, H.; Reuben, J.M.; Qiao, Y.; Komaki, R.; et al. Sequential tracking of PD-L1 expression and RAD50 induction in circulating tumor and stromal cells of lung cancer patients undergoing radiotherapy. Clin. Cancer Res. 2017, 23, 5948–5958. [Google Scholar] [CrossRef]
- Chen, D.; Verma, V.; Patel, R.R.; Barsoumian, H.B.; Cortez, M.A.; Welsh, J.W. Absolute Lymphocyte Count Predicts Abscopal Responses and Outcomes in Patients Receiving Combined Immunotherapy and Radiotherapy: A prospective-retrospective analysis of 3 phase I/II Trials. Int. J. Radiat. Oncol. Biol. Phys. 2020. [Google Scholar] [CrossRef]
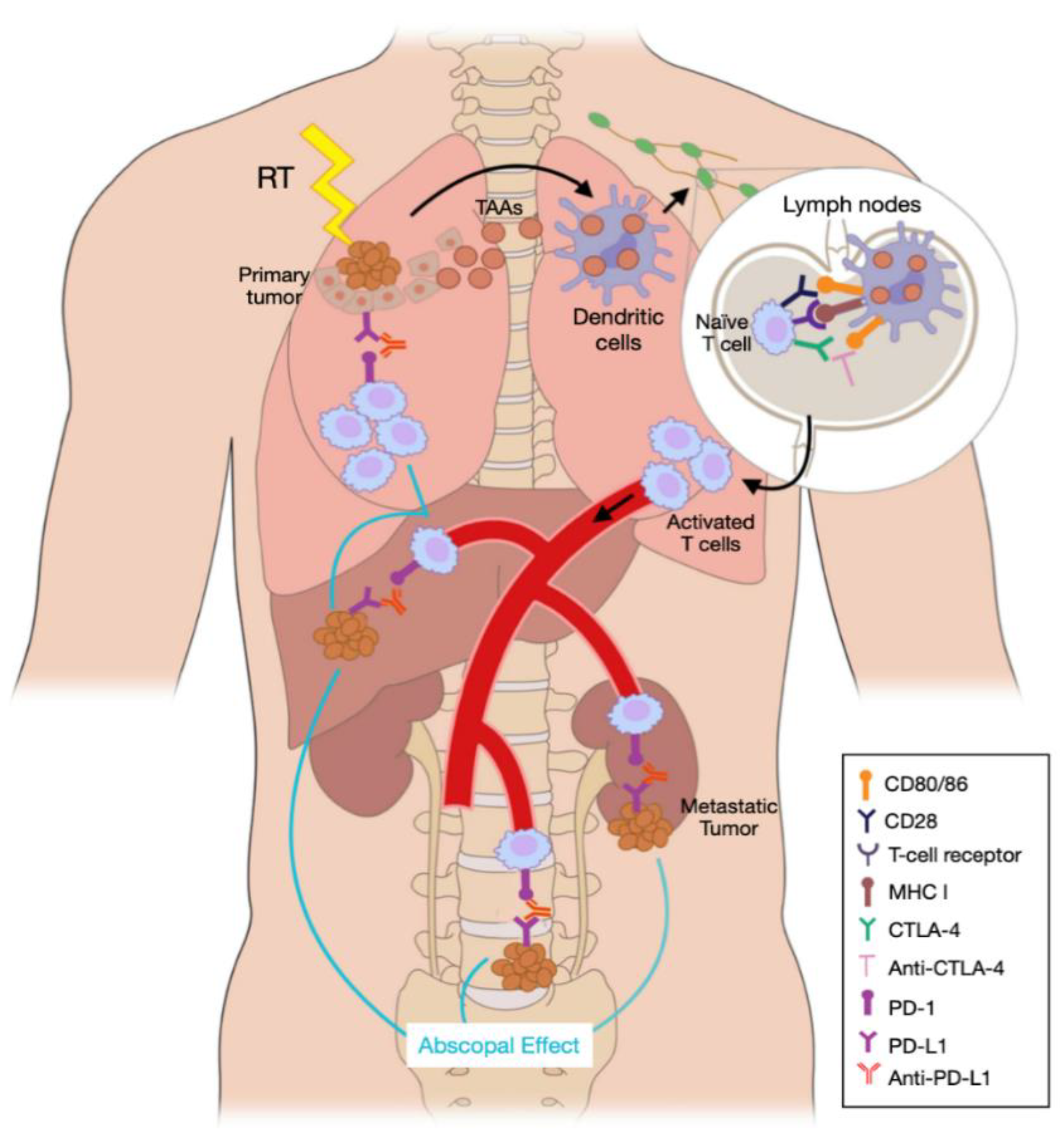
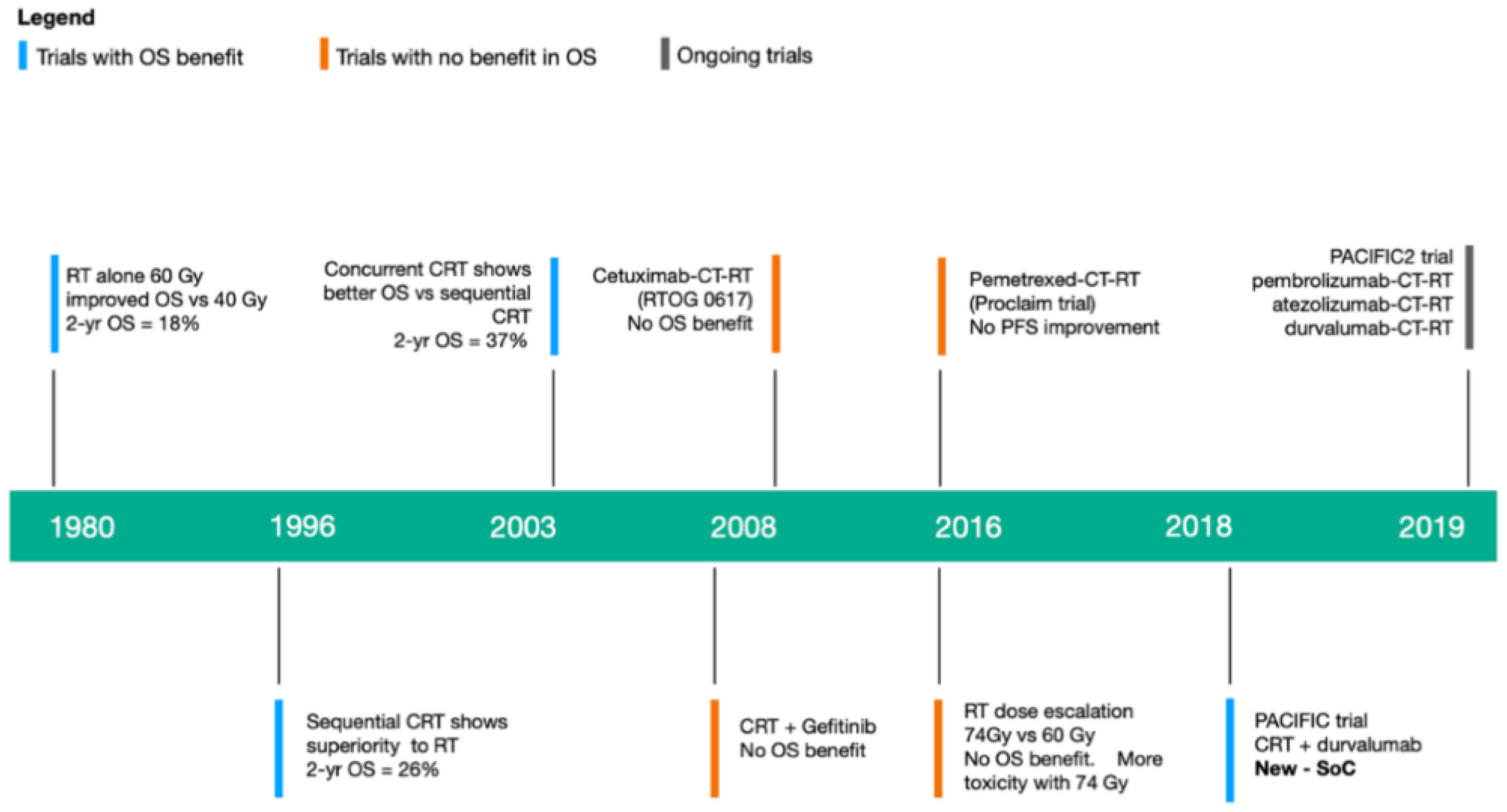
| Indication | Agent | Use | Line |
|---|---|---|---|
| Unresectable, stage III NSCLC | Durvalumab | Monotherapy | Adjuvant after radical chemo-radiotherapy |
| Metastatic NSCLC | Pembrolizumab | Combination with platinum + pemetrexed | 1st line |
| Monotherapy | 2nd line | ||
| Nivolumab | Monotherapy | 2nd line | |
| Atezolizumab | Combination with carboplatin + paclitaxel + bevacizumab | 1st line | |
| Monotherapy | 2nd line | ||
| Metastatic squamous NSCLC | Pembrolizumab | Combination with carboplatin + paclitaxel/nab-paclitaxel | 1st line |
| Monotherapy | 1st line, PD-L1 ≥ 50% | ||
| Metastatic SCLC | Atezolizumab | Combination with carboplatin + etoposide | 1st line |
| Pembrolizumab | Monotherapy | 3rd line | |
| Nivolumab | Monotherapy | 3rd line |
| Trial | Phase | ICI Agent | Design | RT Dose | Primary Endpoint(s) |
|---|---|---|---|---|---|
| NCT03223155 | I randomized | Ipilimumab + nivolumab | SABR + ICI Sequential arm Concurrent arm | 3–5 fx | Number of serious adverse events |
| NCT03158883 | I | Avelumab | ICI + SABR | 50 Gy/5 fx | ORR |
| NCT02239900 | I/II randomized | Ipilimumab | ICI + SABR Multiple arms | 50 Gy/4 fx or 60 Gy/10 fx 1–4 lesions | MTD |
| NCT03176173 RRADICAL | II | Nivolumab, pembrolizumab, atezolizumab | ICI ± SABR | 1–10 fx | PFS at 24 weeks |
| NCT03965468 CHESS | II | Durvalumab | ICI + CT + RT | 1–10 fx | PFS at 12 months |
| NCT03867175 | III randomized | Pembrolizumab | ICI ± SABR (all metastatic lesions) | 3–10 fx | PFS |
| Study | Phase | N | Stage | RT Dose | ICI Agent | ICI Sequence | Status |
|---|---|---|---|---|---|---|---|
| CASE4516 NCT02987998 | 1 | 20 | Resectable IIIA | 45 Gy/25 fx | Pembrolizumab | Neoadjuvant + Adjuvant | Active, not recruiting |
| CLOVER NCT03509012 | 1 | 300 | Unresectable III NSCLC, SCLC, H & N | Conventional RT | Durvalumab | Concurrent | Recruiting |
| NCT03053856 | 2 | 37 | Resectable IIIA N2 | 44 Gy/22 fx | Pembrolizumab | Adjuvant | Not yet recruiting |
| NCT03237377 | 2 | 32 | Resectable IIIA | 45 Gy/25 fx | Durvalumab +/-tremelimumab | Neoadjuvant | Recruiting |
| LUN 16-081 NCT03285321 | 2 | 108 | Unresectable IIIA/B | 59.4–66.6 Gy | Nivolumab +/- ipilimumab | Consolidation | Recruiting |
| CHIO3 NCT04062708 | 2 | 55 | Resectable IIIA/B | 54 Gy | Durvalumab | Neoadjuvant + Adjuvant | Not yet recruiting |
| NCT03871153 | 2 | 25 | Resectable III N2 | 45-61.2 Gy/25–34 fx | Durvalumab | Neoadjuvant + Adjuvant | Recruiting |
| KEYNOTE-799 NCT03631784 | 2 | 216 | Unresectable III | 60 Gy/30 fx | Pembrolizumab | Concurrent + Consolidation | Recruiting |
| NCT03663166 | 1/2 | 50 | Unresectable III | 60Gy/30 fx | Ipilimumab vs. nivolumab | Concurrent vs. Consolidation | Recruiting |
| NCT03102242 | 2 | 63 | Unresectable IIIA/B | 60 Gy/30 fx | Atezolizumab | Neoadjuvant | Active, not recruiting |
| NCT03589547 | 2 | 25 | III | 60 Gy RT followed by 20Gy/2–3 fx SABR | Durvalumab | Consolidation | Recruiting |
| NCT02572843 | 2 | 68 | Resectable IIIA N2 | Conventional RT if R1–2 | Durvalumab | Neoadjuvant + Adjuvant | Active, not recruiting |
| PACIFIC 2 NCT03519971 | 3 | 300 | Unresectable III | Conventional RT | Durvalumab | Concurrent +/- Consolidation | Active, not recruiting |
| PACIFIC 5 NCT03706690 | 3 | 360 | Unresectable III | Conventional RT | Durvalumab | Consolidation | Recruiting |
| PACIFIC 6 NCT03693300 | 2 | 150 | Unresectable III | Conventional RT | Durvalumab | Consolidation | Recruiting |
| MK-3475 NCT03379441 | 2 | 126 | Unresectable IIIA/IIIB | Conventional RT | Pembrolizumab | Consolidation | Not recruiting |
| Which RT technique is more immunogenic? | SABR rather than conventional RT. Every other day rather than consecutive. 6–12 Gy per fraction rather than higher doses. 24 Gy/3 fx and 30 Gy/5 fx are the most frequent in clinical trials. |
| What is the ideal treatment sequence? | Concurrent RT with Anti-PD-1/L1. Sequential RT after Anti-CTLA-4. |
| Which lesions should be treated? | Multisite irradiation rather than single site. Visceral lesions rather than bone. RT to the lymph nodes and bowel could be detrimental. Partial irradiation of bulky tumors can also unleash AEs. |
| Are there any biomarkers that can guide patient selection? | High TGF- β has been associated with worse outcomes. High IFN-I/γ could influence RT effectiveness Lymphopenia could negatively impact the immunogenicity of RT. Currently, no biomarkers are approved for use in clinical practice. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chicas-Sett, R.; Zafra-Martin, J.; Morales-Orue, I.; Castilla-Martinez, J.; Berenguer-Frances, M.A.; Gonzalez-Rodriguez, E.; Rodriguez-Abreu, D.; Couñago, F. Immunoradiotherapy as an Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers 2020, 12, 2178. https://doi.org/10.3390/cancers12082178
Chicas-Sett R, Zafra-Martin J, Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA, Gonzalez-Rodriguez E, Rodriguez-Abreu D, Couñago F. Immunoradiotherapy as an Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers. 2020; 12(8):2178. https://doi.org/10.3390/cancers12082178
Chicago/Turabian StyleChicas-Sett, Rodolfo, Juan Zafra-Martin, Ignacio Morales-Orue, Juan Castilla-Martinez, Miguel A. Berenguer-Frances, Elisa Gonzalez-Rodriguez, Delvys Rodriguez-Abreu, and Felipe Couñago. 2020. "Immunoradiotherapy as an Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent" Cancers 12, no. 8: 2178. https://doi.org/10.3390/cancers12082178
APA StyleChicas-Sett, R., Zafra-Martin, J., Morales-Orue, I., Castilla-Martinez, J., Berenguer-Frances, M. A., Gonzalez-Rodriguez, E., Rodriguez-Abreu, D., & Couñago, F. (2020). Immunoradiotherapy as an Effective Therapeutic Strategy in Lung Cancer: From Palliative Care to Curative Intent. Cancers, 12(8), 2178. https://doi.org/10.3390/cancers12082178





